We consider the process of corrosion of steels in hydrogen-sulfide media of different concentrations with regard for the specific features of the formation of sulfides of different chemical compositions and their influence on the course of redox reactions. We present different points of view on their role in corrosion processes. On the basis of generalization of the influence of iron sulfides on electrode reactions, release and absorption of hydrogen by Armco iron, U8 steel, and 45 steel of different structures, it is established that, despite the fact that iron sulfides mainly reduce the overvoltage of cathodic reactions and increase the amount of hydrogen released under cathodic polarization, they do not always promote the hydrogenation of these materials. Iron sulfides predominantly affect the reaction of hydrogen release, whereas its absorption depends on the structures of steels. By an example of 17G1S-U steel, we show that the corrosion rate is affected by the nature of sulfides formed on the surface. Indeed, for their concentrations varying within the range 25–100 mg/dm3, the corrosion rate decreases by almost an order of magnitude. Moreover, for hydrogen-sulfide concentration CH2S ≥ 500 mg/dm3, we observe the formation of porous sulfides, corrosion defects are localized, and the intensity of absorption of hydrogen by steel becomes almost twice higher than at lower concentrations, which promotes the development of cracking and corrosion cracking. It is shown that the critical concentration for the development of hydrogen- sulfide corrosion cracking of steels of this class is CH2S ≤ 100 mg/dm3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protection of materials of structures against the aggressive influence of external factors is one of the most urgent tasks for various branches of industry. According to the report of the National Association of Corrosion Engineers (NACE), the total amount of losses caused by corrosion throughout the world constitutes 3.4% of the global gross domestic product. Moreover, about 40% of the entire amount of annually produced metals is spent for the compensation of corrosion losses [1, 2]. The major part of corrosion losses corresponds to the oil-and-gas extraction and refining industries. Hydrogen sulfide is the most aggressive component in the composition of extracted products. It causes hydrogen sulfide corrosion, blistering, pitting, hydrogen- initiated cracking, hydrogen-sulfide stress-corrosion cracking, and other defects [3]. At the same time, as the ultimate and yield strengths of the metal increase, the hazard of sulfide stress-corrosion fracture becomes higher [3, 4].
The investigation of the resistance of steels and alloys to stress-corrosion fracture in hydrogen sulfide media is very important for the proper choice of steels intended for operation in the oil-and-gas extraction, oil-refining, and chemical industries. The resistance of steels to hydrogen-sulfide corrosion and its mechanisms, hydrogenation rate, and the phenomenon of accelerated fracture of steels in hydrogen-sulfide media are now extensively investigated in numerous institutions throughout the world, namely, in the National Association of Corrosion Engineers (NACE) (USA), University of Calgary (Canada), China University of Petroleum (China), King Fahd University of Petroleum and Minerals (Saudi Arabia), Egyptian Petroleum Research Institute (Egypt), National Taiwan University (Taiwan), Sharif University of Technology (Iran), Universidad Politécnica de Valencia (Spain), Norwegian University of Science and Technology (Norway), etc. [3,4,5,6,7,8,9,10,11].
According to the NACE standard [12], the influence of hydrogen sulfide on the corrosion cracking of steels is studied in a NACE aqueous solution (5% NaCl + 0.5% CH3COOH saturated with H2S at a temperature of 24 ± 3°С). The application of this highly aggressive solution in our investigations imposes elevated requirements on the choice of materials for the oil-and-gas extraction equipment. However, the content of hydrogen sulfide in the extracted products is, as a rule, lower. Thus, in particular, in Ukraine [13], we discovered ten oil and gas fields in the Western part of the country and 19 fields in the Eastern part, where the H2S concentration does not exceed 3 vol.%. In these fields, it is not necessary to use oil-and-gas extraction equipment made of high-cost structural steels because the processes of corrosion and stress-corrosion fracture differ from the processes observed in a NACE solution. Thus, the analysis of the possibility of application of steels with much lower requirements imposed on their chemical composition and mechanical properties under the analyzed conditions is a quite actual problem.
The main hypotheses concerning the influence of hydrogen sulfide on the corrosion and hydrogenation of metals are based on the formation of [Fe(HS)]ads− surface catalysts facilitating these processes [9]. However, these hypotheses do not relate the course of redox reactions to the formation of various sulfide-containing corrosion products whose compositions depend on numerous factors. In the H2S–H2O system, for different рН values and redox potentials, there exist HS– , S2–, and H2S ions [3, 14]. Depending on the hydrogen-sulfide concentration, temperature, and рН values of the medium, they form corrosion products of different stability. First, we observe the formation of unstable mackinawite Fe(1+x)S, where 0 < x < 0.1 [11]. Then, depending on the рН value of the medium and concentration of sulfide ions, it transforms into pyrite (FeS2), troilite (FeS), or kanzite (Fe9S8). It was established [5, 14] that, on Armco iron, at low concentrations of H2S (up to 2.0 mg/dm3), sulfide films consist of troilite (FeS) and pyrite (FeS2) with crystals of up to 20 nm size. For CH2S = 2 – 20 mg/dm3, the indicated sulfide films consists of troilite with small amounts of kanzite (Fe9S8). Finally, for CH2S > 20 mg/dm3, kanzite with crystals of up to 75 nm in size was predominant in corrosion products. It is believed that kanzite (characterized by imperfect crystal lattice) does not have high protective properties. At the same time, pyrite and troilite have small numbers of defects, which enhances their protective action [14]. However, in [10], it was indicated that the FeS film does not protect metal against corrosion because the corrosion rate is higher than the rate of formation of sulfides. The composition and protective properties of iron sulfides also depend on temperature, the salt composition of solutions, and the hydrodynamics of the flow [3,4,5]. The elevation of temperature promotes the formation of sulfides more enriched with sulfur in the following sequence [15, 16]:
The corrosion products, as a rule, have two layers: an inner layer enriched with iron and an outer layer enriched with sulfur [17,18,19]. In the corrosion products of steels, one may also observe the formation of greigite (Fe3S4). However, it is stable only in the absence of air and moisture [20].
The stability of various iron sulfides depends on the рН value of the solution and the H2S concentration. The highest stability was detected by orthorhombic marcasite FeS2 and cubic pyrite FeS2 [11]. In view of the properties of various sulfides formed on the surfaces of steels, their effect on the formation of corrosion defects, hydrogen release and absorption, and the subsequent development of stress-corrosion fracture can be ambiguous [5,6,7, 17,18,19,20,21,22,23,24].
Depending on the conditions, hydrogen sulfide exerts different influence on the corrosion of steels [25,26,27]. The comparison of the rate of formation of a film of corrosion products with the corrosion rate of Kh65 steel in a 1% NaCl solution with an addition of 9.4 ·10–6 –9.4 ·10–3 mole H2S [18] shows that, as a result of the formation of surface sulfide structures, the corrosion rate of steel decreases but always remains higher than the growth rate of films and unambiguously depends on the concentration of H2S. The sulfide films have a finely divided inner layer with high density and high protective properties and a more porous outer layer with weaker protective action. The corrosion rate of steel depends on the morphology, porosity of the corrosion products, and their adhesion to the surface stronger than on the thickness of the corrosion layer and the rate of formation of iron sulfides participating in the formation of the inner layer [17].
On the other hand, the investigations of the influence of H2S concentration on the electrochemical properties of SAE-1020 steel at a temperature of 90°С demonstrated that the corrosion rate increases as the hydrogensulfide content becomes higher [25]. According to the voltammetric dependences, as the H2S concentration increases from 0 to 408.44 mg/dm3 (∼ 0.012 mole/dm3), the cathodic current density increases, the anodic current density remains unchanged, and the corrosion potential shifts toward the region of more positive values. This reveals a significant influence of cathodic processes on the corrosion potential. Under these conditions, we detect the formation of nonstoichiometric iron-sulfide films consisting mainly of mackinawite Fe(1+x)S, where x = 0.054–0.061, which plays the role of precursor for the other types of sulfides. As the H2S concentration becomes higher, the amount of deposited corrosion products increases but the film is porous and has many structural defects. As a result, local corrosion runs on the surface of carbon steel, which leads to the formation of pits [25].
Atomic hydrogen is one of corrosion products in aqueous solutions of H2S: Fe + 2H+ → Fe2+ + 2H0 [3]. It can be absorbed by metals and affect their mechanical and electrochemical properties. Sulfides inhibit the transformation of atomic hydrogen into the molecular hydrogen, which promotes its diffusion toward the metal surface and absorption. The process of molization of hydrogen atoms inside the metal leads to the appearance of internal stresses promoting both delamination and the formation of cracks and blisters [3, 8, 9, 18].
The hydrogenation ability of the steel varies under the action of mechanical stresses [28,29,30,31,32,33]. It depends on the applied mechanical voltage, material of the specimen, and the medium. It was discovered that the penetration of hydrogen through the membrane under tensile loads becomes more intense as the level of elastic strains increases but less intense as the level of plastic strains becomes higher. Under the conditions of elastic deformation, the interatomic distances in steel increase, and we observe the formation of low-energy dislocations and slip bands facilitating the diffusion of hydrogen. Under the conditions of plastic deformation, numerous dislocations and defects (massive edge dislocations inside grains, grain boundaries, and microcracks caused by the deformation of grain boundaries) play the role of traps for hydrogen, which may inhibit its diffusion into the metal.
It is possible to assume [5] that sulfide films appearing on the metal surface may form barriers blocking the diffusion of hydrogen into steel. The characteristics of blocking depend on the chemical composition and morphology of sulfides. Indeed, it become stronger as the hydrogen-sulfide concentration increases and the рН value of the solution decreases. This is explained by the fact that the corrosion products are semiconductors of the n -type, which form a network of positive charges on the corrosion-film–solution interface. They attract S2– and HS– anions, which interact with Fe2+, form sulfide films, and repel H+ cations from the surface.
As the thickness of the film increases, its inner layer becomes more compact, whereas the outer layer becomes less dense and porous. Since the diffusion of H+ ions toward the surface is retarded by the electrostatic repulsive forces, the reduction of hydrogen ions to Н atoms becomes weaker. The authors of [5] showed that the compositions of mackinawite and FeS on the surface inhibit the process of hydrogenation of carbon steel.
The authors of [29] stated that sulfide films inhibit the process of penetration of hydrogen in steel independently of elastic or plastic deformation. Under the conditions of elastic deformation, the potentials of hydrogen overvoltage (ηH) of steel with sulfide films are lower than for steel without films. Under the conditions of plastic deformation, the potential ηH of steel without films decreases, whereas ηH of steel with films significantly increases. In wet H2S, mackinawite appears on the surface in the form of spherical agglomerates without cracks and delaminations. Under stresses σ = 0.25σ0.2, the changes in the morphology of the film are insignificant. At the same time, for σ > 0.5σ0.2, cracks are formed in films, and individual spherical grains are separated. The thickness of the films does not exceed 5 μm and is independent of tensile stresses.
Influence of Sulfide Films on the Corrosion-Electrochemical Properties of Steels
In view of the existence of different assumptions concerning the action of sulfide-containing corrosion products on the corrosion-electrochemical properties of steels, the researchers of the Karpenko Physicomechanical Institute of NASU studied the influence of various iron sulfides on the surfaces of Armco iron, U8 steel, and 45 steel of different structures on the rate of redox processes and the hydrogenation of these steels in chloride– acetate solutions [34,35,36]. Iron sulfides (pyrite FeS2, troilite FeS, and kanzite Fe9S8) were formed under a potential E = – 400 mV (NHE) for hydrogen sulfide concentrations of 1, 10, and 100 mg/dm3, respectively, in sodium-sulfide solutions with рН = 3.2, 7.2, and 11.2 for 1 h [37, 38]. To identify these sulfides, we used X-ray phase diffraction analysis carried out in a DRON 3,0M diffractometer in the CuKα - and CoKα -radiation. By the method of vacuum extraction [39], we determined the amount of hydrogen absorbed in steel at temperatures of 200 and 800°С (CH200 and CH800) and its total content (CHΣ). Hydrogen desorbed at 200°С (diffusion- mobile) is weakly bound to the crystal lattice and may escape from it for a short time and at room temperature. At the same time, hydrogen desorbed at higher temperatures (residual) has a higher energy of bonding with the metal [40, 41].
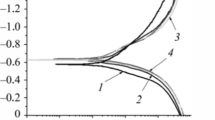
In [34], it was established that the formation of pyrite on the surface of Armco iron decreases the rate of cathodic and anodic processes in a 0.5% CH3COOH + 5% NaCl solution. In the presence of troilite, the rate of cathodic processes increases, whereas the rate of anodic process decreases. In the presence of kanzite, the efficiency of both pyrite and troilite increases, which leads to an increase in the corrosion rate by a factor of ∼ 3.5 (Table 1). On the contrary, pyrite decreases the corrosion rate of Armco iron, which reveals the manifestation of its protective properties. The cathodic processes combine the reduction of oxygen and hydrogen ions. On Armco iron and Fe–FeS2, their rate depends on oxygen depolarization, whereas on Fe–FeS and Fe–Fe9S8, their rate depends on hydrogen depolarization. For current densities much higher than i = 10–1 mA/cm2, the rate of cathodic processes is mainly determined by hydrogen depolarization and, hence, in the first approximation, for i = 1mA/cm2, it was assumed that voltammetric dependences characterize mainly the behavior of hydrogen depolarization. The comparison performed under the outlined conditions of overvoltages of the cathodic reactions that characterize mainly the activation energy of hydrogen depolarization for the volumes of hydrogen released and absorbed by Armco iron under cathodic polarization did not reveal any direct dependence between them [34].
In the presence of Fe–FeS2 pyrite on the electrode, for the highest cathodic overvoltage, the volume of released hydrogen is ∼ 5.5 times higher than for Armco iron and hydrogenation increases by at most ∼ 30%.
At the same time, in the presence of Fe–Fe9S8 kanzite, the cathodic overvoltage is lowest, the hydrogen reduction is ∼ 7.1 times more intense, and hydrogenation increases by a factor of ∼ 2.1.
This shows that sulfides, depending on their composition, exert different influence on the rates of different stages of the reaction of hydrogen release and, in particular, the reaction of catalytic recombination of hydrogen atoms specifying the surface concentration of adsorbed hydrogen atoms and playing the role of prerequisite and driving force of their absorption. In view of the fact that all investigated sulfides promote, to one or another extent, the hydrogenation of Armco iron, we can assume that they decelerate the analyzed reaction.
The formation of sulfides on the surfaces of U8 steel [35] decreases the rates of anodic processes but increases the rates of cathodic processes. Iron sulfides, independently of their composition, reduce the overvoltage of cathodic processes by 10–40% and increase the amount of released hydrogen. Independently of the volume of hydrogen released under the conditions of cathodic polarization of hydrogen, the presence of troilite promotes the hydrogenation of pearlite and sorbite to the greatest degree. At the same time, kanzite weakens hydrogenation of troostite and martensite by 30–35%, which demonstrates that the influence of sulfides on these processes is ambiguous.
The formation of pyrite and troilite on 45 steel [36] leads to a more pronounced increase in the rate of anodic processes as compared with the rate of cathodic processes as a result of which corrosion occurs under cathodic control. Kanzite reduces the corrosion rate of 45 steel with sorbite and troostite structure, and it runs under the anodic control. Under cathodic polarization in chloride–acetate solutions, ferrite–pearlite is hydrogenated to the highest degree, while the degree of hydrogenation of sorbite is twice lower. Pyrite and troilite promote their hydrogenation better than kanzite. Troostite and martensite absorb the lowest amounts of hydrogen, and the influence of sulfides is, in this case, negligible. The analysis of the action of iron sulfides on the electrode reactions of hydrogen release and absorption by Armco iron, U8 steel, and 45 steel with different structures show that, despite the fact that iron sulfides, as a rule, decrease the overvoltage of cathodic reactions and increase the amount of hydrogen released under cathodic polarization, they do not always promote the hydrogenation of the analyzed materials. This confirms the fact that iron sulfides mainly affect the reaction of hydrogen release but its absorption depends on the structure of steels [34,35,36,37].
On the basis of the analysis of the action of various iron sulfides on the corrosion rate, hydrogen overvoltage, and hydrogenation of carbon steels, we supplemented the well-known scheme of the mechanism of influence of hydrogen sulfide on the absorption of hydrogen by metals by the reactions of formation of sulfides of different compositions on the surface (Fig. 1). These sulfides affect the rate of recombination of hydrogen atoms and, hence, the molization of hydrogen and its absorption by the metals [37].
The scheme generalized according to the available literature data (underlined in Fig. 1) terminates by the formation of the [Fe(HS)]ads− surface catalyst, which promotes the ionization of iron and reduction of hydrogen ions [9, 19, 42, 43]. It does not take into account the fact that the oxidation of iron in sulfide-containing media is accompanied by the formation, on the surface, of poorly soluble sulfides whose compositions depend on the conditions of corrosion. It was proposed to supplement this scheme by the reactions of formation of sulfides of different compositions, which would affect the rate of hydrogen release and, in particular, the recombination of its atoms. Thus, the main difference of the proposed scheme is that, as a result of decomposition of the [Fe(HS)]ads− surface catalyst, we observe the formation of unstable mackinawite and its subsequent transformation into pyrite, troilite, or kanzite, which may differently affect the reaction of hydrogen reduction and, hence, its absorption by the metals.
The influence of hydrogen sulfide on the corrosion rate and hydrogenation of the steels was considered by an example of 17G1S-U steel in different media saturated with hydrogen in which kanzite is mainly formed on the surface as a result of corrosion [44]: NACE solution, distilled water, 5% NaCl, and 0.5% CH3COOH. The duration of the tests was 720 h.
The obtained results (Table 2) demonstrate that, in these solutions, hydrogen sulfide causes a significant increase in the level of hydrogenation of steel, which may become as high as 27.0–32.0 ppm independently of the рН value and corrosion rate [45]. At the same time, in the absorbed hydrogen, the concentration of diffusion-mobile component (CH200) is ∼ 2.2–3.5 times higher than the concentration of the residual component (CH800), which is characterized by a higher energy of bonding with the metal. In the absence of H2S, the hydrogenation of steel is ∼ 4–7 times weaker and the concentration of diffusion mobile hydrogen constitutes only ∼ 30–40% of its total content.
Thus, hydrogen sulfide not only intensifies the hydrogenation of steel but also causes a substantial increase in the amount of absorbed diffusion mobile hydrogen as compared with hydrogen with higher energies of bonding with the metal, which may play the role of the main factor of influence on the stress-corrosion fracture resistance of steel.
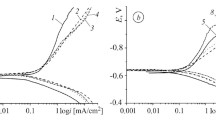
The influence of hydrogen-sulfide concentration (0–2800 mg/dm3) in a chloride–acetate solution with рН = 2.7 on the corrosion-electrochemical properties of 17G1S-U steel was investigated in [46]. It was shown that an increase in the H2S content shifts its electrode potential toward more negative values and leads to an almost linear acceleration of corrosion. At the same time, it does not change the character of electrode reactions. Indeed, the Tafel constants of cathodic reactions are, on the average, equal to 110 mV, whereas the Tafel constants of the anodic reactions constitute 65 mV for ten days. This means that hydrogen depolarization with electron transfer as the control stage is the main cathodic process in these corrosive media [34].
These electrochemical investigations make it possible to determine the rate of redox processes in the initial stage of interaction of metals with media. However, in the course of time, sulfides are formed on the surfaces of steels placed in hydrogen-sulfide solutions. They may affect the course of corrosion and localize it, which promotes the changes in the actual area of the surface. Under these conditions, the gravimetric methods of investigation enable us to trace the time dependences more adequately. As a result of conversion of the electrochemical index into the mass index, we performed the comparative evaluation [45] of corrosion rates in hydrogen- sulfide solutions of different concentration at the beginning and after holding for 150 h in the gravimetric tests (Table 3). It was shown that, in freely aerated chloride–acetate solutions, the corrosion rate increases with time by about two orders of magnitude. For CH2S = 50 mg/dm3, it changes insignificantly but, for 750; 1500, and 2800 mg/dm3, it increases by 20%, by a factor of ∼1.5, and by a factor of 2, respectively. This is an additional indication of the fact that, in order to choose steels for oil-and-gas extraction equipment, it is necessary to take into account the actual chemical composition of working media.
The analyses of the development of corrosion in 17G1S-U steel and its hydrogenation in the indicated media [45, 46] showed that, for CH2S = 25 mg/dm3, the corrosion rate remains practically constant as a function of time and equal to 0.37–0.54 g/(m2∙h). This can be explained by the formation of troilite–mackinawite (FeS– Fe1+xS) mixed dense films with small sizes of crystals.
For CH2S = 100 mg/dm3, the corrosion rate decreases with time and becomes equal to 0.48 g/(m2∙h) after holding for 720 h. For hydrogen-sulfide concentration of 500 and 1500 mg/dm3, it first decreases but then increases up to 1.6–1.8 g/(m2∙h), and steel absorbs a twice larger amount of hydrogen (Fig. 2) as in the case of lower concentrations due to the formation of looser films as a result of elevation of the content of kanzite. For these hydrogen-sulfide concentrations, hydrogen-induced cracking appears on specimens for 400 h (Fig. 3). Cracks are located along the direction of rolling of steel. Thus, for these hydrogen-sulfide concentrations in acid media, 17G1S-U steel is susceptible to stress-corrosion fracture.
The outward appearance of the specimens after investigations in hydrogen-sulfide-containing media shows that only pitting corrosion manifests itself on their surfaces after 400–450 h (Fig. 3). It has the form of chains directed along the direction of rolling of steel but no transformations into cracks were observed. Thus, local corrosion defects are detected despite certain protective properties of sulfides formed under these conditions.
Thus, the increase in hydrogen-sulfide concentration in chloride–acetate solutions at the beginning of holding accelerates the corrosion of 17G1S-U steel almost linearly without changing the character of electrode reactions. In the course of time, the corrosion rate is affected by the nature of sulfides formed on the surface. Thus, at a concentration of 25–100 mg/dm3, sulfides decrease the corrosion rate by an order of magnitude. For hydrogen-sulfide concentrations CH2S ≥ 500 mg/dm3, the corrosion rate is 3–4 times higher, we observe the formation of porous sulfides, corrosion defects are localized, and steel absorbs almost twice larger amounts of hydrogen as for lower concentrations, which promotes the development of cracking and corrosion cracking. The tests of steel for corrosion cracking under static loads σ = 0.8σ0.2 in chloride–acetate solution with CH2S = 100 mg/dm3 for 720 h did not lead to the fracture of specimens [45]. This is why we can state that CH2S ≤ 100 mg/dm3 is the critical concentration for the development of hydrogen-sulfide corrosion cracking of steels from this class.
Prospects of the Investigations of Corrosion and Stress-Corrosion Fracture of Steels in Hydrogen-Sulfide Media
On the basis of the presented results, it can be stated that, for the rational and economically efficient applications of steels in oil-and-gas extraction equipment, it is necessary to take into account the actual chemical compositions of the working media and their hydrogen-sulfide content. In the future, the investigations should be extended by taking into account the content of carbon dioxide in the extracted products. It is necessary to take into account its influence on the development of corrosion and hydrogenation of steels. The problem of carbon-dioxide corrosion appeared relatively recently in connection with the development of deep gascondensate fields with bottom-hole temperatures exceeding 80°С, pressures higher than 30 МPа, and CO2 contents in gases exceeding 1 vol.%. In the presence of CO2 and H2S in media, we observe the formation of films containing iron carbonates and iron sulfides on the steel surface. The appearance of these films is an important factor affecting the corrosion rate because they may inhibit corrosion as a result of formation of diffusion barriers for the components of both the medium and steel. This is why the investigations of corrosion processes in media with different concentrations of CO2 and H2S in combination with hydrogenation of steels and application of loads prove to be of high importance. These tests should be carried out in mineralized media under different partial pressures of corrosive gases at elevated temperatures. This requires the application of autoclave equipment for investigations guaranteeing the possibility to maintaining partial pressures of gases participating in corrosion processes at given levels.
References
R. Pourazizi, M. A. Mohtadi-Bonab, and J. A. Szpunar, “Investigation of different failure modes in oil and natural gas pipeline steels,” Eng. Fail. Anal., 109, 1–14 (2020); https://doi.org/10.1016/j.engfailanal.2020.104400.
International Measures of Prevention, Application, and Economics of Corrosion Technologies Study, Project manager E. Bowman, NACE Int., Houston (2016).
M. Askaria, M. Aliofkhazraeia, and S. Afroukhteh, “A comprehensive review on internal corrosion and cracking of oil and gas pipelines,” J. Nat. Gas Sci. Eng., 71, 1–25 (2019); https://doi.org/10.1016/j.jngse.2019.102971.
M. S. Khoma, “Problems of fracture of metals in hydrogen-sulfide media,” Fiz.-Khim. Mekh. Mater., 46, No. 2, 55–66 (2010); English translation: Mater. Sci., 46, No. 2, 190–200 (2010).
F. Huang, P. Cheng, X. Y. Zhao, J. Liu, Q. Hu, and F. Cheng, “Effect of sulfide films formed on X65 steel surface on hydrogen permeation in H2S environments,” Int. J. Hydrog. Energy, 42, No. 7, 4561–4570 (2017); https://doi.org/10.1016/j.ijhydene.2016.10.130.
M. A. Mohtadi-Bonab, M. Eskandari, K. M. M. Rahman, R. Ouellet, and J. A. Szpunar, “An extensive study of hydrogen-induced cracking susceptibility in an API X60 sour service pipeline steel,” Int. J. Hydrog. Energy, 41, No. 7, 4185–4197 (2016).
J. L. Gonzalez, R. Ramire, J. M. Hallen, and R. A. Guzman, “Hydrogen induced crack growth rate in steel plates exposed to sour environments,” Corrosion, 53, 935–9346 (1997).
E. Legrand, J. Bouhattate, X. Feaugas, and H. Garmestani, “Computational analysis of geometrical factors affecting experimental data extracted from hydrogen permeation tests: II Consequences of trapping and an oxide layer,” Int J. Hydrog. Energy, 37, 13574–13582 (2012).
D. W. Shoesmith, P. Taylor, M. G. Bailey, and D. G. O. Wen, “The corrosion of iron by aqueous hydrogen sulfide at 21°C,” J. Electrochem. Soc., 127, 1007–1015 (1980).
S. Arzola, J. Mendoza-Flores, R. Duran-Romero, and J. Genesca, “Electrochemical behavior of API X70 steel in hydrogen sulfidecontaining solutions,” Corrosion, 62, 433–443 (2006).
D. Abayarathna, A. Naraghi, and N. Obeyesekere, “Inhibition of corrosion of carbon steel in the presence of CO2, H2S and S,” in: Proc. of the NACE Conf. “Corrosion 2003” (March 16–20, 2003, San Diego, California), Paper Number NACE-03340, NACE Int., Houston (2003).
NACE Standard TM 0177-90. Standard Test Method Laboratory of Metals for Resistance to Sulfide Stress Corrosion Cracking in H2S Environments, National Association of Corrosion Engineers (NACE), Houston, TX (1990).
M. M. Ivanyuta (editor), Atlas of Oil Deposits and Gas Fields of Ukraine [in Ukrainian], Tsentr Evropy, Lviv (1998).
N. D. Tomashov and G. P. Chernova, Passivity and Corrosion Protection of Metals [in Russian], Nauka, Moscow (1965).
M. Liu, J. Wang, W. Ke, and E.-H. Han, “Corrosion behavior of X52 anti-H2S pipeline steel exposed to high H2S concentration solutions at 90°C,” J. Mater. Sci. Technol., 30, 504–510 (2014).
G. Genchev and A. Erbe, “Sour gas corrosion – corrosion of steels and other metallic materials in aqueous environments containing H2S,” in: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier (2017), pp. 221–231.
H. Ma, X. Cheng, G. Li, S. Chen, Z. Quan, S. Zhao, and L. Niu, “The influence of hydrogen sulfide on corrosion of iron under different conditions,” Corros. Sci., 42, 1669–1683 (2000).
M. Monnot, R. P. Nogueira, V. Roche, G. Berthomé, E. Chauveau, R. Estevez, and M. Mantel, “Sulfide stress corrosion study of a super martensitic stainless steel in H2S sour environments: Metallic sulfides formation and hydrogen embrittlement,” Appl. Surf. Sci., 394, 132–141 (2017).
Z. A. Iofa, “On the mechanism of action of hydrogen sulfide and inhibitors on the corrosion of iron in acid solutions on iron,” Zashch. Met., No. 3, 275–280 (1980).
H. Vedage, T. A. Ramanarayanan, J. D. Mumford, and S. N. Smith, “Electrochemical growth of iron sulfide films in H2S-saturated chloride media,” Corrosion, 49, 114–121 (1993).
G. T. Park, S. U. Koh, H. G. Jung, and K. Y. Kim, “Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel,” Corros. Sci., 50, 1865–1871 (2008).
C. F. Dong, Z. Y. Liu, X. G. Li, and Y. F. Cheng, “Effects of hydrogen-charging on the susceptibility of X100 pipeline steel to hydrogen-induced cracking,” Int. J. Hydrog. Energy, 34, 9879–9884 (2009).
F. Huang, J. Liu, Z. J. Deng, J. H. Cheng, Z. H. Lu, and X. G. Li, “Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel,” Mater. Sci. Eng. A, 52, 6997–7001 (2010).
H. Ma, X. Cheng, S. Chen, C. Wang, J. Zhang, and H. Yang, “An impedance study of the anodic dissolution of iron in sulfuric acid solutions containing hydrogen sulfide,” J. Electroanal. Chem., 451, 11–17 (1998).
J. Tang, Y. Shao, J. Guo, T. Zhang, G. Meng, and F. Wang, “The effect of H2S concentration on the corrosion behavior of carbon steel at 90°C,” Corros. Sci., 52, 2050–2058 (2010).
H. Y. Ma, X. L. Cheng, S. H. Chen, G. Q. Li, X. Chen, S. B. Lei, and H. Q. Yang, “Theoretical interpretation on impedance spectra for anodic iron dissolution in acidic solutions containing hydrogen sulfide,” Corrosion, 54, 634–640 (1998).
X. L. Cheng, H. Y. Ma, J. P. Zhang, X. Chen, S. H. Chen, and H. Q. Yang, “Corrosion of iron in acid solutions with hydrogen sulfide,” Corrosion, 54, 369–376 (1998).
Z. Cheng, L. Yunhan, B. Yakui, Z. Ruiqian, D. Xun, and C. Yixiang, “Hydrogen permeation characteristic of nanoscale passive films formed on different zirconium alloys,” Int. J. Hydrog. Energy, 41, 7676–7690 (2016).
F. Y. Ge, F. Huang, W. Yuan, Z. Peng, J. Liu, and Y. F. Cheng, “Effect of tensile stress on the hydrogen permeation of MS X65 pipeline steel under sulfide films,” Int. J. Hydrog. Energy, 2, 1–13 (2020); https://doi.org/10.1016/j.ijhydene.2020.02.149.
M. S. Khoma, M. R. Chuchman, B. M. Datsko, and A. I. Dyachuk, “Relationship between the corrosion and hydrogen factors in the course of fracture of pipe steels in hydrogen sulfide media,” Fiz.-Khim. Mekh. Mater., Special Issue No. 10, 5–10 (2014).
M. S. Khoma, V. A. Vynar, M. R. Chuchman, and Ch. B. Vasyliv, “Stress-corrosion failure of pipe steels in hydrogen sulfide environments,” in: Ser. Lecture Notes in Civil Engineering, Vol. 102 (2020), pp. 231–239.
M. S. Khoma, V. R. Ivashkiv, S. A. Halaichak, M. R. Chuchman, and Kh. B. Vasyliv, “Influence of the structure of carbon steels on the corrosion, hydrogenation, and corrosion cracking in hydrogen-sulfide media,” Fiz.-Khim. Mekh. Mater., 55, No. 2, 121–125 (2019); English translation: Mater. Sci., 55, No. 2, 272–276 (2019).
М. R. Chuchman, “Static crack resistance of 20 and 30KhMA steels in a NACE solution,” Fiz.-Khim. Mekh. Mater., 52, No. 4, 76–78 (2016); English translation: Mater. Sci., 52, No. 4, 530–532 (2017).
М. S. Khoma, S. A. Holovei, V. R. Ivashkiv, “Oxidation-reduction processes on Armco iron in sulfide-containing alkaline media,” Fiz.-Khim. Mekh. Mater., 52, No. 5, 38–43 (2016); English translation: Mater. Sci., 52, No. 5, 643–649 (2017).
М. S. Khoma, S. A. Holovei, V. R. Ivashkiv, and Kh. B. Vasyliv, “Effect of sulfides on the hydrogen overvoltage and hydrogenation of U8 steel in chloride-hydrogen-sulfides media,” Fiz.-Khim. Mekh. Mater., 53, No. 6, 16–22 (2017); English translation: Mater. Sci., 53, No. 6, 761–768 (2018).
М. S. Khoma, N. B. Rats’ka, S. A. Holovei, V. R. Ivashkiv, and M. R. Chuchman, “Specific features of corrosion and the microelectrochemical heterogeneity of 45 and U8 steels in hydrogen-sulfide media,” Fiz.-Khim. Mekh. Mater., 54, No. 4, 52–56 (2018); English translation: Mater. Sci., 54, No. 4, 501–505 (2019).
S. A. Halaichak, B. M. Datsko, and Y. Y. Maksishko, “Oxidation-reduction reactions and hydrogenation of steels of different structures in chloride–acetate solutions in the presence of iron sulfides,” in: Proc. of the Young Scientists Conf. on Materials Science and Surface Engineering (MSSE-2019) (September 25–27, 2019, Lviv) [in Ukrainian], Lviv (2019), pp. 61–64.
J. B. Sardisco and R. E. Pitts, “Corrosion of iron in an H2S–CO2–H2O-system. Composition and protectiveness of the sulphide film as a function of pH,” Corrosion, No. 11, 350–354 (1965).
GOST 23338-91. Methods for the Determination of the Content of Diffusion Hydrogen in the Fused Metal and in the Metal of the Weld [in Russian], Izd. Standartov, Moscow (1991).
K. Motomichi, T. Cemal, and А. Eiji, “Hydrogen-assisted decohesion and localized plasticity in dual-phase steel,” Acta Mater., No. 3, 168–173 (2014).
Н. Hagi, “Effect of interface between cementite and ferrite on diffusion of hydrogen in carbon steels,” Mater. Trans. JIM, No. 35, 168–173 (1994).
Z. A. Iofa, “On the mechanism of accelerating action of hydrogen sulfide on the reaction of discharge of hydrogen ions on iron,” Zashch. Met., No. 1, 17–21 (1974).
H. Castaneda, E. Sosa, and M. A. Espinosa-Medina, “Film properties and stability influence on impedance distribution during the dissolution process of low-carbon steel exposed to modified alkaline sour environment,” Corros. Sci., 51, 799–806 (2009).
V. І. Pokhmurskyi, R. K. Melekhov, H. М. Krutsan, and V. H. Zdanovskyi, Stress-Corrosion Fracture of Welded Structures [in Ukrainian], Naukova Dumka, Kyiv (1995).
V. І. Pokhmurskyi, М. S. Khoma, M. R. Chuchman, and B. M. Datsko, “Corrosion and hydrogenation of 17G1S-U steel in hydrogen-sulfide media of different concentrations,” Fiz.-Khim. Mekh. Mater., Special Issue, No. 5, 15–22 (2020); English translation: Mater. Sci., 56, No. 5, 593–601 (2021).
М. S. Khoma, V. R. Ivashkiv, N. B. Ratska, B. М. Datsko, and М. R. Chuchman, “Corrosion-electrochemical properties of 17G1SU steel in chloride–acetate solutions with different concentrations of hydrogen sulfide,” Fiz.-Khim. Mekh. Mater., Special Issue, No. 4, 100–104 (2020); English translation: Mater. Sci., 56, No. 4, 544–549 (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 57, No. 3, pp. 17–27, May–June, 2021.
Rights and permissions
About this article
Cite this article
Khoma, М.S., Vasyliv, K.B. & Chuchman, М.R. Influence of the Hydrogen Sulfide Concentration on the Corrosion and Hydrogenation of Pipe Steels (A Survey). Mater Sci 57, 308–318 (2021). https://doi.org/10.1007/s11003-021-00546-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-021-00546-x