Four different types of electrolyte (aluminate, silicate, borate, and phosphate) solutions were optimized to be used for the surface treatment of aluminum alloy 7075 by plasma electrolytic oxidation. Microstructure, phase composition, and corrosion resistance of ceramic coatings on the surface were analyzed by SEM, X-ray diffraction, and electrochemical work station. It was demonstrated that ceramic coatings prepared in aluminate solution had excellent continuity and a compact structure with micro hardness of HV 0.1 = 1100 MPa. The majority of all coatings consisted of γ-Al 2 O 3 and bits of α-Al 2 O 3 . The corrosion potential was increased to a small extent, while the corrosion current density was significantly reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma electrolytic oxidation (PEO) is an eco-friendly and effective method of the surface treatment [1]. When aluminum alloys were treated by this technique, a plasma arc discharge occurred in the micro-field of the alloy surface where high temperature and pressure were generated to make molten aluminum atoms interact with oxygen atoms in solutions to form ceramic coatings. The coatings have a good adhesion to the substrate with good abrasive resistance and corrosion resistance. So far, the technique was intended to replace hard anodize in the aerospace industry and was widely applied.
The Zn atoms in alloy were usually found to inhibit the PEO process [2, 3]. At present, the aluminum alloys, which have high Zn content, cannot be successfully treated by this technique. However, high strength aluminum alloys are preferred materials for producing components for the air forces.
Therefore, this paper is to study the processing of the high strength aluminum alloy 7075 by PEO in order to optimize electrolyte compositions in aluminate, silicate, borate, and phosphate solutions by orthogonal experiment.
Finally, the data on the microstructure, phase composition, and corrosion resistance of ceramic coatings were obtained.
Experimental procedure
The aluminum alloy 7075 was used as raw material with a composition as, wt.%: 5.1~6.1 Zn, 2.1~2.9 Mg, 1.2~2.0 Cu, 0.5 Fe, 0.4 Si, 0.3 Mn. The dimensional size of samples is 20 × 20 × 6.5 mm3, which were polished and cleaned and then hung in the electrolyte as a positive pole while negative pole was a stainless steel container. The stirred electrolyte was controlled below 30°C. PEO power worked at the parameters of constant current density 10 A/dm2 with ratio of currents I c/I a = 0.7, anode duty cycle 15%, cathode duty cycle 10%, frequency 300 Hz, and oxidation time 45 min.
Orthogonal experiments of three factors and three levels were used in the experiment. The concentration of alkali, sodium hydroxide and triethanolamine were considered as the three factors: the level of alkali was aluminate, silicate, borate, and phosphate solutions; that of sodium hydroxide 0.5, 1.0, and 1.5 g/L; triethanolamine 4, 6, and 8 mL/L. Hydrogen peroxide was added in a content of 2 mL/L to provide abundant oxygen for the electrolyte; boric acid was used as complexing agent to control the pH of electrolyte between 9 and 11.
The thickness of ceramic coatings was measured on two sides of the samples by portable tester and the microhardness was tested on the polished cross-section of the samples. SEM (S-4700) was employed to investigate ceramic coatings. The phase composition was studied with D/max-2200VPC X-ray diffraction with 2θ from 10° to 85°. The corrosion resistance was evaluated by potentiodynamic polarization measurement, which was carried out in 3.5 wt.% NaCl solution for bare and coated samples with CHI604b electrochemical work station.
Results and Discussion
Electrolyte Parameter Optimization. Table 1 shows the results of the orthogonal experiment where the microhardness and thickness of ceramic coatings were defined as index for analysis. Table 2 shows the results of H i (i = 1, 2, 3), h i (i = 1, 2, 3), and R of each factor after calculation, where H i and h i are the sum of the experimental index on level i and the arithmetic mean value, respectively; R is the range.
The factors have the same sequence that is A–B–C, from both hardness and thickness results. The factor A has the biggest influence on the hardness of ceramic coatings, but has a relatively minor influence on the thickness, in any case, the second level should be selected. Based on hardness or thickness data, the second level should be selected for the factor B. The factor C influences on the hardness and thickness of the coating as the factor A does. Therefore, the optimum electrolyte compositions of the aluminate system should be A2B2C2 (for example, the concentration of aluminate 9 g/L, sodium hydroxide 1 g/L, and triethanolamine 6 mL/L). Also, so did alkali, sodium hydroxide, and triethanolamine in silicate, borate, and phosphate solutions. The thicker coatings can be produced in silicate and borate solutions. However, the best hardness of coatings was obtained in aluminate solution. Table 3 lists the optimum electrolyte compositions in different systems.
Microstructure and Phase Composition of the Coating. All coatings showed a crater-like surface morphology with many residual particles on the surface, which were caused by plasma chemical and electrochemical reaction within the area of the micro plasma arc discharge. Each discharge resulted in high temperature and high pressure, and made the molten aluminum erupt from the discharge channel. When the melt met cooled electrolyte, it reacted, quenched, and deposited to form coatings and then, a porous rough surface appeared with pore or particle size ranging from 1 to 5 μm [4]. These pores were a residual discharge channel, which did not close after micro plasma discharge.
Figures 1a , 1c, 1e, and 1g show there are much more and larger pores in the coating produced in silicate solution; “volcanic chimney” morphology of the surface of ceramic coatings is more obvious. The ceramic coatings produced in aluminate had less pores with smaller diameter ranging from 1 to 3 μm. However, the ceramic coatings, formed in borate and phosphate, were not outstanding. The quantity and diameter of pores were at the middle level among the four electrolyte systems and well-distributed.
Figures 1b , 1d, 1f, and 1h show the cross-section structure of ceramic coatings. Ceramic coatings are mainly composed of a compact layer and a loose layer with no obvious boundary between them. It is a metallurgical combination between ceramic coatings and the substrate. The coatings produced in aluminate and borate systems are thin, but continuous and smooth, while the coatings produced in aluminate are relatively smoother. The coatings produced in silicate system are thicker, but rough. The coatings formed in phosphate system are the thinnest and loose among the four solutions.
All ceramic coatings produced in aluminate, silicate, borate, and phosphate were mainly composed of γ-Al2O3 and bits of α-Al2O3. Instant temperature reached up to 8000 K in the plasma discharge area [5], which corresponded to the conditions for the transition from metastable phase γ-Al2O3 to stable phase α-Al2O3 and the transformation spontaneously occurred at above 1200°C. However, the Zn content is rather high in 7075 aluminum alloy, and Zn inhibited the formation of α-Al2O3. Therefore, Fig. 2 demonstrates that ceramic coatings are mainly composed of γ-Al2O3, and the content of α-Al2O3 is very low.
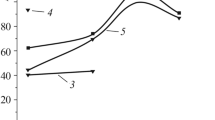
Corrosion Resistance. Corrosion resistance was evaluated by potentiodynamic polarization measurement, which was carried out in 3.5 wt.% NaCl solution for bare and coated samples with CHI604b electrochemical work station. Figure 3 shows the polarization curves.
The substrate of aluminum alloy 7075 had a poor corrosion resistance with the corrosion potential of −0.772 V and corrosion current density of 1.04 ⋅ 10–4 A/cm2. After PEO treatment in different electrolyte systems, the corrosion potential slightly increased and the corrosion current density was significantly reduced. For example, in the case of ceramic coatings produced in borate system: though the corrosion potential (−0.708 V) improved about 64 mV only, the corrosion current density (4.616 ⋅ 10–7A/cm2) was by three orders of magnitude lower than that of the substrate. Thus, PEO treatment can greatly improve the corrosion resistance of aluminum alloy 7075.
Conclusions
Optimum electrolyte compositions were: aluminate 9 g/L (silicate 8 g/L, borate 15 g/L, and phosphate 12 g/L), sodium hydroxide 1 g/L (boric acid 3 g/L), triethanolamine 6 mL/L, hydrogen peroxide 2 mL/L; the micro hardness of ceramic coatings obtained in aluminate optimum compositions can reach up to HV 0.1 = 1100 MPa.
All coatings showed a crater-like surface morphology with many residual particles and pores on the surface, which mainly consisted of compact layer and set up a metallurgical combination with the substrate. The coatings produced in aluminate had less and fine pores on the surface, showing continuous and smooth compact depositing layer.
Ceramic coatings produced in respective electrolyte systems are mainly composed of γ-Al2O3 and bits of α-Al2O3. After plasma electrolytic oxidation treatment in different electrolyte solutions, the corrosion potential of the alloys were increased by just 50–100 mV, while the corrosion current density was (significantly) reduced by 2~3 orders of magnitude.
References
A. L. Yerokhin, A. A. Voevodin, V. V. Lyubimov, et al., “Plasma electrolytic fabrication of oxide ceramic surface layers for tribotechnical purposes on aluminum alloys,” J. Surf. Coat. Techn., 110, 140–146 (1998).
Y.-J. Oh, J.-I. Mun, and J.-H. Kim, “Effects of alloying elements on microstructure and protective properties of Al2O3 coatings formed on aluminum alloy substrates by plasma electrolysis,” J. Surf. Coat. Techn., 204, 141–148 (2009).
K. Tillous, T. Toll-Duchanoy, E. Bauer-Grosse, et al., “Microstructure and phase composition of microarc oxidation surface layers formed on aluminum and its alloys 2214-T6 and 7050-T74,” J. Surf. Coat. Techn., 203, 2969–2973 (2009).
W. Xue, C. Wang, H. Tian, et al., “Corrosion behaviors and galvanic studies of microarc oxidation films on Al–Zn–Mg–Cu alloy,” J. Surf. Coat. Techn., 201, 8695–8701 (2007).
E. Matykina, R. Arrabal, P. Skeldon, et al., “AC PEO of aluminum with porous alumina precursor films,” J. Surf. Coat. Techn., 205, 1668–1678 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Poroshkovaya Metallurgiya, Vol. 54, Nos. 1–2 (501), pp. 124–130, 2015.
Rights and permissions
About this article
Cite this article
Chuanyu, S., Wang, Y. Influence of Electrolyte Parameters on the Properties of the Ceramic Coatings Deposited on Aluminum Alloy by Plasma Electrolytic Oxidation. Powder Metall Met Ceram 54, 101–105 (2015). https://doi.org/10.1007/s11106-015-9685-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9685-8