We study the influence of hydrogen peroxide on the phase composition, thickness, and porosity of oxide-ceramic coatings obtained by plasma electrolytic oxidation on Al–Si–Cu and Al–Cu–Mg aluminum alloys. For these two systems, it is shown that the presence of H2O2, with a concentration of 5 g/liter, makes it possible to get a twofold increase in the thickness of the oxide-ceramic coating as compared with the original electrolyte. A subsequent increase in the concentration of hydrogen peroxide leads to a decrease in the thickness of oxide-ceramic coatings. The maximum content of corundum is obtained for hydrogen-peroxide concentrations of 5 g/liter for the Al–Cu–Mg system and 7 g/liter for the Al–Si–Cu system. The presence of silicon in the composition alloy results in the formation of sillimanite and quartz in oxide-ceramic coatings, which is accompanied by an increase in the volume of oxide-ceramic coatings. As the concentration of hydrogen peroxide in the electrolyte increases, the porosity of the Al–Si–Cu system decreases, whereas the porosity of the Al–Cu–Mg system does not change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silumins are alloys based on aluminum and silicon. The silicon content of silumin products varies from 4 to 22% of the total volume [1,2,3]. At present, silumin is one of the most widely used aluminum alloys. Low cost of silumins in combination with their good technological characteristics makes them quite promising materials for the extensive application in industry, including the mechanical engineering (pistons, components of the bodies, engine cylinders, etc.), aircraft construction (cylinder blocks, cooling pistons, and aircraft units), aerospace engineering (workpieces with low coefficients of linear thermal expansion and high level of mechanical properties), manufacturing of the gas-turbine equipment (generators, heat exchangers), etc. The operating characteristics of silumin depend on its silicon content. The higher the silicon content, the higher the hardness and wear resistance of the alloy, but its strength decreases [4, 5].
As one of the most urgent tasks of contemporary science and technology, we can mention the development of new environmentally friendly technologies of application of highly efficient and reliable coatings aimed at the protection and hardening of metal products. There are numerous ways of increasing the wear and corrosion resistance of light alloys: anodizing, phosphatazing, ion-plasma and laser treatment, etc. [6,7,8,9,10,11,12]. All these methods improve the corrosion resistance but the wear resistance of the obtained coatings remains insufficiently high. At present, there is a relatively new but rapidly developed procedure of surface treatment and hardening of metallic materials, namely, the method of plasma-electrolytic oxidation (PEO). Note that this method originates from traditional anodizing. With the help of this method, it is possible to obtain multifunctional oxide-ceramic coatings on the valve metals (Al, Mg, Ti, Zr, and Ta) [13, 14]. Samples of valve metals are immersed in electrolytes and anode and cathode voltages are applied to them in turn. There are four main stages of formation of oxide-ceramic coatings on these metals: formation of a primary oxide film in the pre-spark stage according to the electrochemical mechanism; breakdown of the primary oxide film and the formation of a plasmoid in the discharge channel; plasma-chemical reactions of formation of the intermediate and final products, and condensation and polymorphic transformations of the oxide phases [15]. The coatings mostly consist of high-temperature oxide phases (Al2O3, TiO2, MgO, and ZrO2) [16].
As a disadvantage of this method, we can mention its quite high energy consumption and, hence, slow formation of coatings [17]. A positive influence of hydrogen peroxide on the rate of synthesis and phase composition of oxide-ceramic coatings synthesized on aluminum alloys of the Al–Cu–Mg system was established in [18,19,20].
The aim of the present work is to study the effect of hydrogen peroxide presence in electrolytes on the phase composition, thickness, and porosity of oxide-ceramic coatings obtained by the method of plasma electrolytic oxidation on aluminum alloys of the Al–Si–Cu and Al–Cu–Mg systems.
Materials and Methods of Investigations
Oxide-ceramic coatings were synthesized on the following aluminum alloys: AK7 (87.6–93.6% Al; 6–8% Si; 1.5% Cu; 0.2–0.5% Mg, and 0.2–0.5% Mn) and D16 (94.7% Al; 3.8–4.9% Cu; 1.2–1.8% Mg, and 0.3–0.9% Mn). The dimensions of the samples were as follows: 20 × 15 × 3 mm. Prior to synthesis, the specimens were polished and washed in distilled water and ethyl alcohol. The coatings were formed by anodic and cathodic pulses applied to the samples in turn. The cathodic and anodic current densities were as follows: jc/ja = 10/10 A/dm2 for D16 alloy and jc/ja = 10/10 A/dm2 or 15/15 A/dm2 for AK7 alloy. As an electrolyte, we used an aqueous solution of KOH (3 g/liter) and Na2SiO3 (2 g/liter) and also the same electrolyte with various concentrations of H2O2.
The X-ray phase diffraction analysis of the coatings was carried out by using a DRON-3.0 X-ray diffractometer in the CuKα-radiation. The content of each phase was found by analyzing the accumulated diffraction patterns with the help of the FullProf software package according to the multiprofile Rietveld method.
The thickness of the coatings was determined by using a CHY TG-05 thickness gauge (with an accuracy of measurements equal to 0–199 μm ± 2 μm). The porosity of the coatings was determined by analyzing the photomicrographs of the PEO coatings obtained in a scanning electron microscope with a magnification of × 500 by the method described in [21,22,23].
Results and Discussion
Plasma-electrolyte coatings were obtained on AK7 and D16 commercial alloys according to the standard procedure. After 1 h of synthesis in the original electrolyte for densities of the anodic and cathodic currents equal to jc/ja = 10/10 A/dm2, an oxide-ceramic coating is formed on AK7 alloy. However, its thickness constitutes only 35–40 μm, which is insufficient to increase the wear resistance of the sample. The yield of the products of the reaction of synthesis of oxide-ceramic coatings can be shifted toward higher amounts of aluminum oxide by increasing the concentrations of oxidizers in plasma discharge channels [18, 23].
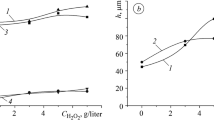
An addition of H2O2 with a concentration of 3 g/liter to the base electrolyte has almost no effect on the thickness of the oxide-ceramic coating. Therefore, in order to increase the thickness, the process of synthesis was performed for the anodic and cathodic current densities equal to jc/ja = 15/15 A/dm2. Although the thickness of the coating in the source electrolyte remains almost constant and equal to 42–47 μm, the increase in the duration of synthesis up to 2 h under the same conditions leads to a twofold increase in thickness (up to 90–100 μm) (Fig. 1).
Dependences of changes in the thickness of oxide ceramic coatings on the D16 (1, 2) and AK7 (3–5) alloys on the concentration of H2O2 in the 3 g/liter KOH + 2 g/liter Na2SiO3 electrolyte for 1 h (2, 3, 5) and 2 h of synthesis (1, 3) and the ratios jc/ja = 10/10 A/dm2 (1–3) and jc/ja = 15/15 A/dm2 (4, 5).
The addition of hydrogen peroxide to the electrolyte leads to an increase in the coating thickness on the Al–Cu–Mg alloy. The maximum thickness (90–100 μm) is observed at a concentration of 5 g/liter H2O2 (Fig. 1, curve 5). Further increase in the concentration of peroxide leads to a decrease in the thickness of the coating. This is explained by a significant increase in the pH value of the electrolyte and the predominance of the processes of dissolution of alumina over the processes of its synthesis [20].
Based on the analysis of diffraction patterns of coatings (Fig. 2) obtained in electrolytes of different composition using FullProf software [25], diffraction reflexes corresponding to six phases were established. The main phases in the coating are as follows: corundum – α-Al2O3; γ-Al2O3; quartz – SiO2; sillimanite – Al2O3·SiO2, as well as traces of silicon and aluminum, which emit from the base metal and inform about the thickness of the oxide-ceramic coating formed on the alloy.
It was found that the elevation of the concentration of hydrogen peroxide leads to an increase in the thickness of the coating, which correlates with the results presented in [19, 20]. The intensity of the reflexes of aluminum and silicon decreases as the thickness of the oxide-ceramic coating on AK7 alloy increases.
We also determined the distributions of the quantitative contents of the phases of oxide-ceramic coating on the alloys of the Al–Si–Cu (Fig. 3a) and the Al–Cu–Mg (Fig. 3b) systems depending on the concentration of hydrogen peroxide.
Phase composition of the oxide-ceramic coating synthesized on AК7 alloy for current densities equal to jc/ja = 15/15 A/dm2 (а) and D16 alloy at jc/ja = 10/10 A/dm2 (b) in the source electrolyte and with additions of H2O2 and the durations of synthesis equal to 1 h (1–3) and 2 h (4–6): (1, 7) α-Al2O3; (2, 8) γ-Al2O3; (3, 9) Al; (4) SiO2; (5) Si; (6) Al2O3·SiO2.
Thus, the content of α-Al2O3 in the coating formed on the alloy D16 is 25–27%, while the same synthesis conditions for AK7 alloy lead to the formation of a coating containing only 11–13% α-Al2O3. Although for these two alloys, the thickness of coatings decreases as the hydrogen peroxide concentration increases to 7 g/liter, the corundum content on AK7 alloy increases to 20%. We should also mention a continuous growth of the contents of both γ- Al2O3 and sillimanite Al2O3·SiO2. A nonuniformity of the amount of quartz in the samples is observed because of a non-uniform distribution of silicon in silumin. The low content of corundum indicates that it is most likely that silicon contributes to the formation of the main phases γ-Al2O3 and Al2O3·SiO2.
An important characteristic of coatings is their porosity. Both corrosion and wear resistance of oxide-ceramics depend on it. The porosity of oxide-ceramic coatings was studied by using 10 micrographs of their surface for each of the modes. The images of the surface of oxide-ceramic coatings on the alloy were analyzed by the segmentation method and the ratio of the pore area to the surface area was calculated.
On the AK7 alloy, the highest porosity (4.7%) was observed at a peroxide concentration of 3 g/liter. Increasing the peroxide content in the electrolyte reduces the porosity to 3.1%. The surface of D16 alloy is covered with the largest number of pores (4.32%) in the initial electrolyte. Changing the concentration of H2O2 slightly reduces the overall porosity to 3.53–3.57%. Analyzing the phase composition of alloys synthesized in different electrolytes, it was found that the increase in the content of sillimanite leads to an increase in the volume of the coating and reduces the relative porosity. This can reduce the access of the corrosive medium to the base metal via the through pores and thus increase the corrosion resistance of the alloy.
Conclusions
It was discovered that, for the Al – Si – Cu and Al – Cu – Mg systems, an increase in the contents of oxidizers (in particular, hydrogen peroxide) in electrolytes leads to the acceleration of growth of oxide-ceramic coatings. The proposed concentration of H2O2 (5 g/liter) makes it possible to increase the thickness of the oxide-ceramic coating almost twice as compared with the original electrolyte for the same energy consumption. The presence of 7 wt.% silicon in the initial alloy AK7 leads to the formation of quartz and sillimanite in the coatings. The maximum content of corundum in oxide-ceramic coatings synthesized on AK7 alloy is 20 wt.%, which is much lower than on D16 alloy (30 wt.%). This means that silicon most likely promotes the formation of Al2O3·SiO2 and γ-Al2O3. The presence of hydrogen peroxide in the electrolyte (5–7 g/liter) lowers the porosity of the Al–Si–Cu system and does not affect it in the Al–Cu–Mg system.
References
D. F. Cherneha, V. S. Bogushev’skyi, Yu. Ya. Gotvyans’kyi, V. S. Tereshchenko, B. M. Boichenko, P. S. Kharlashin, and V. A. Hladkykh, Fundamentals of the Metallurgical Production of Metals and Alloys [in Ukrainian], Vyshcha Shkola (2006).
L. F. Mondolfo, Structure and Properties of Aluminum Alloys [in Russian], Metallurgiya, Moscow (1979).
M. M. Makhloufе and H. V. Guthy, “The aluminum-silicon eutectic reaction: mechanisms and crystallography,” J. Light Metals, 1, No. 4, 199–218 (2001).
O. Mityayev and I. Volchok, “Influence of intermetallic phases on fracture resistance of silumins,” Arch. Foundry Eng., 13, No. 4, 83–86 (2013).
I. P. Volchok, O. A. Mityaev, O. V. Lyutova, O. O. Krulikovs’ka, and T. V. Vanyarkha, “Increasing the resistance to the destruction of secondary silumin,” Metallurg. Metalwork., 20, No. 95, 46–53 (2020).
V. I. Pokhmurskii, I. M. Zin, V. A. Vynar, and L. M. Bily, “Contradictory effect of chromate inhibitor on corrosive wear of aluminium alloy,” Corr. Sci., 53, No. 3, 904–908 (2011).
V. I. Pokhmurskii, I. M. Zin, V. A. Vynar, O. P. Khlopyk, and L. M. Bily, “Corrosive wear of aluminium alloy in presence of phosphate,” Corr. Eng. Sci. Technol., 47, No. 3, 182–187 (2012).
G. W. Critchlow, K. A. Yendall, D. Bahrani, A. Quinn, and F. Andrews, “Strategies for the replacement of chromic acid anodizing for the structural bonding of aluminium alloys,” Int. J. Adhes. Adhesives, 26, No. 6, 419–453 (2006).
I. P. Volchok, V. V. Girzhon, and I. V. Tantsiura, “Increasing of microhardness of Al–Si alloys by laser treatment,” Metallofiz. Noveish. Tekhnol., 33, No. 8, 1111–1118 (2011).
V. Hutsaylyuk, M. Student, K. Zadorozhna, P. Maruschak, and H. Pokhmurska, “Improvement of wear resistance of aluminum alloy by HVOF method,” J. Mat. Res. Technol., 9, No. 6, 16367–16377 (2020).
Yu. F. Ivanov, I. V. Lopatin, O. C. Tolkachev, and M. E. Rygina, “Structure and properties of silumin after electron-ion-plasma multicycle modification,” in: 13th Internat. Conf. “Interaction of Radiation with Solids” (September 30–October 3), Minsk (2019).
I. M. Zin, O. P. Khlopyk, and M. Ya. Holovchuk, “Protective action of inorganic inhibitors on mechanically activated aluminum surfaces,” Mater. Sci., 49, No. 3, 298–303 (2013).
I. V. Suminov, P. M. Belkin, A. V. Epelfeld, V. B. Lyudin, B. L. Krit, and A. M. Borisov, Plasma-Electrolytic Modification of the Surface of Metals and Alloys [in Russian], in 2 Vol., 2, Tekhnosphera, Moscow (2011).
V. Pokhmurskii, H. Nykyforchyn, M. Student, M. Klapkiv, H. Pokhmurska, B. Wielage, T. Grund, and A. Wank, “Plasma electrolytic oxidation of arc-sprayed aluminium coatings,” J. Thermal Spray Technol., 16, No. 5–6, 998–1004 (2007).
M. D. Klapkiv, “Simulation of synthesis of oxide-ceramic coatings in discharge channels of a metal-electrolyte system,” Mater. Sci., 35, No. 2, 279–283 (1999).
H. M. Nykyforchyn, V. S. Agarwala, M. D. Klapkiv, and V. M. Posuvailo, “Simultaneous reduction of wear and corrosion of titanium, magnesium and zirconium alloys by surface plasma electrolytic oxidation treatment,” Adv. Mat. Res., 38, 27–35 (2008).
J. Martin, P. Leone, A. Nominé, D. Veys-Renaux, G. Henrion, and T. Belmonte, “Influence of electrolyte ageing on the plasma electrolytic oxidation of aluminium,” Surf. Coat. Technol., 269, 36–46 (2015).
Yu. A. Kuznetsov, K. V. Kulakov, and V. V. Goncharenko, “Technological features of selection of electrolytes for getting thick-layer ceramic coatings,” New Mater. Technol. Mech. Eng., No. 14, 52–55 (2011).
V. M. Posuvailo, V. V. Kulyk, Z. A. Duriagina, I. V. Koval’chuck, M. M. Student, and B. D. Vasyliv, “The effect of electrolyte composition on the plasma electrolyte oxidation and phase composition of oxide ceramic coatings formed on 2024 aluminium alloy,” Archives Mat. Sci. Eng., 105(2), 49–55 (2020).
M. M. Student, H. H. Veselivska, O. S. Kalakhan, K. R. Zadorozhna, and Y. Y. Sirak, “Influence of the conditions of plasma-electrolytic treatment of D16T aluminum alloy on its corrosion resistance in 3% NaCl solution,” Mater. Sci., 56. No. 4, 550–559 (2021).
L. A. Xiao-Cong, “Hybrid SVM-QPSO model based ceramic tube surface defect detection algorithm,” in: 5th Internat. Conf. on Intelligent Systems Design and Eng. Appl., Hunan (2014), pp. 28–31.
I. Ivasenko, V. Posuvailo, M. Klapkiv, V. Vynar, and S. Ostap’yuk, “Express method for determining the presence of defects of the surface of oxide-ceramic coatings,” Mater. Sci., 45, No. 3, 460–464 (2009).
I. Ivasenko and V. Chervatyuk, “Detection of rust defects of protective coatings based on HSV color model,” in: IEEE 2nd Ukrain. Conf. on Electric and Computer Engineering (UKRCON) (2019), pp. 1143–1146.
M. D. Klapkiv, O. S. Chuchmarev, P. Ya. Sydor, and V. M. Posuvailo, “Thermodynamics of the interaction of aluminum, magnesium and zirconium with components of an electrolytic plasma,” Mater. Sci., 36, No. 1, 66–79 (2000).
J. Rodríguez-Carvajal, Program FullProf.2k. Version 2.20, Laboratoire Léon Brillouin (CEA–CNRS), Grenoble (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 57, No. 6, pp. 127–132, November–December, 2021.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Posuvailo, V.M., Kovalchuk, I.V. & Ivasenko, I.B. Influence of Hydrogen Peroxide on the Composition and Porosity of Oxide-Ceramic Coatings on Alloys of the Al–Si–Cu and Al–Cu–Mg Systems. Mater Sci 57, 894–899 (2022). https://doi.org/10.1007/s11003-022-00619-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-022-00619-5