Abstract
Uncommonly observed behaviors were systematically recorded in a troop (n = 9 individuals) of black howler monkeys (Alouatta pigra) inhabiting a small forest fragment (1.7 ha) in Leona Vicario, Balancán, Tabasco, Mexico. Between February 2002 and January 2003 (n = 499 h), we observed behaviors such as ground travel (85 occasions, total = 269 min/10.8% of total locomotion time), ground foraging (eight occasions, total = 50 min/0.84% of total feeding time) and drinking water pooled in tree holes (20 times, total = 93 min/0.31% of total activity time). Total time (412 min) for these non-resting behaviors (feeding and locomotion on the ground) is almost equivalent to time devoted to social activities (420 min). These behaviors indicate that howler monkeys may be responding to pressures imposed by the small size of the fragment by adopting diverse strategies to cover their basic nutritional needs in this environment. They accomplish this while exposing themselves to potential predation by coyotes (Canis latrans), as was observed once during the study. It is likely that these behaviors are occurring at an increasing rate among monkeys in fragmented landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation may significantly limit the availability of food and water sources in the home ranges of many Mexican primates (Estrada and Coates-Estrada 1996), and may cause changes in foraging and activity patterns, forcing primates to migrate in a matrix of isolated fragments (Mandujano et al. 2004; Bennett 2004). However, troop’s movements in this highly altered landscape imply risks (e.g., endoparasitic infections and presence of potential predators) and limitations on time spent in social activities by individuals trying to find their major survival needs: food and water.
Several studies have reported the high behavioral plasticity of black howler monkeys (Alouatta pigra) when it comes to making efficient use of the resources offered by a particular habitat, even when individuals are found living in extremely reduced forest fragments (Marsh 2002; Pavelka and Knopff 2004). Although it has been suggested that under these conditions having a folivorous diet offers advantages to howler monkeys in terms of tolerating environmental stress (Bicca-Marques 2003), few studies have documented actual alternative behavioral strategies employed by these monkeys to survive under conditions of reduced plant diversity and abundance of basic natural resources. These circumstances lead animals to display behaviors not commonly observed under less-perturbed habitat conditions.
It has been reported that in a fragmented habitat mantled howler monkeys (Alouatta palliata) exhibit behavioral responses that include increased time spent in locomotion and foraging on the ground, where small plants are consumed (Glander 1992; Clarke et al. 2002). Other studies have shown howlers’ adaptability to cope with habitat disturbance and differing isolation conditions by adopting terrestrial behaviors such as movements on the ground (Horwich and Lyon 1998). Although these behaviors (feeding and movements on the ground) have been reported by Carpenter (1965) and Schön-Ybarra (1984), the topic has only been mentioned anecdotally.
Finally, the direct intake of water (principally from tree holes, and occasionally from flowing and standing water on the ground) by Alouatta spp. has been widely reported in the literature (A. palliata: Carpenter 1965; Glander 1975, 1977; Gilbert and Stouffer 1989; A. pigra: Silver et al. 1998; A. guariba: Steinmetz 2001; A. caraya: Bicca-Marques 1992; A. seniculus: Gaulin 1977 and Alouatta guariba clamitans: Almeida-Silva et al. 2005). All concur that under adequate habitat conservation conditions, these animals obtain their necessary water from the food that they consume (mainly young leaves and mature fruit). However, increased habitat disturbance (e.g., isolation, area and plant-diversity reduction) leads to lower availability of new leaves and ripe fruit. Under these conditions, howlers are motivated to eat more mature leaves that contain higher levels of fiber and secondary compounds and low levels of moisture (Glander 1977; Milton 2003). This dietary change may force howlers to find alternative sources of water, especially water deposited in tree holes during the rainy season (Steinmetz 2001), or on the ground (Serio-Silva and Rico-Gray 2000). Nevertheless, some authors suggest that water intake from tree holes may not be indicative of water stress, and may be more common than usually assumed (Clarke et al. 2002). A recent report indicates that, under hydric stress conditions, even howler monkeys (A. guariba clamitans) living in a continuous canopy went to the ground, and this could be due to the fact that they did not found water in the trees during dry season (Almeida-Silva et al. 2005).
The literature contains few reports on these behaviors, and data on how Mexican primate species depart from their habitual behaviors and incur certain risks to satisfy their feeding and drinking needs are scant. Here we report systematic observations on ground movements, foraging on the ground and water intake from tree holes by black howler monkeys (A. pigra) inhabiting a forest patch in Balancán, Tabasco, Mexico.
Methods
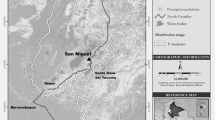
The study site, Leona Vicario, is a private ranch located 14 km from the Balancán—Tenosique highway. Although the ranch covers 32 ha, the vegetation patch inhabited by howler monkeys measures only 1.7 ha and the home range of the howler troop is only 0.2 ha (Pozo-Montuy and Serio-Silva 2006). The mean annual temperature varies from 25 to 28°C. The mean annual rainfall in the State of Tabasco is about 1,380 mm. Three seasons have been identified in the region: the season of the northerly winds or nortes (November—January, mean rainfall = 1,370 mm), dry (February—April, mean rainfall = 570 mm) and rainy (May—October, mean rainfall = 1,780 mm) (CNA 2003).
Vegetation in the fragment included trees (n = 12 spp.), herbaceous plants (n = 3 spp.), bushes (n = 2 spp.) and vines (n = 4 spp.). We defined three categories of arboreal strata based on the vegetation height: high (≥ 15.1 m), medium (3.1—15.0 m) and low (0—3.0 m). Of 12 tree species (n = 698 individuals), Haematoxylum campechianum (Fam. Fabaceae) (n = 350 individuals) had the highest relative density (0.50 individuals/ha). Relative species density is the total number of individuals of a species expressed as a proportion (or percentage) of the total number of individuals of all species.
The study troop consisted of nine individuals (three adult males, two adult females, one juvenile female, one juvenile male, one infant male and one infant female). The troop had been present in the fragment since before its isolation (ca. 10 years ago) (Pozo-Montuy and Serio-Silva 2006). Howlers were easily located, mainly in the lower stratum of the fragment, by listening to vocalizations or by direct observation. Behavioral data (e.g., feeding, locomotion, play and sexual behavior) were systematically collected using focal animal and ad libitum sampling (Altmann 1974) every weekend over the course of 1 year (February 2002—January 2003) in 12-h periods (06:00—18:00). Data recorded for each behavior included: date, season, individual (sex/age), hour and minute, species and plant part consumed when feeding, time spent and distance covered when moving on the ground, and behavior and category of stratum level when drinking water, as well as resting and social activities.
One-way ANOVA tests were used to determine whether season (n = 3), or sex—age category (n = 3; adult male, adult female and immature) had a significant effect on time spent on each activity. Tukey multiple comparisons were used to identify significant differences (Zar 1996). We used the proportional method (Altmann 1974; Bicca-Marques 1992) dividing the observed time that monkeys spent on each behavior (feeding on the ground, moving on the ground and the distance in meters covered, by season and sex–age category) by the total time recorded per troop in each season and age—sex during the sampling period. Finally, we examined whether time ingesting water from tree holes was affected by the stratum occupied by the individual or age—sex category, and we calculated the proportion of time devoted to drink water for each stratum and sex–age category to total time that monkeys spent on each stratum during sampling period. In all cases, tests for normality failed and we used nonparametric statistical tests (Kruskal–Wallis one-way ANOVA on ranks), and Dunn’s method of multiple comparisons test to identify significant values.
Results
The black howler troop was observed during 499 focal hours of observation (nortes season = 146 h, dry season = 86 h, rainy season = 268 h). During this time, individuals moved and foraged on the ground and drank water from tree holes for a total of 412 min or 5.0% of non-resting (feeding and locomotion) activities. A similar amount of time (420 min) was spent in social activities. There were no significant differences among sex—age categories in total observed time (H = 4.636, P = 0.098) and strata use (F = 0.018, P = 0.982), however, we found that the monkey troop spent significantly more time using high and medium strata (F = 188.788, P ≤ 0.001) than lower to perform their activities.
Consumption of plant species from the ground
Monkeys were observed consuming plant parts on the ground eight times (50 min, 0.85% of feeding time), mainly from Pithecellobium lanceolatum (30 min/0.51% feeding time) and an unidentified species of Cucurbitaceae (20 min/0.34% of feeding time). This behavior was more common during the rainy (38 min) than the nortes (12 min) and dry (0 min) seasons. No statistically significant differences were found among sex—age classes (Table 1).
Travel on the ground
On 85 occasions (total = 269 min/10.9% of locomotion time) monkeys were observed locomoting on the ground (mean = 3.04 min per travel bout; max = 30 min/min = 1 min). They spent significantly more time exhibiting this behavior during the rainy season (200 min) than during the nortes (36 min) or dry season (33 min). Again, there were no differences among sex—age categories (Table 1).
Season was also a factor when considering distances traveled on the ground (n = 85 observations, mean distance = 10.4 ± 13.1 m; range 2—80 m). We found significant differences between dry and both the rainy and nortes seasons. Evaluation of sex—age categories showed no differences in travel distance (Table 1).
Water intake from different arboreal strata
Monkeys were seen drinking water from tree holes 20 times (n = 93 min). All records of this behavior were obtained in February, which corresponds to the beginning of the dry season (February—April) in the area. Individuals showed a marked preference for drinking water while in the high tree stratum (70% drinking time) compared to the medium (25% drinking time) and low (5% drinking time) strata. We only found significant differences between high and low strata (Table 1). Comparison of age and sex categories showed no significant differences in stratum used for water intake (Table 1). On only one occasion an adult male was observed going to the ground to drink water from a lagoon near the fragment. This observation was recorded during the dry season.
Discussion
The present study describes behaviors shown by black howler monkeys (A. pigra) that are compatible with adaptations expected for monkeys living under extreme conditions. Early reports of moving and foraging on the ground and drinking water from tree holes have been mainly anecdotal (Carpenter 1965; Schön-Ybarra 1984), or presented as unexpected observations within the basic pattern of Alouatta behavior (Glander 1978; Gilbert and Stouffer 1989; Bicca-Marques 1995; Almeida-Silva et al. 2005). In this study, A. pigra spent the same amount of time engaged in these activities as in social activities over the period of 1 year.
In several sites of Tabasco (Díaz-López and Serio-Silva 2003; Pozo-Montuy and Serio-Silva 2006) and the Yucatan Peninsula (Serio-Silva et al. 2006), habitat fragmentation appears to be increasingly common. Monkey troops inhabiting small fragments are pressed to consume vegetation and travel on the ground and to drink water from alternative sources, potentially exposing themselves to predation, as also reported by Treves (2002).
There were significant differences among seasons with regard to plant consumption on the ground, with the howlers particularly increasing this foraging activity during the rainy season. Three plant species made up 72.6% of feeding records during the rainy season (Pozo-Montuy and Serio-Silva 2006), and all available tree species were used as food sources by the monkeys. Behavioral adaptations included the seasonal selection of plant parts, low activity rates and decreased social interaction rates, in addition to the behaviors reported in the present study (Pozo-Montuy and Serio-Silva 2006). Feeding behavior on the ground may provide a dietary alternative or supplement to satisfy individual nutritional requirements. The same behavioral pattern has been observed occasionally for mantled howlers (A. palliata) in Costa Rica (Clarke et al. 2002) and Panama (J.R. Campbell, personal communication), and for Ateles spp. (Dew 2005). Spider monkeys appear to obtain nutrient supplements from soil (probably obtaining phosphorous, Dew 2005) or rotten wood and salt licks (Campbell et al. 2005).
Increased terrestrial travel during the rainy season is related to feeding on new shoots (P. lanceolatum) in the ground, and feeding in isolated trees in the animals’ reduced home range (Pozo-Montuy and Serio-Silva 2006). Some reports suggest that in such highly fragmented landscapes, this behavior may become more frequent due to the lack of enough trees to provide leaves and fruit to sustain the howler monkey biomass present (Clarke et al. 2002; Marsh 2002). Both the average time and distance of movement involve an increased risk for monkeys on the ground, including the danger of attracting terrestrial predators (Cuaron 1997). During our study, one attempted predation event was observed when an infant female A. pigra was caught moving on the ground by a coyote (Canis latrans).
In addition to the small and perturbed forest patch where the study troop survives, another stressful factor could be the high temperature (29—42°C) (CNA 2003). In such highly fragmented forest patches, these environmental characteristics (temperature record of 45.2°C in May 2006) can be extreme, due to edge effects, proximity to recently burned agricultural fields in March—April, and exposure to hurricane winds and rain impacts during July—November (CNA 2003). Under these conditions, some authors suggest that monkeys cannot obtain enough water from leaves and fruit to maintain basic metabolic function (Glander 1978), due to decreased moisture and increased fiber and secondary compounds in food source leaves (Silver et al. 1998). Our data show that during this season, black howlers used alternative sources of rainwater commonly pooled in holes between branches in the highest tree stratum. These drinking data are equivalent to 8.6% of feeding time during the dry season (Table 1). Without doubt, the dry season was the most stressful period, particularly due to the highest temperature values which could have effects on the small fragments and on howler’s behavioral strategies. For this reason monkeys were spending this amount of time ingesting water from tree holes. This behavior has been reported for other howler species (Glander 1975; Gaulin 1977; Gilbert and Stouffer 1989; Bicca-Marques 1995; Steinmetz 2001) and for other tree-dwelling primates in the Neotropics (Ateles paniscus chamek—Ferrari 1991; Brachyteles arachnoides—Petroni 2000).
Other studies have reported that Mexican mantled howler monkeys (A. palliata mexicana) ingest water directly from the ground under specific conditions (Serio-Silva and Rico-Gray 2000). Our single observation of drinking water from the ground in this study was from a lagoon near the fragment (during dry season with highest temperatures, mean = 42°C). Under these high temperature conditions and particular plant phenology (young leaves and fruits are absent) some howler monkey species are also forced to drink water on the ground in continuous forested areas (970 ha) (Almeida-Silva et al. 2005).
In conclusion, we agree with previous reports that suggest that severe environmental condition and habitat disturbance cause alterations in the forest landscape and the species that interact with it (Serio-Silva and Rico-Gray 2002). In our case, these conditions appear to be causing behavioral changes (Silver and Marsh 2003) that may place individuals at risk mainly due to predation (Treves 2002). The future of the habitat patch and the howler population is uncertain; the survival expectations of these monkeys may be low due to other factors resulting from fragmentation, such as parasitism (Bonilla-Moheno 2002; Rico-Hernández 2005) and physiological stress related to high cortisol levels (Rangel-Negrín 2003; Martínez-Mota et al. 2004). It is likely that this situation will lead to the disappearance of howler monkeys from some perturbed sites, but for those remaining in small forest fragments, there is a pressing need to explore further how they can survive and modify their behavior in this altered landscape. Recently, some authors have suggested the need for management of these endangered troops at a metapopulation level (Mandujano et al. 2006). This proposal is particularly related to habitat rehabilitation, corridor establishment and translocation of troops to fragments with better survival conditions (Mandujano et al. 2004). However, currently, we only have dispersal models which suggest the statistical probabilities of monkey movements if we reduce isolation distance between fragments. It will be interesting in the near future to test how this model can be applied directly to the monkeys’ natural habitat, where we can find a complex interaction between human social factors (poverty, common lands, among others) and monkey population needs (diversity, abundance and nutritional quality of food resources, size and structural heterogeneity in the landscape). More ecological and behavioral studies and specific conservation efforts are required to ensure the persistence of black howlers in such fragmented areas in southern of Mexico.
References
Almeida-Silva B, Guedes PG, Boubli JP, Strier KB (2005) Deslocamento terrestre e o comportamento de beber em um grupo de barbados (Alouatta guariba clamitans, Cabrera, 1940) em Minas Gerais, Brasil. Neotrop Primates 13(1):1–3
Altmann J (1974) Observational study of behavior: sampling methods. Behavior 49:227–267
Bennett AF (2004) Enlazando el paisaje: El papel de los corredores y la conectividad en la conservación de la vida silvestre. Programa de Conservación de Bosques UICN. Conservando los Ecosistemas Boscosos Serie Número 01. UICN, CCAD, SICA, CBM, San José, Costa Rica, 276 pp
Bicca-Marques JC (1992) Drinking behavior in the black howler money (Alouatta caraya). Folia Primatol (Basel) 58:107–111
Bicca-Marques JC (1995) Locomotion of black howlers in habitat with discontinuous canopy. Folia Primatol (Basel) 64:55–61
Bicca-Marques JC (2003) How do howler monkeys cope with habitat fragmentation? In: Marsh LK (ed) Primates in fragments: ecology and conservation. Kluwer Academic/Plenum Publishers, New York, pp 283–303
Bonilla-Moheno M (2002) Evaluación de la incidencia parasitaria de primates silvestres en hábitat fragmentado y conservado en la península de Yucatán. B.Sc. thesis, Facultad de Ciencias, UNAM, México DF
Campbell JR, Aureli F, Chapman CA, Ramos-Fernández G, Matthews K, Russo SE, Suarez S, Vick L (2005) Terrestrial behavior of Ateles spp. Int J Primatol 26:1039–1051
Carpenter CR (1965) The howlers of Barro Colorado Island. In: De Vore I (eds) Primate behavior. Field studies of monkeys and apes. Holt, Rinehart and Winston, New York, pp 250–291
Clarke MR, Collins DA, Zucker EL (2002) Responses to deforestation in a group of mantled howlers (Alouatta palliata) in Costa Rica. Int J Primatol 23:365–381
CNA (2003) Comisión Nacional del Agua, Delegación Tabasco. Estación climatológica Balancán. Villahermosa, Tabasco, México
Cuaron A (1997) Conspecific aggression and predation: cost for a solitary mantled howler monkey. Folia Primatol (Basel) 68:100–105
Dew JL (2005) Foraging, food choice and food processing by sympatric ripe-fruit specialist. Humboldt’s woolly monkey and the white-bellied spider monkey. Int J Primatol 26:1107–1135
Díaz-López H, Serio-Silva JC (2003) Distribución y abundancia de primates silvestres (Alouatta pigra y Ateles geoffroyi vellerosus) en la región de la Sierra, Tabasco, México. In: Memorias del XX Simposio sobre Fauna Silvestre “Grl. MV Manuel Cabrera Valtierra”. UNAM, Mexico DF
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas. Int J Primatol 5:759–783
Ferrari SF (1991) An observation of western black spider monkeys, Ateles paniscus chamek, utilization of an arboreal water source. Biotropica 23:307–308
Gaulin SJ (1977) The ecology of Alouatta seniculus in Andean cloud forest. Ph.D. thesis, Harvard University, Cambridge
Gilbert KA, Stouffer PC (1989) Use of a ground water source by mantled howler monkeys (Alouatta palliata). Biotropica 21:380
Glander KE (1975) Habitat description and resource utilization: a preliminary report on mantled howling monkey ecology. In: Tuttle R (eds) Socioecology and psychology of primates. Mouton, The Hague, pp 37–57
Glander KE (1977) Poison in a monkey’s Garden of Eden. Nat Hist 86:34–41
Glander KE (1978) Drinking from arboreal water sources by mantled howling monkeys (Alouatta palliata Gray). Folia Primatol (Basel) 29:206–217
Glander KE (1992) Dispersal patterns in Costa Rica mantled howling monkeys. Am J Primatol 13:415–436
Horwich RH, Lyon J (1998) A Belizean rain forest—the community Baboon Sanctuary, 3rd edn. Orangutan, Gays Mills
Mandujano S, Escobedo-Morales LA, Palacios-Silva R (2004) Movements of Alouatta palliata among forest fragments in Los Tuxtlas, Mexico. Neotrop Primates 12:126–131
Mandujano S, Escobedo-Morales LA, Palacios-Silva R, Arroyo V, Rodriguez-Toledo EM (2006) A metapopulation approach to conserving the howler monkey in a highly fragmented landscape in Los Tuxtlas, Mexico. In: Estrada A, Garber P, Pavelka M, Luecke L (eds) New perspectives in the study of mesoamerican primates: distribution, ecology, behavior and conservation. Kluwer Academic/Plenum Publishers, New York, pp 513–538
Marsh LK (2002) Section II: behavioral ecology. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Kluwer Academic/Plenum Publishers, New York, pp 159–162
Martínez-Mota R, Valdespino-Quevedo C, Sánchez-Ramos MA (2004) Feacal cortisol levels as a measurement of stress due to habitat fragmentation in wild black howler monkeys (Alouatta pigra) in Mexico. Folia Primatol (Basel) 75(S1):299
Milton K (2003) Micronutrient intakes of wild primates: are humans different? Comp Biochem Physiol A 136:47–59
Pavelka M, Knopff KH (2004) Diet and activity in black howler monkeys (Alouatta pigra) in southern Belize: does degree of frugivory influence activity level? Primates 45:105–114
Petroni LM (2000) Caracterizacao do area do uso e dieta do mono carvoeiro (Brachyteles arachnoides) na Mata atlantica, serra de Paraná Picaiba. Ph.D. thesis, Universidad de Sao Paulo, Sao Paulo, Brazil
Pozo-Montuy G, Serio-Silva JC (2006) Comportamiento alimentario de monos aulladores negros (Alouatta pigra Lawrence, Cebidae), en hábitat fragmentado en Balancán, Tabasco, México. Acta Zool Mex 22(3):53–66
Rangel-Negrín A (2003) Niveles de cortisol fecal en Ateles geoffroyi yucatanensis en diferentes tipos de hábitat de la Península de Yucatán, México. Lab Prim News 42:8
Rico-Hernández G (2005) Endoparasites and forest fragments: implications for howler monkey conservation. Am Soc Primatol Bull 29:9
Schön-Ybarra MA (1984) Arborealism and terrestralism in howler monkeys. Am J Phys Anthropol 57:225
Serio-Silva JC, Rico-Gray V (2000) Use of a stream as water source by a troop of Mexican howler monkeys (Alouatta palliata mexicana) during extreme environmental conditions. Southwest Nat 45:332–333
Serio-Silva JC, Rico-Gray V (2002) Interacting of forest fragmentation and howler monkey foraging on germination and dispersal of fig seeds. Oryx 36:266–271
Serio-Silva JC, Rico-Gray V, Ramos-Fernandez G (2006) Mapping primate populations in the Yucatan peninsula, Mexico: a first assessment. In: Estrada A, Garber P, Pavelka M, Luecke L (eds) New perspectives in the study of mesoamerican primates: distribution, ecology, behavior and conservation. Kluwer Academic/Plenum Publishers, New York, pp 489–511
Silver SC, Marsh L (2003) Dietary flexibility, behavioral plasticity, and survival in fragments: lessons from translocated howlers. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Kluwer Academic/Plenum Publishers, New York, pp 251–265
Silver SC, Ostro LET, Yeager CP, Horwich R (1998) Feeding ecology of the black howler monkey (Alouatta pigra) in northern Belize. Am J Primatol 45:263–279
Steinmetz S (2001) Drinking by howler monkey (Alouatta fusca) and its seasonality at the Intervales State Park, Sao Paulo, Brazil. Neotrop Primates 9:111
Treves A (2002) Predicting predation risk for foraging arboreal monkeys. In: Miler LE (ed) Eat or be eating: predator sensitive foraging among primates. Cambridge University Press, New York, pp 222–241
Zar JE (1996) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgments
We thank Nora Bynum and Julio Cesar Bicca-Marques for their extraordinary support, Nicoletta Righini, Victor Rico-Gray, LeAndra Luecke and Rodolfo Martínez-Mota and reviewers for providing helpful comments. We thank the people in Balancán, Tabasco, Mexico for their support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pozo-Montuy, G., Serio-Silva, J.C. Movement and resource use by a group of Alouatta pigra in a forest fragment in Balancán, México. Primates 48, 102–107 (2007). https://doi.org/10.1007/s10329-006-0026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-006-0026-x