The effect of different concentrations of CO2 and H2S in a chloride-acetate solution on corrosion-mechanical properties of 17G1S-U steel was studied. In a solution saturated with CO2, the corrosion rate of steel was lower than in the presence of H2S, but increased over time due to the absence of protective carbonate films on the surface, plasticity parameters were 2–2.7 times lower than in air due to dimple surface damage. The corrosion rate and hydrogenation of steel was determined primarily by the hydrogen sulfide concentration in the environment. At a concentration of 100 mg/dm3, dense films of the troilite-mackinavite composition were formed, which inhibit corrosion. At higher concentrations, the corrosion rate increased due to the sulfides transformation and the formation of surface layers with defects. With an increase in the H2S concentration from 100 mg/dm3, the strength characteristics of steel decreased in three times, and plasticity decreased in 3–5 times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pipelines today are the cheapest way to transport gas and oil. For the production of pipes, as a rule, low-alloy low-carbon steels are used, which are prone to degradation due to the presence of carbon dioxide and hydrogen sulfide in the transported media. Dissolved gases accelerate corrosion and steel hydrogenation, thus deteriorating its mechanical properties and causing crack initiation [1,2,3,4,5].
Carbon dioxide is an integral component of production gas, gas condensates, as well as attendant gases of oil. Its concentration in gas can vary from 0.01 to 20%, and in formation water vary from 30 to 50%. Gas was considered to contain hydrogen sulfide at a partial pressure of hydrogen sulfide of 0.08%, which, as a rule, is present in deep layers of gas fields. Its content in domestic deposits is 8 × 10−5 to 3.1% [6]. As a result of the dissolution of carbon dioxide in the environment, the concentration of carbonic acid increases and the rate of corrosion can reach several millimeters per year [7,8,9].
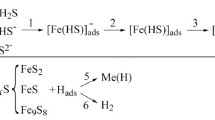
During the interaction of hydrogen sulfide with steels, sulfides with a complex crystal structure are formed, most often these are mackinavite (tetragonal Fe1+ xS), cubic FeS, and troilite (stoichiometric hexagonal FeS) [10]. Under such conditions, hydrogen ions are reduced and steel is hydrogenated, which causes specific damage – hydrogen-induced cracking and delamination of the metal (blistering).
With the simultaneous presence of CO2 and H2S in the environment, films containing iron carbonates and sulfides can be formed on the steel surface [4, 8]. The composition of corrosion products depends on the competitive ability of iron carbonate and mackinawite [8]. Such films affect the rate of corrosion and hydrogenation of steel, as they can retard these processes, creating a diffusion barrier for the components of the environment and steel. Their structure and properties depend on the partial pressures of CO2 and H2S, pH of the environment, solubility of steel components, temperature, etc. The damage character was determined by the residual stresses applied from outside or inside the pipe, as well as the defectiveness of the steel.
The influence of the ratio of CO2 and H2S concentrations in the chloride-acetate solution on the corrosion-mechanical properties and hydrogenetaion of 17G1S-U steel was studied.
Test Method
Ferritic-pearlitic x 17G1S-U steel (mass%: 0.17 C; 0.47 Si; 1.4 Mn; < 0.3 Cr; 0.3 Ni; < 0.3 Cu, Fe is balance) was studied in a 5% NaCl + 0.5% CH3COOH (pH 2.7) solution, which is the basis of the NACE solution which is standard for the study of corrosion and corrosion-mechanical fracture in hydrogen sulfide environments [11]. The solutions were continuously bubbled with argon, carbon dioxide, or hydrogen sulfide. The mixtures of argon or carbon dioxide with hydrogen sulfide were also prepared at different ratios of partial pressures, which were passed through a chloride-acetate solution, maintaining a hydrogen sulfide concentration of 100 or 500 mg/dm3 ± 10%. Tests were carried out at a temperature of 25°C and a total pressure of 0.1 MPa. The corrosion rate (Km) was calculated by the formula Km = (m0 – m) / (S × τ), where m0, m is the weight of the sample before and after the experiment, respectively, g; S is its area, m2; τ is exposure time in a corrosive environment, h. The steel corrosion losses for a year were estimated according to the formula Ky = 1.11Km.
The electrochemical characteristics of corrosion processes were investigated in the potentio-dynamic mode using a PI-2MK-10A potentiostat. The reference electrode was a silver chloride electrode of EVL-1M1 type, platinum electrode was used as the auxiliary electrode. The sweep rate of the potential was 1 mV/s. A LECO DH 603 analyzer determined the concentration of hydrogen in the samples. The effect of the hydrogen sulfide concentration on the mechanical properties of steel under tension at a constant rate of 10−6s−1 in a 5% NaCl + 0.5% CH3COOH solution, saturated with carbon dioxide, was studied on the UIP installation for slow tension. The microstructure, chemical composition, and thickness of corrosion products were measured by an EVO 40XVP scanning electron microscope with a micro-X-ray spectral analysis system, using an INCA ENERGY 350 energy dispersive spectrometer.
Results and Discussion
Electrochemical studies proved that the electrode potential of steel was 0.575 V, and the corrosion current density was 0.091 mA/cm2 in a chloride-acetate solution saturated with carbon dioxide. When adding hydrogen sulfide to the solution, the potential shifts to the cathodic side: at concentrations of 100 and 500 mg/dm3 by approx. 9 and 55 mV respectively, and in a saturated solution – by 70 mV. At hydrogen sulfide concentration of 100 mg/dm3 the corrosion rate does not change, but at a concentration of 500 mg/dm3 and in a saturated solution, it increases slightly – by approx. 6.4 and approx. 20.8% (Fig. 1; Table 1). The Tafel slopes of the polarization curves in all solutions practically do not differ. Therefore, hydrogen sulfide does not change the character of corrosion processes. Carbon dioxide in a chloride-acetate solution with a pH of 2.7 accelerates corrosion more than twice compared to a solution deacidified with argon [12]. Thus, for short-term tests, which are electrochemical studies the effect of hydrogen sulfide on corrosion in the presence of carbon dioxide was negligible. During polarization at potentials, more negative than approx. 750 mV, the rate of cathodic reactions increases due to the direct reduction of hydrogen from the H2S molecule, similar to the case in environments with argon and different concentrations of hydrogen sulfide.
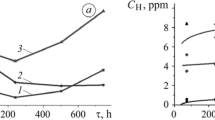
The corrosion rate of steel in a 5% NaCl + 0.5% CH3COOH + CO2 solution without hydrogen sulfide for 720 h is the lowest and equals 0.2 to 0.8 mm/year (Fig. 2a). At hydrogen sulfide concentration of 100 and 500 mg/dm3 , the initial corrosion rate was on average 0.95 and 1.2 mm/year, and in saturated – 2.8 mm/year. During the first 240 to 300 h of exposure, corrosion slows down, the most – in a saturated solution (almost by 60%). In environments with 500 and 2800 mg/dm3 H2S corrosion increases again in 2–2.5 times after 720 h of exposure (Fig. 2a). In a solution containing 100 mg/dm3 H2S , corrosion decreases from 0.95 to 0.46 mm/year during 720 h.
Influence of hydrogen sulfide concentration on the dependence of 17G1S-U steel corrosion rate on exposure time in a 5% NaCl + 0.5% CH3COOH solution with (a) carbon dioxide and (b) argon: (1) CO2 (saturated); (2, 3) CO2 + 100 and 500 mg/dm3 H2S; (4) H2S (saturated); 2′, 3′ are Ar + 100 and 500 mg/dm3 H2S.
In a chloride-acetate solution, containing argon instead of carbon dioxide, the dependence of the corrosion rate on time was similar (Fig. 2b) and depended on the concentration of hydrogen sulfide.
In a chloride-acetate solution with a pH of 2.7, saturated with carbon dioxide, iron carbonates were not formed and the corrosion rate increases with time. In the presence of hydrogen sulfide at the initial stage the rate of general corrosion decreased because of the formation of sulfide films on the surface. At a concentration of 100 mg/dm3 H2S, the rate of corrosion was the lowest. Dense sulfide films were formed on the surface, which inhibit the diffusion of ions. Their structure corresponds to the composition of troilite–makinaivite sulfides (FeS − Fe1+xS) [13, 14]. When the content of H2S was 500 and 2800 mg/dm3, the rate of corrosion of steel slowed down at the beginning of exposure, but increased after some time (Fig. 2a). This was due to the transformation of sulfides and the formation of loose FeS troilite films [13] with a cubic structure and three times greater size of crystallites (Fig. 3).
Thus, the development of corrosion processes in hydrogen sulfide environments were divided into two periods. During the first, sulphide films, which inhibit corrosion, creating a diffusion barrier for the components of both the environment and the steel were formed. The initial rate of corrosion increases with increasing aggressiveness of the corrosive solution. During the second period, after 100 to 300 h, depending on the environment, corrosion accelerated, which was caused by the transformation of sulfides and the appearance of more defective surface layers.
In a solution saturated with carbon dioxide, the concentration of absorbed hydrogen did not exceed 0.8 ppm (Fig. 4, curve 1). At a concentration of 100 mg/dm3 H2S it was 4 to 5 ppm, and with its increase from 500 mg/dm3 to saturation, cathodic reactions became more intense and the concentration reached approx. 7 to 10 and approx. 14 to 18 ppm, respectively (Fig. 4). The increase in the experimental data scattering with an increase in the concentration of hydrogen sulfide can be explained by the capture of hydrogen by traps (structure defects) and its secondary redistribution in the volume of the sample.
Similar dependences of hydrogen absorption by steel on hydrogen sulfide concentration were found in the 5% NaCl + 0.5% CH3COOH solution saturated with argon. In particular, the concentration of absorbed hydrogen in steel after 720 h of exposure to the solution without hydrogen sulfide was 1 ppm, at a concentration of 100; 500 and 2800 mg/dm3 it was approx. 4; approx. 10 and approx. 20 ppm, respectively [12]. Thus, the intensity of steel hydrogenation in a chloride-acetate solution depends, first of all, on the concentration of hydrogen sulfide. Replacing argon with carbon dioxide practically did not affect this process.
After steel exposure to the 5% NaCl + 0.5% CH3COOH solution, saturated with carbon dioxide, argon and their mixtures with different concentrations of hydrogen sulfide, signs of pitting corrosion were recorded (Fig. 5).
In the solution saturated with carbon dioxide, rounded pittings of a depth of up to 150 μm were formed on the steel surface, in the absence of hydrogenation and mechanical stresses, no cracks were detected (Fig. 5a). In the solution with 100 mg/dm3 H2S, the surface was less damaged, there were no signs of hydrogen embrittlement (Fig. 5b). With an increase in H2S concentration to 500 mg/dm3 the depth of pittings increase, the nuclei of cracks appeared at their bottom (Fig. 5c). Numerous subsurface cracks were formed in the saturated hydrogen sulfide solution, which was a sign of hydrogen-induced cracking (HIC) of steel (Fig. 5d). The damage character of steel after exposure to the 5% NaCl + 0.5% CH3COOH solution with argon [13] was similar (Fig. 5e, f). After exposure to the environment with a concentration of 100 mg/dm3 H2S, pitting corrosion with a depth of up to 80 to 100 μm was recorded on the samples surface. At concentrations of 500 mg/dm3 and above, the depth of pittings increases, cracks appear and poropagate, indicating the HIC.
In the solution saturated with carbon dioxide, the strength characteristics of steel were by 3–5% lower than in air, and the plasticity parameters were approx. 2–2.7 times lower, which was probably due to pittings presence on the surface. With the introduction of 100 and 500 mg/dm3 H2S into the solution, the ultimate strength and yield strength decrease by approx. 30%, and the elongation and reduction in area decrease in 3–4.5 times. In the solution saturated with hydrogen sulfide, the strength characteristics decrease by 35 to 45%, and plasticity decrease in 4.4–4.8 times, compared to saturated carbon dioxide (Table 2). The time to samples fracture decreases in 2.6–4.3 times with an increase in the concentration of hydrogen sulfide.
In the chloride-acetate solution saturated with argon, the strength parameters of steel were by 5 to 7% lower than in air, and the plasticity parameters were 1.2 to 2 times lower. With an increase in the concentration of hydrogen sulfide from 100 mg/dm3 to saturation, the ultimate strength decreased by 10 to 30%, and the relative elongation decreased in 2.5–4.5 times. The tests duration before the samples fracture was reduced in 1.8–3.5 times (Table 2).
In the same solution saturated with carbon dioxide, the corrosion rate was lower than in the presence of hydrogen sulfide, but increased with time due to the absence of protective carbonate films on the surface. After corrosion tests, rounded pittings were formed on the steel surface. Under the simultaneous influence of mechanical loads and corrosive environment, the plasticity parameters of steel decreased in 2–2.7 times. The strength parameters decreased by only 3 to 5%, which was probably caused by the low concentration of absorbed hydrogen.
With the addition of 5% NaCl + 0.5% CH3COOH + CO2 hydrogen sulfide to the solution, the character of corrosion processes did not change during short-term tests. In the long-term tests, the corrosion rate, the hydrogenation intensity and the mechanical properties of steel were determined by hydrogen sulfide concentration. The rate of steel corrosion at the initial stage decreased due to the formation of sulfide films on the surface. At a concentration of 100 mg/dm3 H2S, the protective effect of dense troilite–mackinavite films was maintained throughout the entire test time (720 h), and at concentrations of 500 and 2800 mg/dm3 – only for 200–250 h. Then the protective effect increased in 2–2.5 times due to the transformation of sulfides and the formation of more defective surface layers. The formation of sulfide phases was accompanied by steel hydrogenation, which increased from 5 to 18 ppm with an increase in the concentration of hydrogen sulfide from 100 to 2800 mg/dm3.
After corrosion tests in the solution with different concentrations of CO2/H2S, pitting corrosion was detected on the steel surface. With an increase in the concentration of hydrogen sulfide from 100 to 2800 mg/dm3, the depth of pittings increased, and at a concentration of 500 mg/dm3 cracks appeared and propagated indicating the HIC.
When there were no mechanical loads the rate of corrosion, hydrogenation and damage of steel was the lowest in the solution with 100 mg/dm3 of hydrogen sulfide. Steel mechanical properties deteriorated sharply: yield strength and ultimate strength were reduced by a third, and relative elongation and reduction in area decreased in 3–4.8 times, respectively. At higher concentrations, the mechanical properties deteriorated, but to a lesser extent.
As a result of corrosion and hydrogenation, the defectivity of the steel surface layers increased: pittings were formed, internal stresses in the crystal lattice increased and cracks initiated. When external mechanical loads were applied, cracks initiated and developed in the crystal lattice, which could lead to metal fracture [15].
Conclusions
In the chloride-acetate solution saturated with carbon dioxide (pH 2.7), the corrosion rate of 17G1C-U steel was lower than in the presence of hydrogen sulfide, but increased with time due to the absence of protective carbonate films on the surface. The plasticity of steel decreased in 2–2.7 times due to the localization of corrosion, while the strength decreased insignificantly. With the addition of hydrogen sulfide to the 5% NaCl + 0.5% CH3COOH + CO2 solution, the character of corrosion processes during short-term tests did not change. During the long-term tests the rate of corrosion, hydrogenation and mechanical properties of steel were determined by the concentration of hydrogen sulfide. The corrosion rate of steel at the initial stage of long-term research decreased due to the formation of sulfide films on the surface. With an increase in hydrogen sulfide concentration from 500 to 2800 mg/dm3 and test time increase, corrosion rate increase in 2–2.5 times due to the transformation of sulfides and the formation of defective surface layers. With an increase in hydrogen sulfide concentration from 100 to 2800 mg/dm3 steel absorbed from 5 to 18 ppm of hydrogen. After tests in the solution containing carbon dioxide and hydrogen sulfide, pitting corrosion was observed on its surface. When the concentration of hydrogen sulfide increased from 100 to 2800 mg/dm3, the depth of pittings increased, at 500 mg/dm3 cracks initiated and propagated, thus indicating hydrogen-induced cracking. At the hydrogen concentration from 100 mg/dm3 to saturation, the mechanical properties of steel deteriorated sharply as a result of hydrogenation: the yield strength and ultimate strength decreased by 30 to 45%, and the relative elongation and reduction in area decreased in 3–4.8 times.
References
P. Sui, J. Sun, Y. Hua, H. Liu, M. Zhou, Y. Zhang, J. Liu, and Y. Wang, “Effect of temperature and pressure on corrosion behavior of X65 carbon steel in water-saturated CO2 transport environments mixed with H2S,” Int. J. of Greenhouse Gas Control, 73, 60–69 (2018); https://doi.org/10.1016/j.ijggc.2018.04.003
C. Ren, D. Liu, Z. Bai, and T. Li, “Corrosion behavior of oil sulfide in simulant solution with hydrogen sulfide and carbon dioxide,” Mater. Chemistry and Physics, 93, Iss. 2–3, 305–309 (2005); https://doi.org/10.1016/j.matchemphys.2005.03.010
X. H. Zhao, Y. Feng, S. Tang, and J. Zhang, “Electrochemical corrosion behavior of 15Cr–6Ni–2Mo stainless steel with/without stress under the coexistence of CO2 and H2S,” Int. J. Electrochem. Sci., 13, Is. 7, 6296–6309 (2018); https://doi.org/10.20964/2018.07.59
M. Khoma, V. Vynar, M. Chuchman, and C. Vasyliv, “Corrosion-mechanical failure of pipe steels in hydrogen sulfide environments,” in: Degradation Assessment and Failure Prevention of Pipeline Systems, Springer, Cham (2021), pp. 231–239; https://doi.org/10.1007/978-3-030-58073-5_18
M. S. Khoma, S. A. Korniy, V. A. Vynar, B. M. Datsko, Yu. Ya. Maksishko, O. V. Dykha, and R. L. Bukliv, “Influence of hydrogen sulfide on the carbon-dioxide corrosion and the mechanical characteristics of high-strength pipe steel,” Mater. Sci., 57, No. 6, 805–812 (2022); https://doi.org/10.1007/s11003-022-00610-0
M. M. Ivaniuta (Ed.), Atlas of Oil and Gas Deposits of Ukraine: in 6 volumes [in Ukrainian], Tsentr Yevropy, Lviv (1998). ISBN 966-7022-04-8.
C. Plennevaux, J. Kittel, M. Frégonèse, B. Normand, F. Ropital, F. Grosjean, and T. Cassagne, “Contribution of CO2 on hydrogen evolution and hydrogen permeation in low alloy steels exposed to H2S environment,” Electrochem. Communic., 26, Is. 1, 17–20 (2013); https://doi.org/10.1016/j.elecom.2012.10.010
G. A. Zhang, Y. Zeng, X. P. Guo, F. Jiang, D. Y. Shi, and Z. Y. Chen, “Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment,” Corros. Sci., 65, 37–47 (2012); https://doi.org/10.1016/j.corsci.2012.08.007
W. Sun, S. Nesic, and S. Papavinasam, “Kinetics of iron sulfide and mixed iron sulfide/carbonate scale precipitation in CO2/H2S corrosion,” NACE – Int. Corrosion Conference Series, 06644\1–06644\26 (2006).
X. Wen, P. Bai, B. Luo, S. Zheng, and C. Chen, “Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment,” Corros. Sci., 139, 124–140 (2018); https://doi.org/10.1016/j.corsci.2018.05.002
NACE Standard SP0110-2010, Wet Gas Internal Corrosion Direct Assessment Methodology for Pipelines, NACE, Houston, TX (2010).
M. Khoma, V. Vynar, C. Vasyliv, M. Chuchman, B. Datsko, V. Ivashkiv, and O. Dykha, “Tribo-corrosion of 17Mn1Si steel in chloride-acetate environments at the different concentrations of hydrogen sulfide,” J. of Bio- and Tribo-Corrosion, 8, Is. 2, art. no. 53 (2022); https://doi.org/10.1007/s40735-022-00655-3
M. S. Khoma, V. R. Ivashkiv, N. B. Ratska, B. M. Datsko, and M. R. Chuchman, “Corrosion-electrochemical properties of 17G1SU steel in chloride-acetate solutions with different concentrations of hydrogen sulfide,” Mater. Sci., 56, No. 4, 544–549 (2021); https://doi.org/10.1007/s11003-021-00462-0
M. S. Khoma, S. A. Holovei, V. R. Ivashkiv, and Kh. B. Vasyliv, “Effect of sulfides on the hydrogen overvoltage and hydrogenation of U8 steel in chloride-hydrogen-sulfide media,” Mater. Sci., 53, No. 6, 761–768 (2018); https://doi.org/10.1007/s11003-018-0133-z
S. K. Dwivedi, and M. Vishwakarma, “Hydrogen embrittlement in different materials: A review,” Int. J. of Hydrogen Energy, 43, Is. 46, 21603–21616 (2018); https://doi.org/10.1016/j.ijhydene.2018.09.201
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 59, No. 2, pp. 80–87, March–April, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoma, M.S., Pokhmurskii, V.I., Chuchman, M.R. et al. Corrosion-Mechanical Properties and Susceptibility to Hydrogenetaion of Pipe Steel in the Presence of Carbon Dioxide Gas and Hydrogen Sulphide in Environment. Mater Sci 59, 205–212 (2023). https://doi.org/10.1007/s11003-024-00764-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-024-00764-z