Abstract
Microstructure of ceramic coatings formed on commercially pure aluminum by plasma electrolytic oxidation using different amounts of sodium silicate was studied. Analysis of chemical composition and microhardness measurements of the coatings were carried out to characterize the morphology of the coatings. The addition of sodium silicate (1–3 g/L) increased the thickness about 8 orders of magnitude and promotes the growth of the coatings. Chemical analysis of the coatings showed that with increasing sodium silicate concentration in the electrolyte from 1 to 3 g/L, the amount of manganese in the coatings was reduced ∼12%. The contribution of aluminum and silicon changed with a reciprocal relationship. When the concentration of Na2SiO3 changed from 1 to 2 g/L, the aluminium content was reduced about 26% and silicon increased about 80%. When the concentration of Na2SiO3 changed from 2 to 3 g/L, the aluminium content increased about 38% and the concentration of silicon reduced about 60%. The microhardness of the coatings was increased about 2 orders of magnitude by increasing the concentration of sodium silicate from 1 to 3 g/L.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Plasma electrolytic oxidation (PEO) is a well-known process for protecting the surface of aluminum and its alloys via producing ceramic coatings on the surface [1]. The morphology of PEO coatings on Al alloys has been studied in several research papers. Aliofkhazraei et al. [2] investigated the effect of PEO processing time on the morphology and microstructures of ceramic coatings with and without nanoparticles added to the electrolyte. They concluded that the thickness and the diameter of the discharge channels increased with increasing the coating time [2].
Sevidova et al. [3] demonstrated that the sodium silicate concentration is a key factor in PEO of aluminum alloys which correlates to the roughness of the coatings. A decrease in sodium silicate concentration in the electrolyte results in the reduction of the surface roughness [3].
Yerokhin et al. [4] divided the PEO process into four distinctive stages according to the voltage-time response of the process; namely, conventional anodizing, breakdown of the anodized coating, appearance of dispersed discharges, microdischarges, and individual widely spaced sparks [4].
The chemical composition of the electrolyte is one of the most important factors affecting the morphology and microstructure of PEO coatings [5].
The effect of various electrolyte constituents on the morphology of PEO coatings was studied by Xiaohu Huang et al. [7].
It has been demonstrated that sodium silicate is a very useful constituent for PEO electrolytes [5]. Addition of Na2SiO3 to the PEO bath results in the reduction of discharging potentials via increasing the conductivity of the electrolyte [8].
It has been found that silicate-based electrolytes promote the outward growth of PEO coatings in the early stages of the process and when the time of the process is long, the inward growth will be much more dominant [9].
Skeldon et al. [10] investigated the role of concentration of sodium metasilicate in the PEO electrolyte and found that more aluminosilicate are produced phases in the coatings with increasing the concentration of metasilicates in the electrolyte; after the optimal concentration had been reached, two distinctive zones with aluminum and silicon as the dominant elements were formed within the coating microstructure [10].
Addition of (NaPO3)6 to PEO electrolytes containing sodium silicate results in formation of γ-alumina and mullite and improvement of the corrosion resistance of ceramic coatings formed on pure aluminium [11]. it is interesting that when the kind of silicate in the electrolyte changed, the phases present in the PEO coatings on aluminium alloys changed significantly and when the silicate index increased from n = 1 to n = 3, the corrosion resistance of the samples improved. However, the microhardness of the coatings was not influenced by the silicate index of the electrolyte [12].
PEO of aluminium alloys in mixed silicate-borate solutions showed that when the ratio of silicate to borate is 1:1, borosilicate compounds formed within the electrolyte after the coating of the first samples which caused the increase of the roughness and thickness of the coatings across with the decrease of the microhardness of the coatings. Raising the concentration of silicate in the electrolyte results in the reduction of microhardness of the coatings [13].
Some minor impurities such as chlorides and sulphates can be negatively affect the PEO process on aluminium alloys. Presence of these impurities in the electrolyte solution could lead to a decrease in the coating growth rate and therefore in the final thickness of the coatings [14].
PEO coatings can play a two-fold role in preventing corrosion of aluminium substrates. First, they form a physical barrier against the corrosive media, and second, they can behave as local areas of hydrogen ions accumulation which could hinder the progress of pitting corrosion processes [15].
When sodium silicate added to the plasma electrolytic oxidation bath as an additive, it has significant improving effects on the corrosion behavior of the PEO coatings on aluminium [16].
In the present study, PEO coatings were produced on commercially pure aluminum in aqueous solutions containing potassium permanganate, sodium silicate and sodium carbonate. The effect of potassium permanganate on the morphology and corrosion properties of the coatings were previously published by the authors elsewhere [6]. The main focus of the present work is on the role of sodium silicate on the morphology and microstructures of the coatings in terms of microscopic images and EDS analysis.
EXPERIMENTAL
The experimental conditions of the PEO process were discussed in the previously published paper by the authors [6] in detail. Commercially pure aluminum (99.95%) rectangular specimens (50 × 10 × 0.3 mm) were prepared as the substrates for PEO process. PEO process was performed using a 6000VA-DC variable power supply under sparking voltages about 63 V at temperatures near 30°C. Preparation of the specimens was carried out by grinding by emery papers from #400 to #3000, degreasing with commercial detergent solutions, and soaking in concentrated potassium hydroxide solution for 60 s. Between these stages, the specimens were rinsed with dionized water stream. The samples were dried with a hot air blower [6].
The specimens were coated in electrolytes with fixed concentrations of Na2CO3 (6 g/L) and KMnO4 (15 g/L). The concentration of Na2SiO3 was selected among 1, 2, and 3 g/L. All of the chemical reagents were provided from the domestic sources of chemical materials and has laboratory purities.
An MV2300 scanning electron microscope was used to record micrographs of the surface and cross-sections of the coated specimens. Concentrations of the elements within the coatings were determined using an EDS analyzer. The average thickness of the coatings was calculated by Image Tools software. Each thickness value was the average of 5 measurements.
Vickers microhardness tests were performed on the cross-sections of the coated specimens by an INNOVATEST apparatus using a force of 50 gf within 10 s.
RESULTS AND DISCUSSION
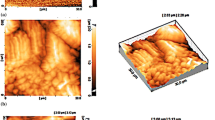
Figure 1 shows the cross-sections and surfaces of the oxide coatings produced using different Na2SiO3 concentrations in the electrolyte.
Surface and cross-sectional Morphologies of the coatings obtained using different amounts of sodium silicate; (a) 1 g/L Na2SiO3 cross-sectional view, (b) 1 g/L Na2SiO3 surface view, (c) 2 g/L Na2SiO3 cross-sectional view, (d) 2 g/L Na2SiO3 surface view, (e) 3 g/L Na2SiO3 cross-sectional view, (f) 3 g/L Na2SiO3 surface view.
The average coating thickness is illustrated in Fig. 2.
As can be seen, the coating thickness increased with Na2SiO3 concentration. The rate of plasma electrolytic oxidation of aluminum increased after sodium silicate had been added to the electrolyte. Also, it can promote the formation of a more dense and uniform coating [5, 17]. The addition of 3 g/L of Na2SiO3 to the PEO bath resulted in about 8 times thickening of the coatings in comparison with 1 g/L of Na2SiO3. Similar evidence was found in earlier research papers [18].
At the same time, when the thickness of the coating increased, the outer layer of the coating became more prone to cracking and had a more porous structure [19]. In the present work, the addition of 3 g/L of sodium silicate to the electrolyte caused an outstanding increase in the thickness of the coatings, but there are larger pores and longer cracks thorough the thickness of the coatings.
Increasing sodium silicate concentration resulted in a smooth and even surface. Samples containing 1 and 2 g/L Na2SiO3 have considerable portions of nodular microstructure on their surface. Increasing Na2SiO3 concentration from 1 to 3 g/L reduced this nodular morphology and promotes a more even and dense microstructure.
Lower amounts of sodium silicate result in a reduction of current density during plasma formation and plasma was uniformly dispersed throughout the surface [5, 17]. As a result, the number of discharge channels decreased while their size increased as a result of local high power discharges. This led to a rough morphology in the coatings produced using 1 and 2 g/L of sodium silicate. Increasing the amount of sodium silicate led to more uniform plasma and smooth and uniform coating surface because the discharges had small sizes and lower powers with lower volumes of molten materials.
Sodium silicate promotes corrosion at the anodic surfaces of aluminum and encourages the formation of ceramic layers [19]. In the present work, the addition of 3 g/L of sodium silicate to the electrolyte caused an outstanding increase in the thickness of the coatings, but there are larger pores and longer cracks thorough the thickness of the coatings. When an electrolyte caused corrosion at the surface, the voltage and current of plasma formation will be reduced significantly [5].
Another interesting result is the variation in concentrations of elements in the coatings obtained using different amounts of sodium silicate.
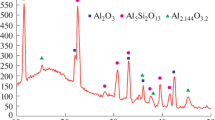
The XRD patterns of the coatings (not included in this study) showed that the coatings contained a mixture of aluminium and manganese oxides [6].
The overall EDS results of the three specimens are summarized in Fig. 3.
The addition of sodium silicate to the electrolyte has changed the concentration of the elements within the coatings. When the amount of sodium silicate increased from 1 to 3 g/L, the content of manganese was decreased. The amount of aluminum was first decreased and then increased to higher levels than the primary level. Oxygen was slightly increased with increasing the concentration of Na2SiO3. The content of silicon was first increased and then decreased to a level higher than the original content.
It seems that the content of oxygen in the coatings was not changed significantly, and the amount of manganese was reduced slightly by increasing the concentration of sodium silicate in the electrolyte. This could be because of the increased ratio of sodium silicate to potassium permanganate in the electrolyte. An interesting trend is observed on the variation of the amounts of Al and Si. When the concentration of Al is decreased, the amount of Si is increased and vice versa. The general conclusion is that the amount of oxygen is approximately constant. This means that the competition was followed by the elements Mn, Al, and Si for incorporation in the coatings. When the concentration of Na2SiO3 was increased in the electrolyte, the contribution of Mn was decreased within the coatings. This could be because of the incorporation of Si in the coatings. At the same time, Al was decreased within the coatings. After a maximum content of Si in the coatings, its concentration was decreased and Al concentration was increased, while the content of Mn was continuously decreased. The addition of sodium silicate to the electrolyte promotes the incorporation of Al and Si in the ceramic coatings.
Variation of microhardness of the coatings formed with different amounts of sodium silicate could be observed in Fig. 4.
The values of microhardness increased with increasing the sodium silicate concentration. This is because of uniform and dense coatings formed with higher concentrations of Na2SiO3.
CONCLUSIONS
• Ceramic coatings were produced on commercially pure aluminum by plasma electrolytic oxidation.
• One of the controlling parameters of the process was sodium silicate concentration as an additive in the electrolyte solution.
• The addition of sodium silicate encouraged the growth and increased the thickness of the coatings (27.6–204.861 μm).
• When the thickness increased, more long cracks and large pores were formed within the body of the coatings.
• When the amount of Na2SiO3 increased, the surface morphology of the coatings changed from a nodular structure to a uniform and dense structure.
• The concentration of manganese was reduced from 62.4 to 54.78 with increasing the concentration of sodium silicate.
• Aluminum and silicon had a reverse competition for incorporating in the coatings. Whenever the concentration of the former increased, the amount of the latter decreased.
• Aluminium was decreased from 11.17 to 8.5 wt % and then increased to 13.68 by changing the amount of sodium silicate (1, 2, and 3 g/L), while silicon increased from 1.09 to 5.46 and then droped to 2.16 wt %.
• The microhardness of the coatings increased continuously from 347.2 to 620.75 HV by increasing the concentration of sodium silicate from 1 to 3 g/L.
REFERENCES
Li, Q., Liang, J., and Wang, Q., Plasma Electrolytic Oxidation Coatings on Lightweight Metals, chapter 4 of Modern Surface Engineering Treatments, InTech, 2013.
Erfanifar, E., Aliofkhazraei, M., Fakhr Nabavi, H., Sharifi, H., and Sabour Rouhaghdam, A., Growth kinetics and morphology of plasma electrolytic oxidation coating on aluminum, Mater. Chem. Phys., 2017, vol. 185, pp. 162–175.
Sevidova, E., Gutsalenko, Y., Rudnev, A., Pupan, L., and Titarenko, O., The study of surface microgeometry and morphology of plasma electrolytic oxidation dielectric coatings on aluminum alloys, in Advances in Design, Simulation and Manufacturing II, Proceedings of the 2nd International Conference on Design, Simulation, Manufacturing: The Innovation Exchange, DSMIE-2019, June 11–14, 2019, Lutsk, Ukraine, Lecture Notes in Mechanical Engineering, Springer, 2020, pp. 302–310.
Yerokhin, A.L., Snizhko, L.O., Gurevina, N.L., Leyland, A., Pilkington, A., and Matthews, A., Discharge characterization in plasma electrolytic oxidation of aluminium, J. Phys. D: Appl. Phys., 2003, vol. 36, pp. 2110–2120.
Yerokhin, A.L., Nie, X., Leyland, A., Matthews, A., and Dowey, S.J., Plasma electrolysis for surface engineering, Surf. Coat. Technol., 1999, vol. 122, pp. 73–93.
Shamsi, F., Khorasanian, M., and Lari Baghal, S.M., Effect of potassium permanganate on corrosion and wear properties of ceramic coatings manufactured on CP-aluminum by plasma electrolytic oxidation, Surf. Coat. Technol., 2018, vol. 346, pp. 63–72.
Famiyeh, L. and Huang, X., Plasma electrolytic oxidation coatings on aluminum alloys: microstructures, properties, and applications, Mod. Concepts Mater. Sci., 2019, vol. 2, no. 1, pp. 1–13.
Polat, A., Makaraci, M., and Usta, M., Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate, J. Alloys Compd., 2010, vol. 504, pp. 519–526.
Li, J., Cai, H., Xue, X., and Jiang, B., The outward-inward growth behavior of microarc oxidation coatings in phosphate and silicate solution, Mater. Lett., 2010, vol. 64, pp. 2102–2104.
Monfort, F., Berkani, A., Matykina, E., Skeldon, P., Thompson, G.E., Habazaki, H., and Shimizu, K., Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte, Corros. Sci., 2007, vol. 49, pp. 672–693.
Guo-Hua, L.V., Wei-Chao, G.U., Huan, C., Li, L., Er-Wu, N., and Si-Ze, Y., Microstructure and corrosion performance of oxide coatings on aluminium by plasma electrolytic oxidation in silicate and phosphate electrolytes, Chin. Phys. Lett., 2006, vol. 23, no. 12, pp. 3331–3333.
Lugovskoy, A., Zinigrad, M., Kossenko, A., and Kazanski, B., Production of ceramic layers on aluminum alloys by plasma electrolytic oxidation in alkaline silicate electrolytes, Appl. Surf. Sci., 2013, vol. 264, pp. 743–747.
Rudnev, V.S., Yarovaya, T.P., Nedozorov, P.M., and Mansurov, Y.N., Wear-resistant oxide coatings on aluminum alloy formed in borate and silicate aqueous electrolytes by plasma electrolytic oxidation, Prot. Met. Phys. Chem. Surf., 2017, vol. 53, no. 3, pp. 466–474.
Terleeva, O.P., Slonova, A.I., Rogov, A.B., Kokovkin, V.V., and Mironov, I.V., Effect of chloride and sulphate anions as minor impurities in silicate alkaline electrolyte on plasma electrolytic oxidation of aluminium alloys, Mater. Res. Express, 2019, vol. 6, p. 015009.
Yang, Z., Wang, R.Q., Liu, C., Wu, Y.K., Wang, D.D., Liu, X.T., Zhang, X.Z., Wu, G.R., and Shen, D.J., The electrochemical corrosion behavior of plasma electrolytic oxidation coatings fabricated on aluminum in silicate electrolyte, J. Mater. Eng. Perform., 2019, vol. 28, no. 6, pp. 3652–3660.
Ali, M.M. and Sathiya, S., Evaluation on physico-chemical and corrosion properties of anodized aluminium surface using sodium oxalate-sodium silicate bath at room temperature, Curr. Sci., 2020, vol. 118, no. 2, pp. 234–242.
Saikiran, A., Hariprasad, S., Arun, S., Rama Krishna, L., and Rameshbabu, N., Effect of electrolyte composition on morphology and corrosion resistance of plasma electrolytic oxidation coatings on aluminized steel, Surf. Coat. Technol., 2019, vol. 372, no. 25, pp. 239–251.
Lee, J.H. and Kim, S.J., Effects of silicate ion concentration on the formation of ceramic oxide layers produced by plasma electrolytic oxidation on Al alloy, Jpn. J. Appl. Phys., 2017, vol. 56, p. 01AB01.
Agureev, L., Savushkina, S., Ashmarin, A., Borisov, A., Apelfeld, A., Anikin, K., Tkachenko, N., Gerasimov, M., Shcherbakov, A., Ignatenko, V., and Bogdashkina, N., Study of plasma electrolytic oxidation coatings on aluminum composites, Metals, 2018, vol. 8, no. 459, pp. 1–12.
Funding
The financial support of Shahid Chamran University of Ahvaz (grant no. 96/3/02/16670) should be kindly appreciated by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest.
About this article
Cite this article
Khorasanian, M., Shamsi, F. & Lari Baghal, S.M. Effect of Sodium Silicate Concentration on Morphology of Ceramic Coatings Produced on Commercially Pure Aluminum Using Plasma Electrolytic Oxidation. Russ. J. Non-ferrous Metals 61, 762–767 (2020). https://doi.org/10.3103/S1067821220060097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821220060097