Low-carbon pipe steel’s corrosion rate and hydrogenation in a chloride-acetate solution with CO2/H2S mixtures at different temperatures and pressures were investigated. The rate of corrosion and hydrogenation depend on the H2S concentration and the properties of corrosion films. In a solution with a pressure ratio of \({P}_{{\text{CO}}_{2}}:{P}_{{\text{H}}_{2}\text{S}}=30:1\) and at the beginning of exposure at a pressure ratio of 3:1 corrosion slows down due to the formation of Fe1+xS mackinawite film. Over time, mackinawite transforms into hexagonal FeS troilite with an acicular structure, and the corrosion rate increases in approx. 2 times. The corrosion rate of steel at 60°C and 5 MPa in a solution with a pressure ratio of \({P}_{{\text{CO}}_{2}}:{P}_{{\text{H}}_{2}\text{S}}=30:1\) was twice as low as at 20°C and 0.1 MPa. A dense layer of cubic iron sulfide FeS crystals was formed on the surface, which reduces corrosion. The adsorption of hydrogen by the steel reduced in approx. 15 times as the temperature increases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural gas from gas-bearing stratums, accompanying gases, and gases from gas-condensate fields contain carbon dioxide and hydrogen sulfide impurities, which are corrosive-active in the presence of moisture. Dissolved CO2 accelerates cathodic reactions and affects the mechanism of steel hydrogenation. Hydrogen sulfide causes corrosion and steel hydrogenation, which is a precondition for their accelerated corrosionmechanical fracture [1,2,3,4]. In the presence of CO2 and H2S in the environment, films containing iron carbonates and sulfides are formed on the steel surface. Depending on the chemical composition and density, films can have different effects on corrosion, hydrogen absorption, and embrittlement of steels [5,6,7].
The purpose of the study is to investigate the effect of H2S and CO2 concentration in a chloride-acetate solution on the corrosion rate and hydrogenation of 17G1S-U steel at different temperatures and pressures.
Research Method
Ferrite-pearlite 17G1S-U steel was studied in a solution of 5% NaCl + 0.5% CH3COOH, pH 2.7 [2]. CO2 or a mixture of CO2 with H2S continuously passing through this solution at a ratio of partial pressures of 30:1 and 3:1 to obtain the hydrogen sulfide concentration in the solution of 100 and 500 mg dm3, respectively. Tests were carried out at a temperature of 20°C and a pressure of 0.1 MPa for 720 h and in an autoclave at 60°C and 5 MPa for 510 h (this exposure was chosen to ensure the stability of the environment’s chemical composition). The corrosion rate Km (g/(m2 × h)) was calculated according to the formula Km = (∆m)/(S × τ), where ∆m is the change in sample weight before and after the experiment; S is its area; τ is the exposure time to a corrosive environment. The hydrogen concentration in the samples was determined using a LECO DH 603 analyzer. The microstructure, chemical composition, and thickness of corrosion products on steel were studied on an EVO 40XVP scanning electron microscope with a micro-X-ray spectral analysis system, using an INCA ENERGY 350 energy dispersive spectrometer.
Results and Discussion
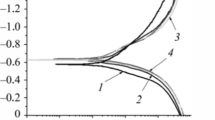
At a temperature of 20°C and a pressure of 0.1 MPa, the initial corrosion rate of steel is the lowest in a solution saturated with CO2 (0.4 g/(m2 × h)). In the presence of hydrogen sulfide in the environment it is 2.3–3 times higher (Fig. 1). The corrosion rate slows down over time in the solution containing C2O and H2S at a ratio of partial pressures of 30:1 and is halved after 720 h. A slight slowdown of corrosion is observed at the beginning of exposure to the solution saturated with carbon dioxide, as well as to the solution with the ratio PCO2: PH2S = 3:1, but after 240–300 h corrosion in these environments increases, reaching 0.79 and 2.16 g/ (m2 × h) after 720 h, respectively (Fig. 1a).
The concentration of absorbed hydrogen does not exceed 0.8 ppm after steel exposure to a solution saturated with carbon dioxide (Fig. 1b). Hydrogen absorption is approx. 4 ppm at the ratio of partial pressures PCO2: PH2S = 30:1. The hydrogen content in steel reaches approx. 7.9 ppm with an increase in the concentration of hydrogen sulfide (PCO2: PH2S = 3:1).
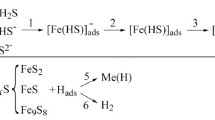
Iron carbonates are not formed in a chloride-acetate solution with pH 2.7, saturated with carbon dioxide. The corrosion rate increases over time due to the formation of local pits which increase the area of the corroding surface [2]. In the presence of hydrogen sulfide in the solution, the rate of general corrosion at the initial stage decreases because of sulfide films formation on the surface. At the ratio of partial pressures PCO2: PH2S = 30:1 the corrosion rate is the lowest, as a dense film of mackinawite ( Fe1+xS), which inhibits corrosion and acts as a diffusion barrier for the environment components [1, 2], is formed (Fig. 2a). At the ratio PCO2: PH2S = 3:1 the rate of steel corrosion slows down at the beginning of exposure, but increases after some time, what is associated with the transformation of mackinawite and the formation of loose films of troilite FeS [1, 2] with an acicular structure (Fig. 2b). These results confirm that the morphology and protective properties of sulfide films on the steel surface depend on the content of hydrogen sulfide in the solution.
The effect of temperature and pressure on the corrosion rate and hydrogenation of 17G1S-U steel in a chloride-acetate solution at different concentrations of CO2 and H2S is illustrated by the diagrams in Fig. 3. At a temperature of 60°C and a pressure of 5 MPa, the corrosion rate of steel after exposure for 510 h to a solution saturated with carbon dioxide and at the ratio of PCO2: PH2S = 30 :1 increases by approx. 17 and approx. 13%, respectively. At a pressure ratio PCO2: PH2S = 3:1 corrosion slows down by half (Fig. 3a).
Influence of H2S and CO2 partial pressures on (a) corrosion rate and (b) hydrogen absorption after exposure of steel to a chloride-acetate solution for 510 h at different temperatures and pressures: (I) CO2 (saturated); (II) PCO2: PH2S = 30:1; (III) PCO2: PH2S = 3:1 [(□) P = 0.1 MPa, t = 20°C; (■) P = 5.0 MPa, t = 60°C].
The hydrogenation of steel was significantly reduced with the increase in temperature and pressure. It is negligible in a solution saturated with CO2, at the ratio PCO2: PH2S = 30:1 the concentration of absorbed hydrogen decreases in approx. 2.7 times, at the ratio PCO2: PH2S = 3:1 it decreases in approx. 15 times compared to the results obtained at 0.1 MPa and 20°C (Fig. 3b).
With the increase in temperature and pressure corrosion products transform. After tests in a solution with a pressure ratio of PCO2: PH2S = 30:1, a dense layer of grains with a size of 1 to 100 μm was revealed on the surface of the steel (Fig. 4). Small crystals have an octahedral shape and a chemical composition which corresponds to cubic iron sulfide FeS (50.58 at.% S and 49.42 at.% Fe) [1]. As the exposure time increases the size of grains increases and a dense barrier layer, which protects the steel from corrosion is formed. No pits and cracks were found under the corrosion products (Fig. 4c).
Sulfide ions adsorbed on the steel surface can inhibit the recombination of hydrogen ions, which promotes their diffusion into the metal. As the temperature increases the mobility of sulfide ions increases and their adsorption slows down. As a result, the formation of hydrogen molecules accelerates and the steel’s hydrogenation slows down. Probably, an increase in pressure affects the adsorption capacity of sulfide ions in the solution less than the temperature.
Conclusions
The corrosion rate of 17G1S-U steel in a chloride-acetate solution saturated with CO2 is lower than in the presence of hydrogen sulfide but increases over time due to the absence of protective carbonate films on the surface. The corrosion rate and hydrogenation of steel were determined by the hydrogen sulfide concentration in the environment. Corrosion slowed down at the ratio of partial pressures PCO2: PH2S = 30:1 and at the beginning of exposure to a solution with ratio PCO2: PH2S 3:1 due formation of the dense films of mackinawite ( Fe1+xS ). Over time mackinawite transformed into hexagonal FeS troilite with an acicular structure, and the corrosion rate doubled. Hydrogen absorption was approx. 4 ppm at a ratio of PCO2: PH2S = 30:1, and it increased to approx. 7.9 ppm at PCO2: PH2S = 3:1. The corrosion rate was reduced by half in a solution with PCO2: PH2S = 3:1 at t = 60°C and P = 5 MPa compared to t = 20°C and P = 0.1 MPa. A dense layer of FeS cubic iron sulfide crystals, which slowed down corrosion and practically eliminated hydrogenation, was formed on the steel surface.
Author contributions. All authors have contributed equally to the work, authors also read and approved the final manuscript.
Conflict of interest. Authors V. I. Pokhmurskii and M. S. Khoma are the Editorial Board Members of Materials Science (translated from Fizyko-Khimichna Mekhanika Materialiv). The paper was handled by another Editorial Board Member and has undergone a rigorous peer review process. Authors V. I. Pokhmurskii and M. S. Khoma were not involved in the journal’s peer review or decisions related to this manuscript.
Funding. The financial support was received from the National Academy of Sciences of Ukraine under the R&D project (Registration No. 0121U108962).
References
X. Wen, P. Bai, B. Luo, S. Zheng, and C. Chen, “Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment,” Corr. Sci., 139, 124–140 (2018). https://doi.org/10.1016/j.corsci.2018.05.002
M. Khoma, V. Vynar, Ch. Vasyliv, M. Chuchman, B. Datsko, V. Ivashkiv, and O. Dykha, “Tribocorrosion of 17Mn1Si steel in chloride acetate environments at the different concentrations of hydrogen sulfide,” J. of Bio- and Tribo-Corrosion, 8, art. no. 53 (2022). https://doi.org/10.1007/s40735-022-00655-3
P. Sui, J. Sun, Y. Hua, H. Liu, M. Zhou, Y. Zhang, J. Liu, and Y. Wang, “Effect of temperature and pressure on corrosion behaviour of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S,” Int. J. of Greenhouse Gas Control, 73, 60–69 (2018). https://doi.org/10.1016/j.ijggc.2018.04.003
C. Ren, D. Liu, Z.Bai, and T. Li, “Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide,” Mater. Chemistry and Phys., 93, Iss. 2–3, 305–309 (2005). https://doi.org/10.1016/j.matchemphys.2005.03.010
X. H. Zhao, Y. Feng, S. Tang, and J. Zhang, “Electrochemical corrosion behavior of 15Cr–6Ni–2Mo stainless steel with/without stress under the coexistence of CO2 and H2S,” Int. J. Electrochem. Sci., 13, Is. 7, 6296–6309 (2018). https://doi.org/10.20964/2018.07.59
M. S. Khoma, S. A. Korniy, V. A. Vynar, B. M. Datsko, Yu. Ya. Maksishko, O. V. Dykha, and R. L. Bukliv, “Influence of hydrogen sulfide on the carbon-dioxide corrosion and the mechanical characteristics of high-strength pipe steel,” Mater. Sci., 57, No. 6, 805–812 (2022). https://doi.org/10.1007/s11003-022-00610-0
C. Plennevaux, J. Kittel, M. Fregonese, B. Normand, F. Ropital, F. Grosjean, and T. Cassagne, “Contribution of CO2 on hydrogen evolution and permeation in low alloy steel exposed to H2S environment,” Electrochem. Communic., 26, Is. 1, 17–20 (2013). https://doi.org/10.1016/j.elecom.2012.10.010
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 59, No. 5, 5–9, September–October, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pokhmurskii, V.I., Khoma, M.S., Chuchman, M.R. et al. Effect of Temperature and Pressure on Corrosion and Hydrogenation of Steel in Chloride-Acetate Environment with Different Concentrations of Hydrogen Sulfide and Carbon Dioxide. Mater Sci 59, 519–523 (2024). https://doi.org/10.1007/s11003-024-00806-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-024-00806-6