Abstract
Woolly monkeys have been considered vulnerable to forest fragmentation. However, we found a population of woolly monkeys that has been living in a small forest remnant in Colombian Amazonia, raising questions about which factors determine the persistence or extinction of woolly monkeys in fragmented forests. The main purpose of this investigation was to describe the behavioral ecology of woolly monkeys (Lagothrix lagothricha) in a 136-ha forest fragment in Guaviare, Colombia. We recorded activity patterns, diet, and use of space for three monkey troops by instantaneous sampling on focal animals, and we quantified interindividual distance, between January and December 2008. This group of woolly monkeys spent on average 31.7 % of their time feeding, 32.4 % resting, 34.2 % moving, and 1.8 % in social interactions. Their main feeding items were fruits (57.2 %), followed by leaves (15.5 %), arthropods (15.8 %), seeds (5.2 %), flowers (5.1 %), and others (1.2 %). The three most used plant families for fruit consumption were Moraceae (23.4 %), Fabaceae (16.3 %), and Ulmaceae (8.3 %), and the most important species was Ampelocera edentula (Ulmaceae). We estimated an average daily travel distance of 2,339 m and a home range of 126 ha. We found a negative relationship between the degree of interindividual distance and group size. Most of the studied ecological parameters were within the reported ranges for woolly monkeys in undisturbed habitats. Thus, our evidence indicates that their persistence in fragments does not require drastic behavioral changes. We suggest that fragmentation represents a threat to woolly monkeys when (1) fragments are not productive enough to sustain the population and/or (2) when it leads to a higher hunting pressure on the population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Habitat degradation usually causes changes in activity patterns , diet, home range, and group cohesion in many primate species (Muruthi et al. 1991; Tutin 1999; Marsh 2003; Irwin 2007). These changes may influence demographic parameters and many species are likely to become locally extinct in small fragments. However, some primate populations are capable of modifying their behavior as a response to habitat disturbance and fragmentation (Tutin 1999; Irwin 2007; Pozo-Montuy and Serio-Silva 2007). For example, in continuous habitats Cercopithecus cephus is mainly frugivorous (Chapman et al. 2002) while groups that are confined to isolated forest fragments consume fewer fruits and more insects (Tutin 1999). In Propithecus diadema, groups that reside in fragmented areas have less intragroup cohesion than groups that occupy continuous habitats, mainly as a response to an increase in intraspecific competition for food, given the reduction and variation in the distribution of their resources (Irwin 2007). This is a similar response to the one found in spider monkeys (Ateles spp.) and chimpanzees (Pan spp.) with less-cohesive and more flexible societies that have adopted a fission–fusion system of social organization (Symington 1990).
Other primates like howlers also show a high degree of dietary and behavioral flexibility that allows them to adjust to different degrees of habitat disturbance (Silver and Marsh 2003; Pozo-Montuy and Serio-Silva 2007). However, they tend to be more vulnerable to hunting, disease, predation, and endogamy in isolated populations (Bicca-Marques 2003). On the other hand, small primates such as Callithrix argentata can show a tendency towards an increase in population densities in fragments. This could be explained by the effect of density compensation, caused by an increase in resource availability when large primates are absent (Peres and Dolman 2000). However, the long-term viability of their populations may depend on genetic diversity, which is probably lower in fragments (Rodríguez-Toledo et al. 2003).
The fact that woolly monkeys (Lagothrix spp.) are absent from most small forest fragments suggests they are one of the most vulnerable primates for habitat disturbance and forest fragmentation (Bernstein et al. 1976; Peres 1994; Defler and Defler 1996; Di Fiore and Rodman 2001; Stevenson 2010). In addition, woolly monkeys have been described as large-bodied frugivores (Stevenson et al. 1994; Peres 1994; Defler and Defler 1996; Di Fiore 2004), a guild usually associated with a high risk of local extinction in fragments. Group size is variable (10–49 individuals; Peres 1996), and groups exhibit some degree of cohesiveness (Stevenson et al. 1994; Stevenson 1998; Dew 2005). Their low reproductive rates (Nishimura 2003) and large activity areas make it unlikely that woolly monkeys will survive in forest remnants (Milton 1993; Tutin 1999; Onderdonk and Chapman 2000; Gilbert 2003; Ferrari et al. 2003; Marsh 2003).
Although it has been suggested that large frugivorous primates are the first to become locally extinct in the smallest forest fragments (Estrada and Coates-Estrada 1996; Gilbert 2003; Michalski and Peres 2005), we found a population of woolly monkeys living in an isolated forest fragment in Colombia for more than 20 years (Stevenson, pers. obs.). Here, we aim to describe for the first time ecological and social variables of woolly monkeys living in forest fragments in order to establish differences and similarities between this population and populations in continuous forest. The specific objectives of the study were: (1) to quantify the activity patterns , diet, use of space, and cohesiveness between woolly monkey groups and their age/sex classes; (2) to quantify the fruit production and to evaluate the influence of monthly fruit production on activity budgets; and (3) to compare the activity budgets in a forest fragment with continuous forest. In addition, we discuss the factors that might have allowed this population to survive and persist in the fragment.
2 Methods
2.1 Study Area
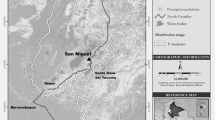
The study was carried out in a forest fragment belonging to the experimental station El Trueno of the Instituto Amazónico de Investigaciones Científicas (SINCHI) located near El Retorno town (Departamento del Guaviare, 2° 22ʹ N, 72° 41ʹ W; Fig. 13.1a) in Colombia. The station includes a fragment of 136 ha including both flooded and terra firme rainforest. The fragment contains high plant species richness with dominance of pioneer species (mostly Croton matourensis and Cecropia sciadophylla; Stevenson and Rodríguez 2008). Other important plant species include Iriartea deltoidea, Oenocarpus bataua, Pourouma minor, and Pourouma bicolor, and the most common families are Moraceae, Fabaceae, Urticaceae, Euphorbiaceae, and Burseraceae. The site has a mean temperature and rainfall of 25.9 °C and 2,448 mm, respectively (IDEAM 1999).
(a) Map of “El Trueno” experimental station, Instituto Amazónico de Investigaciones Científicas SINCHI. (b) Total area used by all groups of woolly monkeys in the forest fragment (136 ha). The gray patterns represent the frequency of use for each of the 1-ha quadrants. This scale is based on instantaneous samples every 30 min, where lighter patterns represent less frequently used quadrants and darker patterns represent more frequently used quadrants
Eight primate species inhabit the fragment (Lagothrix lagothricha, Alouatta seniculus, Saimiri sciureus, Callicebus torquatus lugens, Saguinus inustus, Cebus albifrons, Sapajus apella, and Aotus sp.) of which woolly monkeys are the most common, with a density of 50 individuals/km2. This estimate is similar to what has been found in continuous forest with high densities of woolly monkeys such as Tinigua National Park (41–50 ind/km2; Stevenson 2007) and Ecuadorian Amazonia (31 ind/km2; Dew 2005).
2.2 Data Collection
We studied all three groups of woolly monkeys that lived in the forest fragment (Table 13.1). The large “L” group was followed from January to December of 2008; the medium-sized “M” group was followed from January to April; and the small “S” group was followed from May to December. The M group was impossible to monitor all year, because its home range included an area (29.4 ha) that was regularly flooded.
We followed two of the three groups during 5 days for each group, usually in consecutive days at the beginning of each month. All members of the groups were individually recognized. Genital marks, injuries, particular facial expressions, and body size were used to distinguish individuals. We collected data on activity patterns and diet (see below) using instantaneous sampling on focal individuals every 10 min (Stevenson et al. 1994), a method that has been recommended (González and Stevenson 2009). Each focal individual was followed continuously for 12 h (72 instantaneous samples) for each day of data collection, usually between 0600 and 1800 h. Thus, we recorded 360 instantaneous samples in 5 days of sampling each month, totaling 4,320 instantaneous samples for a group in 12 months (8,640 in total). Three different age/sex classes were observed in each group: two adult males, two adult females without infants and one adult female with dependent infant (< 1-year-old). All focal individuals selected were habituated and the same animals along the study, but the sequence in which they were followed each month was not established a priori.
Activity was classified as moving, resting, feeding, or social interactions (as defined by González and Stevenson 2009). Different food items were identified and classified as fruits (ripe and unripe), seeds, leaves, flowers, arthropods, and other types rarely ingested (termite nest, water, or vertebrate prey). In addition, we quantified the time each focal animal spent feeding on each of the food types (except for arthropod feeding) in a continuous fashion (Stevenson et al. 1994). The moment when we detected food ingestion was considered as the initial time, and the time at which the animal stopped consuming items for more than 1 min, or when the animal moved away from the feeding source, was considered as the ending time (Stevenson et al. 1994).
Using a global positioning system (GPS; Garmin 76 CSX map), we registered the location of the focal animal every 30 min, and we referenced these positions in a 1-ha grid (Stevenson 2006). We used these positions to estimate daily traveled distance using Mapsource version 6 (2008).
2.3 Interindividual Distance
To quantify the distance between individuals, we selected another five focal animals from the same groups and from the same age/sex classes as the main focal animals. These additional focal animals were simultaneously followed by a second observer, who gathered information on the position of the animal using another GPS at 30-min intervals. The selected focal animals were individually recognized and the pair chosen each month was determined randomly each month. Thus, to quantify the degree of separation between individuals, the linear distance between the main focal animals and a secondary focal animal was estimated using Mapsource version 6 (2008). In total, we measured 1,440 sample points of linear distance between simultaneously followed focal individuals in the same group during the study.
2.4 Fruit Production
Fruit production estimates were calculated following the methodology used by Stevenson and Link (2010), using 10 km of phenological transects. The transects were monitored twice a month during 12 months, looking for fruits or parts of them on the trail floor and estimating crop size visually (number of fruits in the plant) by counting the fruits of several tree branches and then multiplying by the number of branches in the plant. First, we estimated the average dry weight of fruits (> 5 fruits) of every species in transects. We multiplied dry fruit weight by the total crop size in each individual plant sampled. Then, individual fruit production was assigned to the effective sampling area, by estimating the effective transect width, using information on the perpendicular distance from the producing trees to transect because larger plants are detected in linear transects at larger distances than smaller plants (Stevenson 2002). For this purpose, we regressed diameter at breast height (DBH) of fruiting trees on the perpendicular distance to transect and selected six of the farthest points of the distribution to estimate the effective width for each plant size (distance = (0.028 × DBH) + 5.45 m). Finally, we calculated a community-wide estimate of fruit abundance in units of production (fruit mass.ha−1.mo−1), summing individual productions, which take into account the effective sampling area.
2.5 Data Analyses
Comparisons between pairs of groups were made only considering those months when both groups were followed (January–April, L Group vs. M Group; May–December, L Group vs. S group).
Activity patterns and Diet
We calculated the proportion of instantaneous samples in each activity and diet category every day, and we used each day as a sampling unit for statistical comparisons between groups and age/sex classes . We used binomial Z tests for two proportions to assess these comparisons, except in cases of infrequent behaviors (when we used analysis of variance (ANOVA) or the Kruskal–Wallis test (in the cases with nonparametric values) and T tests). In order to compare among age/sex classes, we combined data from the 2 days following adult males and adult females without dependent offspring. We used ANOVA or the Kruskal–Wallis test (in the cases with nonparametric values) to evaluate monthly variations in activity patterns and diet, using the percentage of each sampling day as statistical units. We performed Pearson correlation analysis to determine the influence of monthly fruit production on activity budgets. All analyses were done using the Statistical Product and Service Solutions (SPSS) program (SPSS 17 2008).
Use of space
To evaluate if there were differences between the daily traveled distances between groups, we used T tests each month, using daily path length of each individual followed as sampling units. Comparisons were only made between the periods when the two different group pairs were followed. We performed a one-way ANOVA for each group to evaluate whether there were monthly differences between the daily traveled distances along time. To compare home range areas, we constructed accumulation curves between the number of 1-ha quadrants used by the different woolly monkey groups, as a function of instantaneous sampling points recorded in each quadrant. For this purpose, we used the EstimateS program (Colwell 2005).
Interindividual distances
To establish whether the interindividual distance distribution differed among groups, we constructed a frequency distribution curve for each group. We grouped interindividual separation distances in categories of 20 m. We evaluated differences between groups using Kolmogorov–Smirnov tests (Sokal and Rohlf 1995).
3 Results
3.1 Activity Patterns and Diet
Throughout the study year, individuals of the L group were seen resting 32.4 % of the time, 33.8 % feeding, 32.6 % moving, and 1.2 % on other activities (n = 4,320 instantaneous samples) . Activity budgets for the M group were 24.4 % resting, 31.1 % feeding, 41.3 % moving, and 3.2 % on other activities (n = 1,440) and for the S group 40.3 % resting, 30.2 % feeding, 28.6 % moving, and 0.9 % on other activities (n = 2,880). We did not find significant differences in the type of activity between groups L and M (Fig. 13.2a). However, feeding was more frequent in group L than in group S (t = 2.460, df = 8; P = 0.039). We found few differences in activity budgets among age/sex classes (Fig. 13.3), and the main difference was in feeding behavior, which was more frequent for adult females with a dependent infant in the L group (adult female with a dependent infant vs. male and female adult: Z = 2.689, P = 0.006; Z = 2.085, P = 0.031). Although this tendency was evident for all three groups, we did not find a significant difference between age/sex classes and behavior for the M and S groups (Appendix 13.1a).
Between-group comparison of the percentage of instantaneous samples registered for each of the different activities (error bars = standard error). (a) L group vs. M group: compared between January and April, (b) L group vs. S group: compared between May and December. The asterisk (*) above an activity denotes significant differences (T test for two independent samples; p < 0.05)
Comparison of the percentage of instantaneous samples assigned to the different activities for each age/sex class in the L group (error bars show the standard error). An asterisk (*) above an activity denotes significant differences and the dash (−) shows a P score of 0.05 (Z test for two proportions)
In general, the most frequent items in the diet were ripe fruits (57.2 %), followed by young leaves (15.5 %), arthropods (15.8 %), seeds (5.2 %), flowers (5.1 %), and others (1.2 %). We did not find significant differences in diet composition between groups (L vs. M groups and L vs. S groups). However, young leaves consumption was more frequent in the L group (t = 2.507, df = 8, P = 0.036), and the consumption of arthropods was more frequent in the M group (t = − 2.852, df = 8, P = 0.021). We did not find significant differences in diet composition between the age/sex classes in the groups (Appendix 13.1b). However, in group L, the adult females with dependent infants had a tendency to consume more young leaves than other age/sex classes (Fig. 13.3) .
Diet composition varied over time (Fig. 13.4). Group L showed temporal differences in the frequency of consumption of all feeding items (Appendix 13.1c), and group S showed lesser temporal differences, because they were followed for a lesser period of time. We estimated annual fruit production to be 685 kg/ha/year, and it varied throughout the year. We found that monthly fruit production was a good predictor of feeding time (P = 0.005, N = 12, r 2 = 0.746) and ripe fruit consumption (P = 0.009, N = 12, r 2 = 0.74). In general, ripe fruits were the most consumed item through time, but, in periods of low fruit availability (January, and August–October), woolly monkeys tended to increase their consumption of secondary items such as leaves and flowers (Fig. 13.4). However, fruit production was not a good predictor of leaf or flower consumption (P = 0.68, N = 12, r 2 = 0.132; P = 0.27, N = 12, r 2 = − 0.34, respectively) .
The population of woolly monkeys in the fragment used a total of 229 plant species of 130 genera in 55 families. They used 133 species for fruit consumption (65 % of the total feeding time), 4 for seeds (8.0 %), 116 for leaves (17.5 %), 25 for flowers (8.5 %), and 11 for other parts (1.9 %). The most important species used for ripe fruit were Ampelocera edentula (13 %; Ulmaceae), Spondias mombin (5.0 %; Anacardiaceae), Iriartea deltoidea (4.0 %; Arecaceae), Cecropia sciadophylla (3.5 %; Urticaceae), and Brosimum acutifolium (3.2 %; Moraceae; Table 13.2). The most important families in terms of feeding time on unripe fruits or seeds were Malvaceae (49.7 %), Moraceae (46.3 %), and Bignoniaceae (3.1 %). When comparing the plant families used by the three groups, Moraceae and Fabaceae were the most common for all groups. However, there was variation in the use of plant genera and species among groups. Theobroma cacao and Pseudolmedia hirsuta were the more-used species for group L, Dialium guianense and Virola peruviana for group M, and Ampelocera edentula and Spondias mombin for group S .
3.2 Use of Space and Degree of Cohesiveness
The average distance traveled per day for the population of woolly monkeys in the fragment was 2,339 m (range 784–4,841 m). We did not find significant differences between groups (group L vs. M: t = − 0.481, df = 8, P = 0.642; group L vs. S: t = −0.772, df = 8, P = 0.462). However, there was a tendency to find shorter daily traveled distances for the smallest group (L: 2,306 ± 812 m; M, 2,777 ± 909 m; S: 2,169 ± 529 m). Overall, the L, M, and S groups used a total of 72, 55, and 48 ha, respectively (128 ha of total area; Fig. 13.1b). This seems to reflect the number of samples obtained for each group. In fact, the home range for the three groups increased as sample size increased and it did not reach an asymptote for any of the groups (Fig. 13.5). Finally, group L showed the highest interindividual separation compared to the other groups (Fig. 13.6; Kolmogorov–Smirnov test: L group: 96.3 ± 45 m vs. S group: 64 ± 16 m, D = 2.34, P < 0.01; L group: 72.9 ± 22 m vs. M group: 31.2 ± 21 m, D = 5.23, P < 0.01).
4 Discussion
4.1 Intraspecific Comparisons
In general, our results are within the ranges reported for other populations of woolly monkeys in undisturbed habitats (Di Fiore and Campbell 2007; Table 13.3). This indicates that woolly monkeys are in fact capable of inhabiting forest fragments, but their persistence is possibly not related to an extraordinary ecological or behavioral plasticity because the conditions at the fragment are adequate for the maintenance of the population. In fact, our annual fruit production estimate (685 kg/ha) turned out to be higher than the one calculated for an undisturbed site in Colombian Amazonia using the same methodology (Estación Biológica Caparú: interannual range = 106–471 kg/ha; González and Stevenson 2009; Vargas and Stevenson 2009). This seems to be a consequence of the low fertility of soils at Caparú (Defler 1995).
We found similar activity budgets for woolly monkeys in the fragment with Caparú (González and Stevenson 2009), where fruit production and population density are lower (Palacios and Peres 2005). In Tinigua, which has richer soils, it has been estimated that fruit production is the highest reported using our method (636–1,129 kg/ha, Stevenson, unpublished). In Tinigua, despite the high density of woolly monkeys, these rest for long periods of time and still show high feeding rates (Stevenson 2006). These comparisons suggest that activity budgets may be determined by feeding needs, which depend on resource availability and competition. Therefore, where fruit supply is low and/or frugivore population densities are high, woolly monkeys should travel more to get enough resources.
Tutín (1999) and Riley (2007) compared the effect of fragmentation on Cercopithecus cephus and Macaca tonkeana, respectively and found that these monkeys compensate the reduction of space and low productivity in a fragment by increasing insect and leaf consumption rates. In our case, we found that the consumption of leaves and flowers is in the upper range of the reported values for the genus, while the consumption of insects was not substantially different from the one reported for continuous forests (Table 13.3b). Diet composition was similar to the one found at Caparú (González and Stevenson 2009). This suggests that medium fruit production levels and high population density promote the search for secondary items to compensate for the lack of preferred items. Then, the feeding behavior becomes similar to the one in places with lower resources, but with a low population density.
When comparing fruit diet composition of woolly monkeys at different study sites (Stevenson et al. 1994; Peres 1994; Defler and Defler 1996; Di Fiore 2004; Stevenson 2006), we found that woolly monkeys in our study fragment share two of the three most important plant families used by woolly monkeys at other sites (Moraceae and Fabaceae). However, the third most used family in the fragment (Ulmaceae) had not been previously identified as an important resource in their diet. At the species level, two of the most used species Ampelocera edentula (Ulmaceae) and Theobroma cacao (Malvaceae) had not been reported in the diet of the woolly monkeys previously. These results may be explained by the fact that fruit production of A. edentula coincided with the months of overall low fruit productivity (September–October), and to the fact that the fragment has a plantation of T. cacao. This shows that woolly monkeys can exhibit an opportunistic behavior, and may compensate to some degree for the variation in the preferred fruit available with other non-preferred items. For instance, T. cacao in Tinigua may show high fruit production, but it has never been reported in the diet of woolly monkeys inhabiting undisturbed forests (Stevenson 2002).
The average daily travel distance and home range of woolly monkeys at the fragment are within the reported range for the genus (Table 13.3c). The average travel distance resembles the one reported by Defler (1996) in Caparú, while the home range area is similar to the one found in places with higher soil fertility (Stevenson et al. 1994; Di Fiore 2003). The relatively small home range area found in the fragment seems to reflect the restrictions imposed on populations that inhabit a small forest remnant. Defler (1996) suggested that soil fertility is the main factor explaining such variation. In other words, the large home ranges that woolly monkeys exhibit at Caparú would be a result of areas with poor soils, lower fruit production, and the need to travel more to reach nutritional demands. For example, Tinigua has fertile soils and high fruit production, and the home range of woolly monkeys is close to 200 ha and the mean daily traveled distance is 2,000 m (Stevenson 2006). Stevenson et al. (1994) suggested that such a pattern is explained by the high productivity of the forest, where woolly monkeys do not need to travel great distances to access their preferred resources. All this suggests that home ranges and daily traveled distances of woolly monkeys inhabiting a forest fragment are mainly a result of fruit production and fragment size.
4.2 Intrapopulation Comparisons
We found few differences in activity budgets and diet composition among groups. However, arthropod feeding was more frequent in group M than in group L. We suggest that this difference represents higher arthropod availability in the core areas of group M. In addition, we found a higher feeding frequency in individuals of group L compared to individuals of group S. We suggest the high number of births in group L at the end of the study (n = 11) might have caused an increase in nutritional demands for the females. It has been estimated that protein and mineral consumption in pregnant and lactating females may increase about 25 and 50 %, respectively, in primates (Coelho 1974; Altmann 1980). This is also consistent with the result showing that females with infants tend to ingest more leaves than females without infants; however, other patterns have been observed in other woolly monkey populations (Stevenson 2006). Nonetheless, in other primate’s species (e.g., lemurs) during lactation the females ingest a high proportion of young leaves in order to acquire calcium and protein, as well as some energy, which would be crucial for offspring development (Sauther 1998).
During periods of high fruit production (February–July and November–December), woolly monkeys in the fragment moved more and fed mainly on ripe fruits. However, when productivity was low (September–October), woolly monkeys did not move lesser than in periods of high productivity. This pattern does not support a hypothesis of energy minimization during food scarcity periods (Rosenberger and Strier 1989), but it is similar to previous findings for woolly monkeys in Caparú (González and Stevenson 2009). We observed long daily travel distance and repetitive circuits on a single day, in spite of relatively short distances among fruit resources. Thus, we believe that in periods of fruit scarcity woolly monkeys maintain the same movement frequency because they forage for alternative resources such as leaves and insects.
Initially, it was described that woolly monkeys lived in fission–fusion groups similar to Ateles and Brachyteles (e.g., Terborgh and Janson 1986), smaller group units allowing for a reduction in intragroup competition (Symington 1988). However, at least in one site, woolly monkeys live in much more cohesive groups than sympatric spider monkeys (Stevenson et al. 1998). It has been proposed that arthropod feeding may have relaxed the negative effects of food competition, leading to the evolution of a more cohesive social structure in Lagothrix (Stevenson et al. 1994). However, Stevenson and Castellanos (2000) found that intragroup competition for fruits is an important limiting factor in determining foraging group size. In our study, the largest group showed a higher spatial separation among its individuals compared to the other two groups (Fig. 13.6). It is probable that, similar to what was found for Propithecus diadema in Madagascar (Irwin 2007), woolly monkeys at the fragment reduce competition by increasing intragroup distance. Thus, our results support the idea that woolly monkeys living in a forest fragment could reduce intragroup competition for food, by reducing their cohesiveness. However, groups with many individuals must compensate for their size by traveling longer distances or by increasing foraging distances (Janson and Goldsmith 1995; Stevenson 2006; but see Chapman and Chapman 2000), which was not evident in our study, probably due to a small sample size for the M group.
4.3 Implications for Conservation
Our study suggests that woolly monkeys do not exhibit drastic behavioral changes in response to fragmentation. Similar to what has been reported for undisturbed habitats, woolly monkeys at the studied fragment adjusted their behavioral patterns mainly as a response to primate density and resource availability. Even though woolly monkeys consumed non-preferred items in the remnant, our study suggests that their populations show low behavioral plasticity. Thus, their persistence in the remnant seems to be determined by favorable conditions met in the fragment, and not by a particular behavioral response. This suggests that a higher reduction in resources and the loss of space can affect their survival. We suggest that woolly monkeys are rarely found in fragmented forests because: (1) Remnants may not be productive enough to sustain the populations, which can be a result of their small size and/or low fertility; and (2) fragmentation may be associated with an elevated hunting rate (Peres and Palacios 2007), which would be the main negative effect for woolly monkey populations at fragments with sufficient resource production (which should include arthropod abundance and foliage quality, as well as fruit production) .
References
Altmann J (1980) Baboon mothers and infants. Harvard University , Cambridge
Bernstein IS, Balcaen P, Dresdale L et al (1976) Differential effects of forests degradation on primate populations. Primates 17:401–411
Bicca-Marques JC (2003) How do howler monkeys cope with habitat fragmentation? In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 283–303
Chapman CA, Chapman LJ (2000) Determinants of group size in primates: the importance of travel costs. In: Boinski S, Garber PA (eds) On the move: how and why animals travel in groups. University of Chicago, Chicago, pp 24–42
Chapman CA, Chapman LJ, Gautier-Hion A et al. (2002) Variation in the diet of Cercopithecus Monkeys: differences within forests, among forests, and across species. In: Glenn M, Cords M (eds) The Guenons: diversity and adaptation in African monkeys. Plenum Press, New York, p 319–344
Coelho AM (1974) Socio-bioenergetics and sexual dimorphism in primates. Primates 15:263–269
Colwell RK (2005) Statistical estimation of species richness and shared species from Samples (Software and User’s Guide). EstimateS version 7.5. University of Conneticut
Defler TR (1995) The time budget of a group of wild woolly monkeys (Lagothrix lagothricha). Int J Primatol 16:107–120
Defler TR (1996) Aspects of ranging in a group of wild woolly monkeys (Lagothrix lagothricha). Am J Primatol 38:289–302
Defler T, Defler SB (1996) Diet of a group of Lagothrix lagothricha lagothricha in Southeastern Colombia. Int J Primatol 17:161–190
Dew JL (2005) Foraging, food choice, and food processing by sympatric ripe-fruit specialists: Lagothrix lagothricha poeppigii and Ateles belzebuth belzebuth. Int J Primatol 26:1107–1135
Di Fiore A (2003) Ranging behavior and foraging ecology of lowland woolly monkeys (Lagothrix lagothricha poeppigii) in Yasuní National Park, Ecuador. Am J Primatol 59:47–66
Di Fiore A (2004) Diet and feeding ecology of woolly monkeys in a western Amazonian rain forest. Int J Primatol 25:767–801
Di Fiore A, Campbell CJ (2007) The atelines: Variation in ecology, behavior, and social organization. In: Campbell CJ, Fuentes A, MacKinnon KC et al (eds) Primates in perspective. Oxford University , New York, pp 155–185
Di Fiore AF, Rodman P (2001) Time allocation patterns of lowland woolly monkeys (Lagothrix lagothricha poeppigii) in a neotropical terra firme forest. Int J Primatol 22:413–480
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas. Int J Primatol 5:759–783
Ferrari SF, Iwanaga S, Ravetta AL et al (2003) Dynamics of primates communities along the Santarem-Cuiaba highway in south-central Brazilian. In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 123–4142
Gilbert KA (2003) Primates and fragmentation of the amazon forest. In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 145–156
González M, Stevenson PR (2009) Patterns of daily movement, activities and diet in woolly monkeys (genus Lagothrix): a comparison between sites and methodologies. In: Potoki E, Krasinski J (eds) Primatology: theories, methods and research. Nova Science Publishers Inc., New York, pp 171–186
IDEAM (1999) Cartas Climatológicas-Medias Mensuales. San José del Guaviare. http://bart.ideam.gov.co/cliciu/guavi/tabla.htmAccessed 1 July 2008
Irwin M (2007) Living in forest fragments reduces group cohesion in diademed sifakas (Propithecus diadema) in Eastern Madagascar by reducing food patch size. Am J Primatol 69:434–447
Janson CH, Goldsmith ML (1995) Predicting group size in primates: foraging costs and predation risks. Behav Ecol 6:326–336
Mapsource (2008) Garmin International Inc., Rel. 6.11.6. 2008. Garmin International Inc., Kansas
Marsh LK (2003) The nature of fragmentation. In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 1–10
Michalski F, Peres CA (2005) Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol Conserv 124:383–396
Milton K (1993) Diet and primate evolution. Science 269:86–93
Muruthi P, Altmann J, Altmann S (1991) Resource base, parity, and reproductive condition affect females feeding time and nutrient intake within and between groups of a baboon population. Oecologia 87:467–472
Nishimura A (2003) Reproductive parameters of wild female Lagothrix lagothricha. Int J Primatol 24:707–722
Onderdonk DA, Chapman CA (2000) Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int J Primatol 21:587–611
Palacios E, Peres CA (2005) Primate population densities at three amazonian terra firme forests of southeastern Colombia. Folia Primatol 76:135–145
Peres CA (1994) Diet and feeding ecology of grey woolly monkeys (Lagothrix lagothricha cana) in central Amazonia: comparisons with other atelines. Int J Primatol 15:332–372
Peres CA (1996) Use of space, spatial group structure, and foraging group size of gray woolly monkeys (Lagothrix lagothricha cana) at Urucu, Brazil. A review of the Atelinae. In: Norconk MA, Garber PA, Rosemberger AF (eds) Adaptive radiations of neotropical primates. Plenum Press, New York, p 467–488
Peres CA, Dolman PM (2000) Density compensation in neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122:175–189
Peres CA, Palacios E (2007) Basin-wide effects of game harvest on vertebrate population densities in amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39:304–315
Pozo-Montuy G, Serio-Silva JC (2007) Movement and resource use by a group of Alouatta pigra in a forest fragment in Balancán, Mexico. Primates 48:102–107
Riley EP (2007) Flexibility in diet and activity pattern of Macaca tonkeana in response to anthropogenic habitat alteration. Int J Primatol 28:107–133
Rodríguez-Toledo EM, Mandujano S, García- OF (2003) Relationships between characteristics of forest fragments and howler monkeys (Alouatta palliata mexicana) in southern Veracruz, Mexico. In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 79–97
Rosenberger AL, Strier KB (1989) Adaptive radiation of the ateline primates. J Human E 18:717–750
Sauther ML (1998) Interplay of phenology and reproduction in ring-tailed lemurs: implications for ring-tailed lemur conservation. Folia Primatol 69:309–320
Silver SC, Marsh LK (2003) Dietary flexibility, behavioral and survival in fragments. In: Marsh LK (ed) Primates in fragments. Kluwer Press, New York, pp 252–265
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman, New York
SPSS Inc (2008) SPSS for Windows, Rel. 17.0.0. 2008. SPSS Inc., Chicago
Stevenson PR (1998) Proximal spacing between individuals in a group of woolly monkeys (Lagothrix lagothricha) in Tinigua National Park, Colombia. Int J Primatol 19:299–311
Stevenson PR (2002) Frugivory and seed dispersal by woolly monkeys at Tinigua National Park, Colombia. Ph.D. dissertation. State University of New York at Stony Brook, Stony Brook
Stevenson PR (2006) Activity and ranging patterns of Colombian woolly monkeys in North-Western Amazonia. Primates 47:239–247
Stevenson PR (2007) Estimates of the number of seeds dispersed by a population of primates in a lowland forest in western Amazonia. In: Dennis AJ, Schupp EW, Green RJ, Westcott DW (eds) Seed dispersal: theory and its application in a changing world. CAB International, Wallingford, pp 340–362
Stevenson PR (2010) Efectos de la fragmentación y de la producción de frutos en comunidades de primates neotropicales. In: Pereira-Bengoa V, Stevenson PR, Bueno M, Nassar-Montoya F (eds) Primatología en Colombia: avances al principio del milenio. Fundación Universitaria San Martín, Bogotá, p 229–258
Stevenson PR, Castellanos MC (2000) Feeding rates and daily path range of Colombian woolly monkeys as evidence for between and within group competition. Folia Primatol 71:399–408
Stevenson PR, Link A (2010) Fruit preferences by white-bellied spider monkeys (Ateles belzebuth) in Tinigua Park, North-Western Amazonia. Int J Primatol l31:393–407
Stevenson PR, Rodriguez ME (2008) Determinantes de la composición florística y efecto de borde de un Fragmento de bosque en el Guaviare, Amazonia Colombiana. Colombia Forestal 11:5–17
Stevenson PR, Quiñones MJ, Ahumada J (1994) Ecological strategies of woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Colombia. Am J Primatol 32:123–140
Stevenson PR, Quiñones MJ, Ahumada JA (1998) Effects of fruit patch availability on feeding subgroup size and spacing patterns in four primate species, at Tinigua National Park, Colombia. Int J Primatol 19:313–324
Symington MM (1988) Food competition and foraging subgroup size in the black spider monkey (Ateles paniscus chamek). Behaviour 105:117–134
Symington MM (1990) Fission-fusion social organization in Ateles and Pan. Int J Primatol 11:47–61
Terborgh J, Janson CH (1986) The socioecology of primate groups. Ann Rev Ecol Evol Syst 17:111–135
Tutin CEG (1999) Fragmented living: behavioural ecology of primates in a forest fragment in the Lope Reserve, Gabon. Primates 40:249–265
Vargas IN, Stevenson PR (2009) Patrones fenológicos en la estación biológica Mosiro Itajura-Caparú: producción de frutos estimada a partir de transectos fenológicos trampas de frutos. In: Alarcón G, Palacios E (eds) Estación biológica Mosiro Itajura-Caparú: Biodiversidad en el territorio del Yaigojé-Apoporis. Conservación Internacional, Bogotá, p. 99–114
Acknowledgments
This study was possible thanks to the collaboration during the fieldwork of Maria Carolina Santos-Heredia and Manuel Zabala. We thank the Instituto Amazónico de Investigaciones Científicas SINCHI, for their collaboration in the field phase of the project, especially to Dr. Mauricio Zubieta, and to Mr. Milton Oidor. We also thank Drs. Brent White, Daniel Cadena, Ellen Andresen, and Victor Arroyo-Rodríguez for their valuable comments on the manuscript. This study was funded by the Woolly Monkey Preservation Foundation, Primate Conservation Inc, and Universidad de Los Andes.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix 13.1
Appendix 13.1
Statistical comparisons of activity (a), and diet (b), between different age/sex classes in three groups of woolly monkeys inhabiting a fragment in Colombian Amazonia. The monthly variation in diet is also shown (c; adult male = AM; adult female = AF; adult female dependent infant = AFI)
L group | M group | S group | |
|---|---|---|---|
Activity budgets among age/sex classes (Z test for two proportions: ANOVA and Kruskal–Wallis test) | |||
Feeding | |||
AM vs. AF | Z = 0.657; P = 0.453 | Z = 0.495; P = 0.582 | Z = − 0.207; P = 0.802 |
AM vs. AFI | Z = 2.689; P = 0.006* | Z = 0.385; P = 0.652 | Z = 0.129; P = 0.880 |
AF vs. AFI | Z = 2.085; P = 0.031* | Z = 0.892; P = 0.395 | Z = 0.369; P = 0.652 |
Resting | |||
AM vs. AF | Z = − 0.061; P = 0.960 | Z = 0.644; P = 0.515 | Z = 0.866; P = 0.342 |
AM vs. AFI | Z = 1.857; P = 0.062 | Z = − 0.178 P = 0.802 | Z = 0.173; P = 0.802 |
AF vs. AFI | Z = 1.86; P = 0.051 | Z = 0.46; P = 0.582 | Z = 0.424; P = 0.652 |
Moving | |||
AM vs. AF | Z = 0.657; P = 0.453 | Z = 0.579; P = 0.515 | Z = 0.489; P = 0.582 |
AM vs. AFI | Z = − 0.076; P = 0.960 | Z = − 0.429; P = 0.0.652 | Z = 0.138; P = 0.880 |
AF vs. AFI | Z = 0.514; P = 0.582 | Z = − 0.116; P = 0.880 | Z = 0.138; P = 0.880 |
I. Socials | F = 0.26; df = 2; P = 0.26 | F = 1.13; df = 2; P = 0.36 | X2 = 1.50; df = 2; P = 0.17 |
Diet composition between the age/sex classes (Z test for two proportions) | |||
Mature fruits | |||
AM vs. AF | Z = 0.416; P = 0.652 | Z = 0.396; P = 0.652 | Z = 0.730; P = 0.453 |
AM vs. AFI | Z = 1.32; P = 0.177 | Z = 0.261; P = 0.726 | Z = 0.591; P = 0.582 |
AF vs. AFI | Z = 1.759; P = 0.080 | Z = − 0.166; P = 0.802 | Z = − 0.139; P = 0.880 |
Seeds | |||
AM vs. AF | Z = 0.626; P = 0.515 | – | – |
AM vs. AFI | Z = 0.844; P = 0.359 | – | – |
AF vs. AFI | Z = 0.301; P = 0.726 | – | – |
Young leaves | |||
AM vs. AF | Z = 0.147; P = 0.880 | Z = − 0.339; P = 0.726 | Z = 0.148; P = 0.880 |
AM vs. AFI | Z = 1.112; P = 0.250 | Z = − 0.198; P = 0.802 | Z = 0.460; P = 0.582 |
AF vs. AFI | Z = 1.436; P = 0.147 | Z = − 0.259; P = 0.726 | Z = 0.075; P = 0.880 |
Arthropods | |||
AM vs. AF | Z = − 0.293; P = 0.802 | Z = 0.199; P = 0.802 | Z = − 0.187; P = 0.802 |
AM vs. AFI | Z = 0.185; P = 0.802 | Z = 0.075; P = 0.880 | Z = − 0.144; P = 0.880 |
AF vs. AFI | Z = 0.2; P = 0.802 | Z = − 0.262; P = 0.726 | Z = − 0.366; P = 0.652 |
Flowers | |||
MA vs. FA | Z = 0.207; P = 0.802 | – | Z = − 0.380; P = 0.652 |
MA vs. FAI | Z = 0.123; P = 0.0.880 | – | Z = − 0.545; P = 0.582 |
FA vs. FAI | Z = − 0.382; P = 0.726 | – | Z = − 0.435; P = 0.652 |
Temporal variation in diet composition (ANOVA and Kruskal–Wallis test) | |||
Mature fruits | F = 4.678; df = 11; P = 0.001* | F = 2.778; df = 3; P = 0.075 | F = 4.351; df = 7; P = 0.002* |
Seeds | F = 4.703; df = 11; P > 0.001* | – | X2 = 11.865; df = 7; P = 0.105 |
Young leaves | F = 2.351; df = 11; P = 0.021* | F = 1.232; df = 3; P = 0.331 | F = 1.679; df = 7; P = 0.150 |
Arthropods | F = 3.418; df = 11; P = 0.001* | F = 1.039; df = 3; P = 0.402 | F = 0.580; df = 7; P = 0.767 |
Flowers | F = 4.309; df = 11; P > 0.001* | – | F = 2.956; df = 7; P = 0.017* |
Rights and permissions
Copyright information
© 2014 Springer New York
About this chapter
Cite this chapter
Zárate, D., Stevenson, P. (2014). Behavioral Ecology and Interindividual Distance of Woolly Monkeys (Lagothrix lagothricha) in a Rainforest Fragment in Colombia. In: Defler, T., Stevenson, P. (eds) The Woolly Monkey. Developments in Primatology: Progress and Prospects, vol 39. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0697-0_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0697-0_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0696-3
Online ISBN: 978-1-4939-0697-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)