Abstract
Exosomes, the extracellular secretary nano-vesicles, act as carriers of biomolecules to the target cells. They exhibit several attributes of an efficient drug delivery system. Curcumin, despite having numerous bioactive and therapeutic properties, has limited pharmaceutical use due to its poor water solubility, stability, and low systemic bioavailability. Hence, this study aims to enhance the therapeutic potential of curcumin, a model hydrophobic drug, by its encapsulation into milk exosomes. In the present study, we investigated the stability of free curcumin and exosomal curcumin in PBS and in vitro digestive processes. Additionally, their uptake and trans-epithelial transport were studied on Caco-2 cells. Curcumin in milk exosomes had higher stability in PBS, sustained harsh digestive processes, and crossed the intestinal barrier than free curcumin. In conclusion, the encapsulation of curcumin into the exosomes enhances its stability, solubility, and bioavailability. Therefore, the present study demonstrated that milk exosomes act as stable oral drug delivery vehicles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes are the lipid bilayered nano-vesicles, which carry the cell-specific load of biomolecules like microRNA (miRNA), mRNA, DNA, and proteins, released by the cells in the extracellular environment [1]. They are the functional delivery vehicles that can act as the medium of communication between the cells via transferring their cargo to the target cells influencing their cellular environment [2]. Exosome signaling is known to influence various cellular functions like immunity [3], movement [4], inflammation [5], immunosuppression [6], differentiation [7], proliferation [8], and metastasis [9]. Recently, they have emerged as the efficient delivery nano-vehicles for the variety of drug molecules, including nucleic acids like miRNA [10], siRNA [11], proteins like catalase [12], small molecules like doxorubicin [13], and paclitaxel [14]. These nano-vehicles may possess a great potential in the treatment of severe diseases as a drug delivery vehicle like cancer [13], Parkinson’s disease [12], inflammatory diseases [3], cardiovascular diseases [15], and viral infections [16]. Exosomes have revolutionized the pharmaceutical field as they possess the various properties of an effective drug delivery system. Exosomes can be used as the medium to load unstable drug molecules like curcumin, which can ultimately lead to the enhanced therapeutic effect. It has been widely reported that curcumin, a symmetric molecule with three reactive functional groups, i.e., one di-ketone moiety and two phenolic groups, is anti-inflammatory in nature [17]. It has also been shown to possess the anti-cancer potential, as it inhibits the action of mammalian target of rapamycin complex 1 (mTORC 1), by dissociating raptor from the complex [18]. But it is not able to exhibit its potential therapeutic activity due to its instability, limited solubility, and poor absorption [19]. So, there is a need to develop a delivery vehicle which can potentiate the therapeutic effects of curcumin. In the present study, we made an attempt to use milk exosomes as a delivery vehicle using curcumin as a therapeutic molecule, as bovine milk is the most suitable source of exosomes [20]. The major quest about these nano-vesicles in milk is whether they can be used as an oral drug delivery vehicle which can release the natural contents/encapsulated drug in the blood circulation. There is a need for more focused research that can confirm exosomes as an oral drug delivery vehicle. In this study, we have used an in vitro approach to elucidate the effect of different digestive processes on the stability of curcumin and exosome encapsulated curcumin along with their absorption across intestinal cells to blood circulation. We used a validated in vitro digestion model for free curcumin and exosomal curcumin digestion and then Caco-2 cells (human intestinal epithelial cell model) to demonstrate their stability, uptake, and further their trans-epithelial transport across the monolayer. Our results confirm that curcumin encapsulated into milk exosomes are stable against gastrointestinal digestion and can transfer their contents to the blood circulation crossing the intestinal barrier, which ultimately opens up a new horizon of their application as a stable oral drug delivery vehicle.
Methods
Chemical and Reagents
All the chemicals and enzymes for in vitro digestion were purchased from Sigma-Aldrich (St. Louis, MO, USA), except bile extract, which was purchased from Santa Cruz Biotechnology. For cell culture, Dulbecco’s modified Eagle’s medium (DMEM), non-essential amino acids, glutamine, and amphotericin were purchased from Sigma-Aldrich (St. Louis, MO, USA), whereas antibiotics (penicillin-streptomycin) were purchased from Gibco™ (Grand Island, NY).
Collection of Milk and Preparation of Milk Whey
Milk was collected from the healthy, non-pregnant, and early lactating Murrah buffaloes of National Dairy Research Institute, Karnal. The animals were fed with similar diet and maintained in the same herd. The milk samples were collected in 15-ml tubes and then transferred to the laboratory on ice. Milk was centrifuged at 1200g at 4 °C for 10 min using Hettich Mikro 22 R centrifuge (Tuttlingen, Germany). The fat layer and pellet fractions were carefully eliminated. The supernatant was carefully transferred into a separate tube and centrifuged at 21,500g at 4 °C for 30 min. The supernatant was transferred into the separate tubes and again, centrifuged at 21,500g at 4 °C for 1 h. Discarding the fat and pellet fraction, the supernatant was filtered through 0.45-μm filter to get the clear whey fraction. The milk whey fraction was stored at −80 °C until use.
Incorporation of Curcumin Into Milk Whey
Five-milligram curcumin was added and mixed in 1.5-ml filtered clear whey fraction. Then, it was kept undisturbed to incubate overnight at 4 °C.
Isolation of Exosomes and Exosomal Curcumin
miRCURY™ Exosome Isolation Kit (Exiqon) was used for the isolation of exosomes from milk. Milk whey incubated with the curcumin was centrifuged at 2000g for 10 min at 4 °C to remove the free curcumin. The supernatant was transferred into a sterile tube for the isolation of curcumin-positive exosomes. Filtered milk whey fraction was used for the isolation of curcumin-negative exosomes. Precipitation buffer (400 μl) was added to both the 1-ml whey fractions and incubated at 4 °C for 3 h. After 3 h, they were centrifuged at 10,000g for 30 min at 20 °C to get the exosome pellet for both, i.e., exosomes and exosomal curcumin. The supernatant was discarded and the pellet was resuspended in 100 μl PBS. The resuspended exosomes were stored at −20 °C until analysis.
Loading Efficiency of Curcumin
The loading efficiency of curcumin on exosomes was calculated as per a reported procedure [21]. The curcumin-encapsulated exosomes were prepared as described above. The supernatant was collected and the encapsulated curcumin was quantified spectrophotometrically. From the free curcumin available in the supernatant, the entrapped curcumin was calculated and was expressed as loading efficiency.
BCA Protein Assay
Exosomal protein estimation was done using Pierce™ BCA Protein Assay Kit. Ten microliter of exosomal suspension (diluted in 1:10 ratio with PBS) was used for the protein estimation, and absorbance was compared with the serially diluted BSA standards provided in the kit following manufacturer’s instructions. Values of exosomal protein concentrations were extrapolated using standard curve with r 2 = 0.997 plotted using diluted BSA standards.
Characterization of Exosomes
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was performed for exosomes using a concentration of ∼20 μg/ml. Ten microliter of diluted exosomal solution was applied on coverslip and allowed to air dry completely. The dried exosomal smear was fixed with 200 μl glutaraldehyde (2%) for 4 h and then serially dehydrated with 30, 50, 60, 70, 80, and 90% for 10 min. Final dehydration was done with 100% ethanol for overnight. The samples were then air dried followed by gold coating. Gold coating was done using ion coater (Hitachi IB-3, Japan) with an ion current at 6–8 mA and a vacuum of 0.05–0.07 torr for 2–4 min. Scanning electron microscope (EVO® 18, Carl ZEISS Special Edition-UK) was used to image the coated exosomes.
Dynamic Light Scattering Analysis of Exosomes
Size measurements of exosomes were done using the Zetasizer (Malvern Instruments Limited, UK). Exosomes diluted in PBS (1:100) were analyzed in the equilibration time of 120 s at a constant temperature at 25 °C. A laser beam at 632.8 nm was applied to the exosomal suspension, and scattered light was detected by an avalanche photodiode detector (APD) at 173° and non-invasive backscattering (NIBS) optics. An average of the three measurements was considered for determining the size of exosomes.
Analysis of Curcumin Concentration
The concentration of curcumin in samples was determined using a nano-photometer (Implen GmbH, Germany). Standard curve of the curcumin was plotted to evaluate the concentration of curcumin. Briefly, a stock of curcumin (100 μg/ml) was prepared by dissolving 1 mg of curcumin in 10 ml acetonitrile. Standard solutions were prepared by diluting stock solution with acetonitrile to obtain the solution in the concentration range of 8–100 μg/ml. Absorbance was measured at 420 nm to calculate the concentration of curcumin.
Determination of In Vitro Solubility of Curcumin and Exosomal Curcumin
In vitro solubility of curcumin: 1 mg curcumin was dissolved in 1 ml PBS. It was allowed to incubate on ice for 30 min, followed by centrifugation at 5000 rpm for 5 min to remove the free curcumin. The supernatant was transferred into a sterile centrifuge tube. Absorbance was measured at wave scan in the range of 250–800 nm.
In vitro solubility of exosomal curcumin: 1 mg curcumin was added and mixed with 10-μl exosomes. The exosomal solution and curcumin mixture were vortexed and spun for 5 s. Then, the mixture was incubated for 30 min at room temperature. After incubation, 1 ml 1× PBS was added to it and then incubated further on the ice for 30 min, followed by centrifugation at 5000 rpm for 5 min to remove the free curcumin. The supernatant was transferred into the sterile centrifuge tube, and the absorbance was measured using wave scan in the range of 250–800 nm. The spectrograph was obtained using a spectrophotometer.
Determination of Stability of Free Curcumin and Exosomal Curcumin
Identical concentrations of curcumin and exosomal curcumin were taken in separate tubes. PBS was added to both the tubes and incubated in the dark at 37 °C water bath. At 30, 60, 90, 120, and 150 min, respectively, samples were taken and absorbance was measured at a wavelength of 420 nm. The concentrations of in vitro curcumin and exosomal curcumin at the beginning were set as 1.0. The fold reduction of the curcumin concentration at each time point was compared to the beginning.
In Vitro Digestion of Free Curcumin and Exosomal Curcumin
Salivary, gastric, pancreatic, and bile juices were prepared, respectively, as described earlier [22]. Using a rotating wheel at 37 °C, 2.25 ml of curcumin entrapped in exosomes and identical amount of free curcumin (control) were initially mixed with 3 ml of salivary juice and incubated for 5 min. Further, the mixture was incubated with 6 ml of gastric juice for 120 min. Then, 6 ml of pancreatic juice and 3 ml of bile juice were simultaneously added to the earlier mixture and incubated for 120 min. After incubation, absorbance of both the solutions was measured at 420 nm to determine the fraction of free curcumin.
Caco-2 Cell Culture
The human adenocarcinoma cell line, Caco-2 cells, was purchased from National Centre for Cell Science (Pune), India. Caco-2 cells were cultured in DMEM along with 10% FBS, 2 mM glutamine, non-essential amino acids, and antibiotics (penicillin-streptomycin (1%) and amphotericin (25 μg/ml)) in a humidified atmosphere at 37 °C with 5% CO2 in air. Cells were sub-cultured in T-25-cm2 flask with seeding density 1 × 106 cells/flask and took 8–9 days to form a confluent monolayer. Replacement of media for cells was done after every 2–3 days. Cells between the passages 37 to 55 were used for the experiments in this study.
Fluorescence Microscopy
Caco-2 cells were seeded with the density of 10,000 cells/well for the detection of uptake of exosomal curcumin. Exosomes encapsulating curcumin were added to the Caco-2 cells with a concentration of 200 μg exosomal protein/200 μl media and incubated at 37 °C for different time intervals for 3 h. The cells were washed with PBS at the respective time intervals. The cells were fixed with 4% formaldehyde for 20 min and washed with PBS for four times. Later, permeabilization of the cells was performed with 0.2% Triton-X 100 for 10 min; the cells were washed again with PBS for four times and stained with DAPI solution (Sigma-Aldrich, St. Louis, MO, USA) to stain the nuclei. The cells were visualized by a fluorescence microscope (Nikon Eclipse Ti, Japan).
Trans-Epithelial Transport Studies
To carry out the transport studies of curcumin, Caco-2 cells were seeded with density 1 × 105 cells/well in 24-well plate with permeable supports having membrane pore size of 0.4 μm coated with fibrillar collagen (Corning). Experiments were performed post-21 days culture of Caco-2 cells on permeable supports to allow the formation of the differentiated confluent monolayer. The integrity of the Caco-2 cell monolayer on the permeable supports was checked using phenol red dye test [23]. Both apical as well as basolateral chamber were washed with PBS three times, followed by adding 500 μl DMEM containing phenol red in the upper chamber and 500 μl PBS in the basolateral chamber. Absorbance was measured at 558 nm to detect phenol red using 100-μl aliquots from apical as well as basolateral chamber after incubation at 37 °C for 60 min. PBS was added to the both apical and basolateral chambers in control as well as treated wells. The identical concentrations of curcumin and exosomal curcumin were added to the apical chamber in PBS and incubated for 3 h. After 3 h, the fluid was collected from the basolateral chamber and absorbance of both the solutions was measured at 420 nm to determine the fraction of curcumin undergoing trans-epithelial transport.
Results
Solubility, Stability, and Characterization of Curcumin-Encapsulated Milk Exosomes
Curcumin encapsulated into exosomes was found to be more soluble and stable as compared to free curcumin. The loading efficiency of milk exosomes to load the curcumin was found to be 70.46%. Exosomes and curcumin-encapsulated exosomes were characterized by using various techniques. Scanning electron microscopy revealed no change in the morphology of curcumin-encapsulated exosomes as compared to exosomes without curcumin (Fig. 1A). The size of both (exosomes with and without curcumin) was found to be in between the range of 30–100 nm. Zetasizer analyzer confirmed the size of milk exosomes and curcumin-encapsulated exosomes. The polydispersity index was less than 0.5 for these nano-structures when analyzed according to the number weighted distribution at 37 °C in the Zetasizer (Fig. 1B). These studies indicate that curcumin-encapsulated exosomes maintain their characteristic features. In addition, curcumin encapsulated into exosomes was found to be more soluble and stable as compared to free curcumin when assessed in PBS (Fig. 2). In addition, it was also found that exosomal curcumin was more stable after in vitro digestion (Fig. 3). These findings indicate that milk exosomes efficiently carry the curcumin, protecting against the harsh action of digestive juices.
Enhanced stability and solubility of exosomal curcumin than free curcumin. a Identical concentrations of curcumin and exosomal curcumin were taken in separate tubes. PBS was added to both the tubes and incubated in the dark at 37 °C water bath. At 30, 60, 90, 120, and 150 min, respectively, samples were taken and absorbance was measured at a wavelength of 420 nm. The concentration of in vitro curcumin and exosomal curcumin at the beginning were set as 1.0. The fold reduction of the curcumin concentration at each time point was compared to the beginning. b Solubility studies were conducted, and the graphs of curcumin and exosomal curcumin were obtained using spectrophotometer scanned between 250 and 800 nm. An absorbance maximum of curcumin was observed at 420 nm
Uptake and Trans-Epithelial Transport of Curcumin Encapsulated in Milk Exosomes Across Intestinal Epithelium in Vitro
Fluorescence microscopy studies revealed the localization of curcumin in the Caco-2 cells via milk exosomes (Fig. 4). Trans-epithelial transport of curcumin was confirmed by the analysis of the curcumin from the fluid collected from basolateral chambers of treated (encapsulated into exosomes) and control (without exosome encapsulation) wells. The concentration of curcumin was significantly (P < 0.05) higher in the treatment wells compared to the control (Fig. 5). This study confirmed that exosomal curcumin can cross the intestinal barrier surviving intestinal peptidases and gets secreted into the basolateral chamber. In addition, it also confirmed that encapsulated curcumin is more bioavailable than free curcumin.
Visualization of exosomal curcumin in Caco-2 cells. Exosomes encapsulating curcumin were added to the Caco-2 cells with a concentration of 200 μg exosomal protein/200 μl media and incubated at 37 °C for 2 h. After washing, the cells were fixed with 4% formaldehyde for 20 min and washed with PBS for three times. Later, permeabilization of the cells was performed with 0.2% Triton-X 100 for 10 min; the cells were washed again with PBS for three times and stained with DAPI solution. Uptake of exosomal curcumin was determined using fluorescence microscope. The scale bar is 100 μm
Increased transport of curcumin across intestinal epithelial layer in vitro via exosomes. Caco-2 cells were seeded with density 1 × 105 cells/well on transwell permeable supports for 21 days. Equivalent amounts of curcumin and exosomal curcumin were added to the cells in the upper chamber. Incubation was done for 3 h. Transepithelial transport of curcumin was measured using spectrophotometer from the PBS collected from the basolateral chamber of treated (exosomal curcumin) and control (free curcumin) wells. Y axis represents the fraction of curcumin left after trans-epithelial transport across Caco-2 monolayer, and the X axis represents the comparison between treated wells and the control wells. Different alphabets represent the significant difference of trans-epithelial transport of curcumin in control and treated wells (P < 0.05)
Discussion
Eukaryotic cells release exosomes into the extracellular space by fusion of internal multivesicular compartments [24]. These nano-vesicles are constitutively released from many cell types and found in different body fluids like saliva [25], milk [26], semen [27], urine [28], and cerebro-spinal fluid [29]. The membrane of exosomes is rich in lipids such as ceramide, sphingolipid, and cholesterol with a density between 1.15 and 1.19 g/ml [30]. They play an eminent role in cell-cell communication [31]. Exosomes, being naturally present in the biological system, have long circulating half-life and are non-immunogenic in nature. Recently, it has been demonstrated that these exosomes are very stable even under harsh conditions like low pH and high temperature [32, 33]. Recognizing these attributes, in the present study, we made an attempt to use milk exosomes as a delivery vehicle while curcumin was taken as the therapeutic molecule. Zetasizer measurements indicated a unimodal size distribution of milk-derived exosomes and exosomes encapsulating curcumin. Scanning electron microscopy analysis found that the size and shape of milk-derived exosomes and curcumin-incorporated exosomes were almost similar. Our results corroborate well with previous studies where it has been shown that incorporation of curcumin into EL-4-derived exosomes did not change the shape and morphology of exosomes [34]. We concluded that the sample preparation of the present study contains a homogeneous population of exosomes and devoid of other microvesicles. The shape and morphology of exosomes were maintained because curcumin gets self-assembled into the exosomes.
Various curcumin delivery vehicles like liposomes [35] and ferritin [36] have been used to increase the solubility of the curcumin, but synthetic delivery vehicles have certain limitations. Synthetic carriers are sensitive to the attack by complement system, coagulation factor, opsonin, and antibodies in the circulation [37]. Due to their inherent limitations, we emphasized on exosomes which are more stable and non-immunogenic in nature. The present results show that solubility of exosomal curcumin is higher in PBS (pH 7.4) than free curcumin. Our results corroborate well with previous studies where it has been shown that incorporation of curcumin into EL-4 (mouse lymphoma cell line) derived exosomes increases the solubility of curcumin [34]. Previous studies support our results, which showed that after incorporation in lipid nano-particles, the solubility of curcumin becomes higher than that of untreated curcumin [38]. Curcumin is a hydrophobic molecule which makes it insoluble in water [39]. Water-insoluble compounds can become water soluble with higher dissolution velocity and saturation solubility, if they are in the form of nano-dispersion with a particle size in the nano-meter range [40]. The solubility of curcumin is improved slightly in basic conditions [41]. The higher solubility of exosomal curcumin is due to the binding of curcumin to exosomes [34]. From previous studies and our results, we can conclude that higher solubility of curcumin when encapsulated in exosome is due to the binding of curcumin to the exosomes because the rate of dissolution of curcumin is increased by increasing its surface area. After encapsulation of curcumin into exosomes, the surface area of curcumin is increased as it gets self-assembled into nano-sized vesicles, i.e., exosomes [39]. Non-covalent interactions are possibly involved in the interaction between curcumin molecules and inner surface of the exosomes.
Curcumin has three important functionalities: an aromatic o-methoxy phenolic group, α,β-unsaturated β-diketone moiety, and a seven-carbon linker [42]. Curcumin undergoes rapid hydrolytic degradation at neutral and basic pH, which becomes a significant disadvantage in its therapeutic use [43, 44]. Our results demonstrated that exosomal curcumin was more stable than that of free curcumin in PBS (pH 7.4). The present results corroborate well with previous studies where it has been shown that encapsulation of curcumin into EL-4-derived exosomes increases the stability of curcumin [34]. From previous studies and our results, we can conclude that exosomes contain a lipid bilayer load curcumin through physical entrapment. Curcumin can be self-assembled into the lipid bilayer of exosomes through the hydrophobic interaction between the hydrophobic tail and hydrophobic drug. Curcumin participates in nucleophilic addition reaction, i.e., Michael addition which occurs through unsaturated ketone as an acceptor (α,β-unsaturated β-diketone moiety of curcumin) and anions of –OH and −SH as donors (proteins in exosomes). Through this reaction, α,β-unsaturated β-diketone moiety of curcumin is protected from degradation after incorporation into exosomes.
Next, we evaluated whether curcumin incorporated in exosomes is stable to the in vitro digestion. Our results showed that exosomal curcumin is more stable than free curcumin after in vitro digestion. Previous studies have been demonstrated that these exosomes are very stable even under harsh conditions like low pH and high temperature [32, 33, 45]. Accordingly, our results suggested that curcumin encapsulated in these stable exosomes is stable at optimum temperature, high and low pH, and different enzymatic treatments. However, free curcumin is highly susceptible to pH change and leads to the destruction of its structure under in vitro digestion. As per our knowledge, this is the first report studying the effect of all digestive processes in determination of stability of exosomal curcumin.
Milk exosomes encapsulating curcumin were taken up by human intestinal cells and crossed the barrier of intestinal epithelium in vitro. We studied the uptake of curcumin encapsulated in the milk exosomes in the human intestinal cell model, Caco-2 cells, after ensuring their stability to the digestive processes. The Caco-2 cell culture system represents the well characterized in vitro system that closely mimics the process of absorption and the transport of drugs as well as nutrients across the human intestinal epithelium for carrying out the nutritional and pharmacological research [46, 47]. The uptake of bovine milk exosomes is mediated via endocytosis [48]. We visualized the dynamic uptake phenomena of the exosomal curcumin in Caco-2 cells by using fluorescence microscopy. Specifically, exosomal curcumin was found to be present in the cytosol of treated Caco-2 cells. Curcumin uptake studies were also conducted in human pancreatic adenocarcinoma cells [49] and endothelial cells [50]. Caco-2 cells in monolayer, cultured on the permeable inserts for 21 days, behave like fully differentiated epithelium acting as an intestinal barrier, a best suited model for trans-epithelial transport and bioavailability studies [51]. The present results of transwell experiments conducted with Caco-2 cells demonstrated that the curcumin encapsulated in bovine milk exosomes can pass through the differentiated intestinal monolayer. These results further indicated that free curcumin is more vulnerable to degradation, whereas association with exosomes makes it more stable in a physiological environment. The present results well corroborate with the earlier results [34]. Our experimental cascade elucidated that the exosomal curcumin is more resistant to digestion processes and can pass through the intestinal barrier into the blood circulation, as compared to free curcumin. Considering the previous reports, buffalo milk exosomes also contain miRNAs. They can transfer its encapsulated miRNAs along with its encapsulated drug to the target cells. miR-21 is one of the abundant miRNAs present in the buffalo milk exosomes [45]. Interestingly, curcumin shows anti-cancer effects, which may be via regulating the expression of miR-21 [52]. Curcumin inhibits the transcription of MIR-21 gene by binding to its promoter and also increases its exclusion from the cells via exosomes [52]. Curcumin affects various processes in the cancerous cells like proliferation, apoptosis, metastasis, and anti-cancer drug resistance, mediated by miR-21. These effects can be mediated via different pathways like phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), programmed cell death protein 4 (PDCD4) [53] and NF-κB pathways [52]. Thus, in addition to the therapeutic effects of curcumin encapsulated in exosomes, the transfer of milk exosomal miRNAs can also lead to the adverse effects to the target cells.
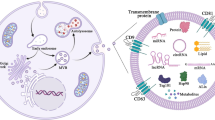
In conclusion, curcumin encapsulated in exosomes is stable enough to resist the harsh environment of human digestive system and can cross the barrier of intestinal epithelium efficiently, with respect to the free curcumin as summerised in Fig. 6. The protective environment provided by the exosomes to the encapsulated curcumin leads to the potential of exosomes as an efficient oral drug delivery vehicle.
Schematic representation of the methodology and concept representing a) Incorporation of curcumin in the milk exosomes., b) Enhanced solubility of exosomal curcumin in PBS and stability of exosomal curcumin than free curcumin in PBS and against digestive processes. c) Enhanced trans-epithelial transport of exosomal curcumin than free curcumin across intestinal barrier
References
Mathivanan, S., Ji, H., & Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics, 73, 1907–1920.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9, 654–659.
Wang, J., Yao, Y., Xiong, J., Wu, J., Tang, X., & Li, G. (2015). Evaluation of the inflammatory response in macrophages stimulated with exosomes secreted by Mycobacterium avium-infected macrophages. BioMed Research International, 2015, 1–9.
Sung, B. H., Ketova, T., Hoshino, D., Zijlstra, A., & Weaver, A. M. (2015). Directional cell movement through tissues is controlled by exosome secretion. Nature Communications, 6, 1–14.
Holder, B., Jones, T., Sancho, S. V., Rice, T. F., Donaldson, B., Bouqueau, M., Forbes, K., & Kampmann, B. (2016). Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic, 17, 168–178.
Wang, J., De, V. K., Faict, S., Frassanito, M. A., Ribatti, D., Vacca, A., & Menu, E. (2016). Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. The Journal of Pathology, 239, 162–173.
Karlsson, T., Lundholm, M., Widmark, A., & Persson, E. (2016). Tumor cell-derived exosomes from the prostate cancer cell line TRAMP-C1 impair osteoclast formation and differentiation. PloS One, 11, 1–12.
Harada, T., Yamamoto, H., Kishida, S., Kishida, M., Awada, C., Takao, T., & Kikuchi, A. (2016). Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Science, 108, 42–52.
Wu, L., Zhang, X., Zhang, B., Shi, H., Yuan, X., Sun, Y., Pan, Z., Qian, H., & Xu, W. (2016). Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumour Biology, 37, 12169–12180.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L., & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy- Nucleic Acids, 2, 1–11.
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., & Wood, M. J. A. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29, 341–345.
Haney, M. J., Klyachko, N. L., Zhao, Y., Gupta, R., Plotnikova, E. G., He, Z., Patel, T., Piroyan, A., Sokolsky, M., Kabanov, A. V., & Batrakova, E. V. (2015). Exosomes as drug delivery vehicles for Parkinson’s disease therapy. Journal of Controlled Release, 207, 18–30.
Tian, Y., Li, S., Song, J., Ji, T., Zhu, M., Anderson, G. J., et al. (2013). A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 35, 2383–2390.
Kim, M. S., Haney, M. J., Zhao, Y., Mahajan, V., Deygen, I., Klyachko, N. L., Inskoe, E., Piroyan, A., Sokolsky, M., Okolie, O., Hingtgen, S. D., Kabanov, A. V., & Batrakova, E. V. (2016). Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine, 12, 655–664.
Davidson, S. M., Takov, K., & Yellon, D. M. (2016). Exosomes and cardiovascular protection. Cardiovascular Drugs and Therapy, 31, 77–86.
Kouwaki, T., Fukushima, Y., Daito, T., Sanada, T., Yamamoto, N., Mifsud, E. J., Leong, C. R., Tsukiyama-Kohara, K., Kohara, M., Matsumoto, M., Seya, T., & Oshiumi, H. (2016). Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Frontiers in Immunology, 7, 1–13.
Ferreira, V. H., Nazli, A., Dizzell, S. E., Mueller, K., & Kaushic, C. (2015). The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PloS One, 10, 1–18.
Beevers, C. S., Chen, L., Liu, L., Luo, Y., Webster, N. J., & Huang, S. (2009). Curcumin disrupts the mammalian target of rapamycin-raptor complex. Cancer Research, 69, 1000–1008.
Gordon, O. N., Luis, P. B., Sintim, H. O., & Schneider, C. (2015). Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. The Journal of Biological Chemistry, 290, 4817–4828.
Sun, Q., Chen, X., Yu, J., Zen, K., Zhang, C. Y., & Li, L. (2013). Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein & Cell, 4, 197–210.
Athira, G., & Jyothi, A. (2014). Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. International Journal of Pharmacy and Pharmaceutical Sciences, 6, 171–176.
Kopf-Bolanz, K. A., Schwander, F., Gijs, M., Verge’res, G., Portmann, R., & Egger, L. (2011). Validation of an in vitro digestive system for studying macronutrient decomposition in humans. Journal of Nutrition, 142, 245–250.
Vij, R., Reddi, S., Kapila, S., & Kapila, R. (2016). Transepithelial transport of milk derived bioactive peptide VLPVPQK. Food Chemistry, 190, 681–688.
Colombo, M., Raposa, G., & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289.
Lasser, C., Alikhani, V. S., Ekstrom, K., Eldh, M., Paredes, P. T., & Bossios, A. (2011). Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. Journal of Translational Medicine, 9, 1–8.
Munagala, R., Aqil, F., Jeyabalan, J., & Gupta, R. (2016). Bovine milk-derived exosomes for drug delivery. Cancer Letters, 371, 48–61.
Madison, M. N., Roller, R. J., & Okeoma, C. M. (2014). Human semen contains exosomes with potent anti-HIV activity. Retrovirology, 11, 1–15.
Dear, J. W., Street, J. M., & Bailey, M. A. (2013). Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signaling. Proteomics, 13, 1572–1580.
Street, J. M., Barran, P. E., Mackay, C. L., Weidt, S., Balmforth, C., & Walsh, T. S. (2012). Identification and proteomic profiling of exosomes in human cerebrospinal fluid. Journal of Translational Medicine, 10, 1–7.
Thery, C., Ostrowski, M., & Segura, E. (2009). Membrane vesicles as conveyors of immune response. Nature Reviews Immunology, 9, 581–593.
Toro, J. D., Herschlik, L., Waldner, C., & Mongini, C. (2015). Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutics applications. Frontiers in Immunology, 6, 1–12.
Ban, J., Lee, M., Im, W., & Kim, M. (2015). Low pH increases the yield of exosome isolation. Biochemical and Biophysical Research Communication, 461, 76–79.
Malik, Z. A., Kott, K. S., Poe, A. J., Kuo, T., Chen, L., Ferrara, K., & Knowlton, A. (2013). Cardiac myocyte exosomes: stability, HSP60 and proteomics. American Journal of Physiology Heart and Circulatory Physiology, 304, H954–H965.
Sun, D., Zhuang, X., Xiang, X., Liu, Y., Zhang, S., Liu, C., Barnes, S., Grizzle, W., Miller, D., & Zhang, H. (2010). A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy, 9, 1606–1614.
Choudhury, S., Das, N., Ghosh, S., Ghosh, D., Chakroborty, S., & Ali, N. (2016). Vesicular (liposomal and nanoparticulated) delivery of curcumin: a comparative study on carbon tetrachloride mediated oxidative hepatocellular damage in rat model. International Journal of Nanomedicine, 11, 2179–2193.
Chen, L., Bai, G., Yang, R., Zhao, G., Xu, C., & Leung, W. (2014). Encapsulation of curcumin in recombinant human H-chain ferritin increases its water-solubility and stability. Food Research International, 62, 1147–1153.
Boorn, J., Schlee, M., Coch, C., & Hartmann, G. (2011). siRNA delivery with exosome nanoparticles. Nature Biotechnology, 29, 325–326.
Anand, P., Kunnumakkara, A., Newman, R., & Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics, 4, 807–818.
Lee, W., Loo, C., Bebawy, M., Luk, F., Masen, R., & Rohanizadeh, R. (2013). Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Current Neuropharmacology, 11, 338–378.
Sutradhar, B., Khatun, S., & Luna, I. (2013). Increasing possibilities of nanosuspension. Journal of Nanotechnology, 12, 204–217.
Priyadarsini, K. I. (2014). The chemistry of curcumin: from extraction to therapeutic. Molecules, 19, 20091–20112.
Priyadarsini, K. (2013). Chemical and structural features influencing the biological activity of curcumin. Current Pharmaceutical Design, 19, 2093–2100.
Wang, Y. J., Pan, M. H., Cheng, A. L., Lin, L. I., Ho, Y. S., Hsieh, C. Y., & Lin, J. K. (1997). Stability of curcumin in buffer solutions and characterization of its degradation products. Journal of Pharmaceutical and Biomedical Analysis, 15, 1867–1876.
Tonnesen, H. H., & Karlsen, J. (1985). Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Zeitschrift fur Lebensmittel- Untersuchung- Forschung, 180, 402–404.
Baddela, V. S., Nayan, V., Rani, P., Onteru, S. K., & Singh, D. (2015). Physicochemical biomolecular insights into buffalo milk-derived nanovesicles. Applied Biochemistry and Biotechnology, 178, 544–577.
Hidalgo, I. J., Raub, T. J., & Borchardt, R. T. (1989). Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology, 96, 736–749.
Meunier, V., Bourrié, M., Berger, Y., & Fabre, G. (1995). The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biology and Toxicology, 11, 187–194.
Wolf, T., Baier, S. R., & Zempleni, J. (2015). The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. Journal of Nutrition, 145, 2201–2206.
Osterman, C. J., Lynch, J. C., Leaf, P., Gonda, A., Ferguson, B. H. R., Griffiths, D., & Wall, N. R. (2015). Curcumin modulates pancreatic adenocarcinoma cell-derived exosomal function. PloS One, 10, 1–17.
Taverna, S., Fontana, S., Monteleone, F., Pucci, M., Saieva, L., De Caro, V., Cardinale, V. G., Giallombardo, M., Vicario, E., Rolfo, C., Leo, G. D., & Alessandro, R. (2016). Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenicphenotype via exosomal miR-21. Oncotarget, 7, 30420–30439.
Artursson, P., Palm, K., & Luthman, K. (2001). Caco-2 monolayers in experimental and theoretical predictions of drug transport. Advanced Drug Delivery Reviews, 46, 27–43.
Chen, J., Xu, T., & Chen, C. (2015). The critical roles of miR-21 in anti-cancer effects of curcumin. Annals of Translational Medicine, 3, 330.
Mudduluru, G., George-William, J. N., Muppala, S., Asangani, I. A., Kumarswamy, R., Nelson, L. D., & Allgayer, H. (2015). Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Bioscience Reports, 31, 185–197.
Acknowledgments
We are thankful to the Director, NDRI, Karnal, for providing the necessary facilities for this work. We are grateful to CRP on Nanotechnology-ICAR for the support. We acknowledge Dr. S. K. Tomar for providing the SEM facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vashisht, M., Rani, P., Onteru, S.K. et al. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl Biochem Biotechnol 183, 993–1007 (2017). https://doi.org/10.1007/s12010-017-2478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2478-4