Abstract
Ultrasound-assisted vacuum (USV) drying is a promising method to increase heat and mass transfer rate. This study aimed to examine effects of USV drying on the drying rate and some quality parameters of red peppers at 45, 55, 65, and 75 °C. The results were compared with USV control drying (vacuum drying without ultrasound treatment) and the other drying methods. The USV drying shortened the drying period to 25% and increased the effective moisture diffusivity (Deff) to 89% when compared by USV control drying. The dehydration kinetics of the red peppers were successfully described using seven thin layer drying models for all of the dehydration methods, and the logarithmic model had the best fit for the USV drying, with highest R2 and lowest RMSE values. The total yeast and mold counts were significantly reduced following the USV drying in comparison with the USV control drying. This study suggested that USV drying could be used effectively in drying of red pepper with high drying rate and no significant bioactive compound degradation compared to USV control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Red peppers (Capsicum annuum L.) are a highly consumed vegetable because of both having specific aroma, flavor, and color and being rich source of vitamins (A, C, and E) and minerals [1]. In some countries, it is believed that they prevent or cure asthma, rheumatism, neuralgia, lumbago, and pharyngitis, and they are also believed to increase a person’s appetite [2]. Drying is one of the most commonly used preservation methods, and red peppers are primarily dried to obtain a spice that is mainly used in meat products and instant soup; the spice is also added to bakery products, spice mixes, and seasonings and sauces in the food industry [3]. Generally, peppers have been dried in the open air by exposing to sunlight for 8–10 days, in Turkey, or they are dried by other conventional drying methods. However, there are significant shortcomings such as long drying times, the contamination risk of dust, soil, and insects, the risk of rain and quality loss. Therefore, ultrasound-assisted vacuum drying can be used as combination to overcome the shortcomings of both each other and traditional methods.
Vacuum drying takes place in the absence of oxygen and provides effective dehydration at mild temperature so that most of the food nutritive and sensory characteristics can be preserved [4]. Therefore, a combination of or pre-treatment with other drying methods is needed because the deficiencies of traditional drying methods might be compensated for by using other methods, resulting in a more effective drying process [5, 6].
Ultrasound is a form of mechanical energy generated by sound (i.e., really pressure) waves with a frequency of more than 20 kHz. In recent years, a growing number of various stages of research and development on ultrasound have been in the food industry due to the fact that ultrasound is a green novel technology [7]. There are a lot of ultrasound applications such as cooking, cutting, de-foaming, degassing, drying, emulsification, filtration, freezing, oxidation in food processing [8].
Ultrasonic dehydration is very promising because the effects of power ultrasound are more significant at low temperature which reduces the probability of food degradation. Cavitation, explained with bubble formation and its subsequent collapse in a liquid, can be supported by ultrasonic waves so that microscopic cavities can be created in the tissues of vegetables and fruit [9]. This results in the removal of moisture that is strongly attached to the solid center of the tissues [10]. Microscopic channels improve diffusion, increase convective mass transfer, and reduce drying time [11].
When food is dried, water availability is reduced so that microbial activity and the rate of chemical and biochemical reactions are decreased. However, the yeast and molds found in red peppers can cause discoloration of products as well as negative taste and malodor. Moreover, molds can cause the formation of toxic metabolites (especially aflatoxins), so they can have an adverse effect on health. In this study, the formation of toxic metabolites was not determined, but the toxic metabolites were not supposed to be formed by using USV drying method.
USV drying is a novel method that uses ultrasound to increase heat and mass transfer in vacuum drying. A few research studies have been conducted using the technique on some types of food, such as beef meat, chicken [12], fish [13] and green beans [14] and non-food materials, such as Chinese fir wood [15] and Catalpa wood [11]. Those studies have indicated that the USV drying has the potential to be a faster drying process for foods and other materials.
The objective of the present study is to examine the effects of the USV drying on red pepper dehydration. Toward this end, the study investigated the efficacy of USV drying in comparison with different drying methods by examining drying kinetics, rehydration behavior, total yeast and mold count, bioactive compounds, such as phenolic compound and antioxidant capacity, and color properties.
Experimental
Materials
Red pepper (Capsicum annuum var. abbreviata Fingerh.) samples were bought from a market in Istanbul, Turkey and stored at 4 °C before the drying process. The peppers were not peeled, but the seeds and the placenta were removed. The peppers were then cut into 1.5 ± 0.1 cm2 square pieces. Samples weighing approximately 50 g were used for the drying process.
Drying methods
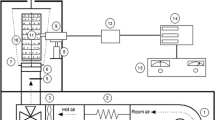
Red peppers (approximately 50 g) were dried using a vacuum oven with a vacuum pump (for vacuum drying), a drying oven (for oven drying), the combination system of an ultrasonic water bath and vacuum pump (for USV drying), and the combination system of water bath and vacuum pump (for USV control drying) until the water content decreased to 10% (moist basis) at 45, 55, 65, and 75 °C. Three repetitions were performed for each type of drying at each temperature, and also three repetitions were carried out for each analysis.
A vacuum oven (DaihanWOV-30, Gangwondo, South Korea) combined with a vacuum pump (EVP 2XZ-2C, Zhejiang, China) with 60 mbar pressure was used for the vacuum drying. Oven drying was performed using a forced-air circulation oven (Memmert UF110, Germany) with 1.3 m s−1 constant air velocity. The USV drying, mainly used in this study, consisted of an ultrasonic water bath (Daihan-WUC-D10H, South Korea) (amplitude 100%, power 590 W) and a vacuum pump (KNF N838.3KT.45.18, Germany) with 15 mbar pressure linked to a hose. The temperature of the ultrasonic water bath was measured using a thermocouple (k-type, Omega Engineering Inc., USA). The water bath (Daihan, WUC-D10H, South Korea) (amplitude 100%, power intensity ~ 1 W cm−2, volume 10 L) and a vacuum pump (KNF N838.3KT.45.18, Germany) with 15 mbar pressure linked to a hose for USV control drying.
The pepper samples, weighing approximately 50 g, were placed into a filtering flask, which was then closed with a stopper. The drying process took place under negative pressure with ultrasonic waves. The USV control drying is a vacuum treatment process in which the ultrasonic treatment is not applied. This USV drying was detailed and schematized in our previous studies [12, 13].
Comparison with empirical equations
The moisture ratio (MR) was calculated using Eq. (1):
where MR stands for sample moisture ratio (dry basis), Xt moisture content at t (kg-water kg-dry matter−1), Xe moisture content at equilibrium (kg-water kg-dry matter−1) and X0 moisture content at initial (kg-water kg-dry matter−1). The equilibrium moisture content was considered zero (Xe = 0), because the value of Xe is relatively small compared with X0 [16].
Seven thin layer models were used to express the drying kinetics of the red peppers (Table 1). Nonlinear regression was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA) software. The determination coefficient (R2) was determined using SPSS; however, Chi-square (χ2) and the root-mean-squared error (RMSE) were calculated according to Doymaz [17].
Mass transfer with diffusion was defined according to Fick’s laws of diffusion. In general, by using Fick’s 2nd Law, moisture transfer in the crop drying process is denoted by Eq. (2) [18]:
However, the diffusion equation can be modified for food dehydration. Effective moisture diffusivity was calculated by using SPSS program according to using Eq. (3):
where Deff is the effective moisture diffusivity (m2 s−1) and L the thickness of red pepper samples (m).
Arrhenius equation defines the relationship between moisture content and the temperature parameters according to Eq. (4):
where D0 represents the pre-exponential constant (m2 s−1), Ea the activation energy (kJ mol−1), R the ideal gas constant (8.314 J mol−1 K−1), and T the drying temperature (K).
Determination of dry matter content
The samples were dehydrated by using vacuum drier at 70 °C until constant mass and the dry matter content determined according to the mass.
Determination of the drying rate
The drying rate (DR) (kg-water h−1m−2) of red pepper dried at constant drying conditions for any time (t) was calculated using Eq. (5) [19]:
where X symbolizes the moisture content (kg-water kg-dry matter−1), t the drying time (h) and Ls the dry matter of the peppers (kg), S the total surface area of all red peppers in one container (m2).
Determination of the rehydration ratio
The dried peppers were rehydrated with distilled water at 25 °C in amounts equal to four times the mass of the pepper sample before the drying process. After waiting 5 h, the rehydration ratio (RR) was calculated using Eq. (6) [20]:
where m1 and m2 are the mass before rehydration and after rehydration, respectively.
Color measurement
The color parameters (L*, a*, b*) of the red pepper samples were obtained using a colorimeter (CR-400 Konica, Minolta, Tokyo, Japan) calibrated at standard illuminant D65 (representing daylight). The chroma value defines the color saturation, and that value decreases with shaded colors (gray, black and white) and increases with vivid colors. The total color differences (ΔE*) were calculated by using ΔL*(lightness difference: L *sample − L *standard ), Δa*(red/green difference: a *sample − a *standard ), and Δb*(yellow/blue difference: b *sample − b *standard ) values and the chroma (C*) values were calculated by using a and b values according to Eqs. (7–8), respectively [21]:
Determination of the total yeast and mold counts
Dichloran rose bengal chloramphenicol (DRBC) agar was used to determine the total yeast and mold counts, which were calculated after incubation at 25 °C for 4–5 days. The detection limit of yeast and mold was 2 log10 cfu mL−1.
Determination of the total phenolic content
The dried samples were first rehydrated using the water that had evaporated during the drying process because it was important to ensure that the same conditions were utilized for all of the drying methods. The fresh and rehydrated red pepper samples were weighed at approximately 50 g and then mixed with aqueous methanol (80%) (1:4 methanol to samples ratio). They were then homogenized with a homogenizer (Daihan, 230 V, 50/60 Hz, 280 W, Korea) at about 1250 rpm for 5 min. Each sample was then incubated using a shaker for 2 h at 25 °C. The mixture was centrifuged at 4000 rpm for 10 min, and the supernatants were filtered. The total phenolic content of the filtered red pepper supernatants was determined using the Folin–Ciocalteu method according to Singleton and Rossi [22], with some alterations [23]. The results were expressed as gallic acid equivalents (GAE) mg g-dry matter−1.
Determination of DPPH free radical scavenging capability
The supernatants filtered for determination of total phenolic compounds were also used to determine DPPH free radical scavenging capability. The 2,2 diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capability was determined according to analysis recommended by Singh et al. [24]. The results were expressed as trolox equivalents mg g-dry matter−1.
Results and discussion
Drying kinetics
The initial moisture content of the fresh red peppers was 91.623% ± 0.852 [25], and the peppers were dried to reach 10% in wet basis. In some research studies, the dried pepper samples had a similar final moisture content, such as 12% [26], 11% [27], and 10% [28]. In the present study, the red pepper samples were dried using USV drying and USV control drying methods at four different temperatures, and the moisture ratio versus time graphs are shown in Fig. 1.
The drying times of the samples decreased as the drying temperature increased for all drying methods. The drying times of the samples are shown in Table 2. While the drying times of the samples dried using USV drying were 570, 450, 270, and 240 min, the drying times of samples dried using the USV control drying were 750, 510, 360, and 270 min at 45, 55, 65, and 75 °C, respectively. Employing of USV significantly reduced drying time at same temperature. Thanks to USV drying, food materials can be dried at lower temperature.
With regard to other techniques, the drying times ranged between 270 min and 690 min for the samples dried using the oven drying and between 270 min and 540 min for the samples dried using the vacuum drying. Similarly, in a thesis study about red peppers dried with 2 ± 0.1 m s−1 air velocity at 55, 60, 65, and 70 °C, the drying times ranged between 495 and 960 min [3]. Akpinar et al. [29] reported that the drying times for red peppers ranged from 160 to 330 min at 55–70 °C of oven. In another study, the USV drying decreased the drying time of beef meat and chicken by 7.4 and 27.4%, respectively, according to vacuum drying [12].
The energy consumption changes based on the applied methods and the drying temperature in drying systems, and it decreases as the temperature increases. In the present study, the total energy consumption ranged between 1.2 and 1.5 kWh with oven drying, between 1.7 and 2.7 kWh with vacuum drying, between 1.6 and 1.9 kWh with USV drying, and between 1.1 and 1.8 kWh with USV control drying. The total energy consumption of the samples dried using USV drying was higher than the samples dried using the USV control drying; however, the drying times using the USV drying were shorter, so the USV drying could be considered to be more economical than the USV control drying.
Drying rate
Drying of most foods under different constant drying conditions will generally give curves with different shapes in the falling rate period [19, 30]. If cell structure of foods is damaged, they may also give curves in the constant drying rate period [31]. The drying rate can be improved with ultrasonic treatment because ultrasound provides the positive effects of heating, thanks to attenuation and adsorption, with the mechanical effects of pressure waves [32]. Figure 2 shows that the drying rate values for USV drying and USV control drying changed with time. As seen in these graphs, the drying rate values obtained using the USV drying were generally higher than those obtained using the USV control drying in the falling rate period because of the ultrasound positive effects. In addition, the drying rate values increased with all of the drying methods as the temperature increased. This means that a shorter time is required to dry the material in order to reach equilibrium moisture content.
Effective moisture diffusivity and activation energy
In this study, the Deff increased with increasing temperature for all of the drying processes. The Deff values of the dried samples changed from 2.62 × 10−9 to 8.10 × 10−9 m2 s−1, from 1.82 × 10−9 to 6.31 × 10−9 m2 s−1, from 3.44 × 10−9 to 6.78 × 10−9 m2 s−1, and from 2.65 × 10−9 to 7.82 × 10−9 m2 s−1 for the USV drying, the USV control drying, the vacuum drying, and the oven drying, respectively. The Deff values and the activation energy (Ea) values for all four drying methods and for all temperatures are shown in Table 2. The Deff values of the USV-dried samples are higher than the Deff values of the USV control dried samples. From the data presented in Table 2, it is clear that Deff increases as the temperature increases because the moisture content of the red pepper samples decreased due to the rapid evaporation of water molecules. Doymaz [33] and Huang and Chen [34] found that the effective moisture diffusivity increased with increasing temperature for mint leaves and sewage sludges, respectively.
The Ea values indicate the correlation of the diffusivity against temperature. When the Ea values increase, the correlation of the apparent diffusivity of red peppers against temperature increases [35]. There is a direct correlation between Ea and the energy needed to facilitate diffusion. When less Ea is required, less energy is needed to facilitate diffusion. The Ea value of the Deff was less for USV drying than it was for the USV control drying; thus, USV drying required less energy consumption. The Ea values of the dried samples were found to be 35.911 kJ mol−1 (R2 = 0.980), 33.921 kJ mol−1 (R2 = 0.990), 24.047 kJ mol−1 (R2 = 0.926), and 36.744 kJ mol−1 (R2 = 0.925) for the USV drying, the USV control drying, the vacuum drying, and the oven drying, respectively.
Gupta et al. [36] dried the pre-treated red peppers at 55, 60, 65, and 70 °C using a tray drier, and they determined that the Ea values were 41.95 and 41.06 kJ mol−1 for the samples pre-treated with blanching and a solution of gum Arabic (acacia gum), respectively. Kaleemullah and Kailappan [37] reported that the Ea and the Deff of samples dried at 50, 55, 60, and 65 °C using a thin layer drier were 37.76 and 3.78–7.10 × 10−9 m2 s−1, respectively. In the literature review, the moisture diffusivity constant was found to range between 5.86 × 10−11 and 1.16 × 10−8 m2 s−1 at 25–80 °C [38] and the Ea was found to range between 12.7 and 110.0 kJ mol−1 [39]. In the present study, the results are in conformity with the literature results which are between 5.86 × 10−11 and 1.16 × 10−8 m2 s−1 for Deff and between 12.7 and 110.0 kJ mol−1 for Ea.
Evaluation of the empirical equations
In the present study, dehydration behavior was modeled using thin layer drying models, as shown in Table 1. The obtained data were analyzed using SPSS software (Tables 3, 4). To determine the best model, the highest R2 value and the lowest RMSE and Chi-square values were selected. The best models selected were the logarithmic model [42] and the Wang and Singh model [40] (R2 0.995–0.997) for the USV drying; the Wang and Singh model (R2 0.994–1.000) for the USV control drying; the Page model [41] and the Wang and Singh model (R2 0.992–0.999) for the oven drying; and the Page model and the Wang and Singh model (R2 0.992–0.999) for the vacuum drying.
Doymaz and Pala [27] reported that the Page model was the best fit model for red pepper that was dried using hot air because the coefficient value was the highest for the Page model (it ranged between 0.998 and 0.999). Akpinar [29] stated that the best fit model for thin layer drying of red peppers was ‘the approximation of the diffusion model’ with the highest determination coefficient (0.9987). Sunil et al. [43] selected Wang and Singh model the best model for solar drying (bottom tray and top tray) and open sun drying of fenugreek leaves because of the highest value of R2 and the lowest values of SSE, MSE, and RMSE.
Rehydration ratio
The pepper samples that were dried to reach 10% (wb) final moisture were placed in distilled water at room temperature for 5 h to calculate the rehydration ratio. Figure 3 shows the changes in rehydration ratio with time. As expected, the rehydration ratio increased as the time increased. However, this increase in the rehydration ratio began to decrease as the experiment time increased. In general, the rehydration ratio of the USV-dried red peppers was similar to or slightly less than the ratio for the USV control drying. In USV drying, moisture inside the capillaries of the material being dehydrated creates microscopic channels [44]. Thanks to these microscopic channels, the drying rate can be increased when using ultrasonic waves, but the porous solid material could also become deformed. Therefore, the rehydration ratio could slightly decrease for red peppers dried using the USV drying.
Evaluation of bioactive properties
Total phenolic compound
The total phenolic compounds tend to decrease as the drying temperature increases. The total phenolic compound content of the dried samples was shown in Table 5. Total phenolic content of the fresh red pepper was 2.674 mg g−1. As seen in Table 5, the drying process caused a significant loss in total phenolic content, especially at high temperatures. Similar results were also reported from previously published studies [45, 46]. The USV drying provided to reduce drying time and drying temperature. As we know, phenolic compounds are sensitive to thermal process and can be easily degraded during drying process, especially at high temperature and long time. Same drying time was observed with the sample dried at USV drying at 65 °C and the samples dried with USV control at 75 °C. Phenolic content of the samples dried at lower temperature was higher than that of the sample dried at higher temperature. Therefore, bioactive content of the food can be preserved by USV drying method by reducing drying time.
DPPH free radical scavenging capability
The DPPH free radical scavenging capability of red peppers (mg-trolox-equivalent g-dry matter−1) is shown in Table 6. The antioxidant activity of the red peppers decreased as the temperature increased. The lowest value was obtained with the USV drying at 45 °C (8.305 ± 0.76 mg-trolox-equivalent g-dry matter−1). At 45 °C, no significant difference in the antioxidant activity was found between the fresh red peppers and the samples dried using the vacuum drying and between the samples dried using the USV control drying and the oven drying (P > 0.05). At 75 °C, the antioxidant activity of the samples dried using the USV drying and oven drying was not significantly different from the sample dried using the vacuum drying, but they were significantly different from the samples dried using the USV control drying (P < 0.05). However, there was no significant difference in the antioxidant activity of the samples dried using the vacuum drying and the USV control drying (P > 0.05).
In their study of dehydrating red peppers, Kim et al. [47] observed that drying conditions had a strong effect on antioxidant activity. They also stated that there was a correlation among antioxidant activity, the degradation of ascorbic acid, and the loss of phenolic content, and they found that red peppers were significantly affected by the drying conditions, such as drying time and temperature. Tannenbaum and Young [48] stated that ascorbic acid degradation was closely related to the non-enzymatic browning reaction. In our study, the antiradical activity of peppers was usually caused by the loss of phenolic components and vitamin C. Therefore, it is believed that the decrease in the antiradical activity of red peppers dried using USV drying is caused by the degradation of vitamin C and carotenoids in addition to the loss of phenolic components.
Color change
Table 7 shows the color values of the red pepper samples examined in this study. As seen, the increase or decrease in the color was not regularly obtained with changes in temperature or changes in the drying method. The ∆E* values ranged between 3.260 ± 0.654 and 8.224 ± 0.589, 2.757 ± 0.437 and 9.716 ± 0.551, 2.989 ± 0.086 and 7.277 ± 0.508, and 3.907 ± 0.645 and 7.544 ± 1066 for the USV drying, USV control drying, vacuum drying, and oven drying, respectively.
The chroma (C*) values provide more information about the spatial distributions of the colors than the direct values of the tristimulus colorimeter measurements [49]. The chroma values for the USV drying ranged from 41.021 ± 0.323 to 41.798 ± 0.322; the chroma values for the USV control drying ranged between 37.233 ± 5.092 and 44.981 ± 0.617. The chroma values for the vacuum drying and oven drying ranged between 36.862 ± 0.229 and 41.999 ± 0.118 and between 44.461 ± 0.032 and 38.107 ± 1.859, respectively.
Vega-Ga´lvez et al. [46] studied red peppers dried at 50, 60, 70, 80, and 90 °C and reported that the ∆E* values ranged from 5.78 ± 2.91 and 7.79 ± 2.16. Similar results were obtained by Miranda et al. [49]. The ∆E* values were expected to increase as the temperature increased since heat sensitive compounds, such as proteins and carbohydrates, are affected by high temperatures. The autoxidation of the carotenoids caused the loss of the red color in the pepper samples. In carotenoids, the degradation rate increases as the drying temperature and drying time increase [50]. Therefore, the USV drying provided shorter drying times than the USV control drying and that could result in less loss of the red color.
Total yeast and mold count
The total yeast and mold count in the fresh red pepper samples was 3.072 ± 0.154 log cfu g−1. At 75 °C, the total yeast and mold count of the samples dried using the USV drying was undetermined (< 2 log); however, the count of the samples dried using the USV control drying was calculated as 2.301 ± 0.151 log cfu g−1. As the temperature decreased, the decontamination of the yeast and mold began to diminish. At 45 °C, the total count calculated for the USV drying was 2.602 ± 0.138 log cfu g−1, while the total count was 2.903 ± 0.201 log cfu g−1 for the USV control drying. The results are shown in Table 8. At 45 and 55 °C, no significant difference in the total yeast and mold count was observed between the control samples and the samples dried using the USV drying and USV control drying (P > 0.05). At 65 °C, no significant difference in the total yeast and mold count was observed between the samples dried using the USV drying and USV control drying, but the counts in those samples were significantly different from the counts in the fresh red peppers (P < 0.05). The yeast and mold counts for the control, the samples dried using the USV drying, and the samples dried using the USV control drying were significantly different at 75 °C (P < 0.05). It is clear that USV drying processing contributes to the reduction in microbial loads because ultrasound is an effective nonthermal method used to prevent microbial growth and decrease biochemical reactions [51, 52].
Conclusions
In the present study, USV drying was performed as a novel technique for enhancing the dehydration rate and the quality of red peppers. USV drying was found to provide a shorten drying period and increased moisture diffusion; therefore, it could be possible for USV drying to become the first choice in food dehydration. It has the potential to be used to speed up heat and mass transfer in food drying, either for part of the process or for the entire process. Moreover, this study concluded that the best empirical models for describing the dehydration kinetics of red peppers for the drying methods were, generally, the Wang and Singh model, the Page model, and the logarithmic models. Ultrasound waves could significantly reduce microbial content in some USV applications. USV drying increased energy consumption and caused a loss of antiradical activity. In this present study, it also caused a slight decrease in the rehydration rate or it did not change the rehydration quality of the pepper samples.
Abbreviations
- D eff :
-
Effective moisture diffusivity/m2 s−1
- R 2 :
-
Correlation coefficient
- RMSE:
-
Root-mean-square error
- MR:
-
Moisture ratio/dry basis
- X t :
-
Moisture content at t/kg-water kg-dry matter−1
- X e :
-
Moisture content at equilibrium (kg-water kg-dry matter−1)
- X 0 :
-
Moisture content at initial/kg-water kg-dry matter−1
- χ 2 :
-
Chi-square
- L :
-
The thickness of red pepper samples/m
- D 0 :
-
The pre-exponential constant/m2 s−1
- E a :
-
The activation energy/kJ mol−1
- R :
-
Ideal gas constant/8.314 J mol−1 K−1
- T :
-
Drying temperature/K
- DR:
-
Drying rate/kg-water h−1m−2
- t:
-
Drying time/h
- h:
-
Hours
- X :
-
Moisture content/kg-water kg-dry matter−1
- L s :
-
Dry matter of the peppers/kg
- S :
-
Total surface area of all red peppers in one container/m2
- RR :
-
The rehydration ratio
- ΔE* :
-
The total color differences
- ΔL* :
-
Lightness difference/L *sample − L *standard
- Δa* :
-
Red/green difference/a *sample − a *standard
- Δb* :
-
Yellow/blue difference/b *sample − b *standard
- C* :
-
The chroma
- GAE:
-
Gallic acid equivalents/mg g-dry matter−1
- 0:
-
Initial
- e:
-
Equilibrium
- eff:
-
Effective
References
Duke JA. Handbook of medicinal herbs. 3rd ed. Boca Raton: CRC Press; 1986.
Arikan BC. The investigation of the acute effects of hot pepper (Capsicum annuum L.) on serum leptin and serum nitric oxide levels. M.Sc. Thesis. University of Kahramanmaras Sutcu Imam, Institute of Natural and Applied Sciences. Turkey: Kahramanmaras; 2004.
Kocayigit F. Investigation of drying characteristics of some vegetables. M.Sc. Thesis, T.C. Yildiz Technical University, Institute of Science and Technology, Department of Chemical Engineering, Turkey: Istanbul; 2010.
Reis FR. Vacuum drying for extending food shelf-life. Berlin: Springer; 2014.
Arevalo-Pinedo A, Murr FEX. Kinetics of vacuum drying of pumpkin (Cucurbita maxima): modeling with shrinkage. J Food Eng. 2006;76:562–7.
Wu L, Orikasa T, Ogawa Y, Tagawa A. Vacuum drying characteristics of eggplants. J Food Eng. 2007;83:422–9.
Chemat F, Chemat MKK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–35.
Ercan SŞ, Soysal Ç. Use of ultrasound in food preservation. Nat Sci. 2013;5:5–13.
Garcia-Noguera J, Oliveira FIP, Gallão MI, Weller CL, Rodrigues S, Fernandes FAN. Ultrasound-assisted osmotic dehydration of strawberries: effect of pretreatment time and ultrasonic frequency. Dry Technol. 2010;28:294–303.
Sabarez HT, Gallego-Juarez JA, Riera E. Ultrasonic-assisted convective drying of apple slices. Dry Technol Int J. 2012;30(9):989–97.
He Z, Yang F, Peng Y, Yi S. Ultrasound assisted vacuum drying of wood: effects on drying time and product quality. BioResources. 2013;8(1):855–63.
Baslar M, Kilicli M, Toker O, Sagdic O, Arici M. Ultrasonic vacuum drying technique as a novel process for shortening the drying period for beef and chicken meats. Innov Food Sci Emerg Technol. 2014;26:182–90.
Baslar M, Kilicli M, Yalinkilic B. Dehydration kinetics of salmon and trout fillets using ultrasonic vacuum drying as a novel technique. Ultrason Sonochem. 2015;27:495–502.
Tekin ZH, Baslar M, Karasu S, Kilicli M. Dehydration of green beans using ultrasound-assisted vacuum drying as a novel technique: drying kinetics and quality parameters. J Food Process Preserv. 2017. https://doi.org/10.1111/jfpp.13227.
He Z, Zhao Z, Yang F, Yi S. Effect of ultrasound pretreatment on wood prior to vacuum drying. Maderas-Ciencia y tecnología. 2014;16(4):395–402.
Kingsly ARP, Singh DB. Drying kinetics of pomegranate arils. J Food Eng. 2007;79:741–4.
Doymaz I. Experimental study on drying of pear slices in a convective dryer. Int J Food Sci Technol. 2013;48:1909–15.
Crank J. The mathematical of diffusion. 2nd ed. London: Oxford University Press; 1975.
Geankoplis CJ. Transport processes and unit operations. 3rd ed. Upper Saddle River: Prentice-Hall International; 1993.
Cemeroglu B. Food analyses. 2nd ed. Ankara: Food Technology Association Publication; 2011.
Sahin S, Sumnu SG. Electromagnetic properties. In: Heldman DR, editor. Physical properties of foods. San Marcos: AVI Pub. Co; 2006. p. 167–71.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–58.
Ozturk I, Karaman S, Baslar M, Cam M, Caliskan O, Sagdic O, Yalcin H. Aroma, sugar and anthocyanin profile of fruit and seed of mahlab (Prunus mahaleb L.): optimization of bioactive compounds extraction by simplex lattice mixture design. Food Anal Methods. 2014;7(4):761–73.
Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–6.
AOAC, VA: Association of official analytical chemists, 15th ed., Arlington. Reference to a chapter in an edited book: Official Methods of Analysis; 1990.
Nasiroglu S. The use of infrared drying technology in the drying of red peppers, apples and gypsum. M.S. Thesis. T.C. Canakkale Onsekiz Mart University. Institute of Science and Technology. Department of Agricultural Machinery. Turkey: Canakkale; 2007.
Doymaz I, Pala M. Hot-air drying characteristics of red pepper. J Food Eng. 2002;55:331–5.
Akpinar EK. Energy and exergy analyses of drying of red pepper slices in a convective type dryer. Int Commun Heat Mass Transf. 2004;1(8):1165–76.
Akpinar EK, Bicer Y, Yildiz C. Thin layer drying of red pepper. J Food Eng. 2003;59:99–104.
Mulet A, Berna A, Borras M, Pinaga F. Effect of air flow rate on carrot drying. Dry Technol. 1987;5:245–58.
Labuza TP, Simon IB. Surface tension effects during drying. Air drying of apple slices. Food Technol. 1970;24:712–5.
Schossler K, Jager H, Knorr D. Novel contact ultrasound system for the accelerated freeze-drying of vegetables. Innov Food Sci Emerg Technol. 2012;16:113–20.
Doymaz I. Thin-layer drying behaviour of mint leaves. J Food Eng. 2006;74(3):370–5.
Huang YW, Chen MQ. Thin-layer isothermal drying kinetics of municipal sewage sludge based on two falling rate stages during hot-air-forced convection. J Therm Anal Calorim. 2017;129:567–75.
Kaymak-Ertekin F. Drying and rehydrating kinetics of green and red peppers. J Food Sci. 2002;67(1):168–75.
Gupta P, Ahmed J, Shivhare US, Raghavan GSV. Drying characteristics of red chilli. Dry Technol. 2002;20:1975–87.
Kaleemullah S, Kailappan R. Modelling of thin-layer drying kinetics of red chillies. J Food Eng. 2006;76:531–7.
Panagiotou NM, Krokida MK, Maroulis ZB, Saravacos GD. Moisture diffusivity: literature data compilation for foodstuffs. Int J Food Prop. 2004;7(2):273–99.
Zogzas NP, Maroulis ZB, Marinos-Kouris D. Moisture diffusivity data compilation in foodstuffs. Dry Technol. 1996;14:2225–53.
Wang CY, Singh RP. A single layer drying equation for rough rice. Washington: ASAE; 1978.
Page G. Factors influencing the maximum rates of air-drying shelled corn in thin layer. M.S. Thesis. Purdue University West Lafayette. Indiana: USA; 1949.
Yaldız O, Ertekin C. Thin layer solar drying of some vegetables. Dry Technol. 2001;19(3):83–97.
Sunil V, Sharma N. Experimental investigation of the performance of an indirect mode natural convection solar dryer for drying fenugreek leaves. J Therm Anal Calorim. 2014;118:523–31.
De la Fuente-Blanco S, Riera-Franco de Sarabia E, Acosta-Aparicio VM, Blanco-Blanco A, Gallego-Juárez JA. Food drying process by power ultrasound. Ultrasonics. 2006;44(s1):523–7.
Ozgur M, Ozcan T, Akpinar-Bayizit A, Yilmaz-Ersan L. Functional compounds and antioxidant properties of dried green and red peppers. Afr J Agric Res. 2011;6(25):5638–44.
Vega-Gálvez A, Scala KD, Rodriguez K, Lemus-Mondaca R, Miranda M, López J, Perez-Won M. Effect of air drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. Var. Hungarian). Food Chem. 2009;117:647–53.
Kim S, Lee KW, Park J, Lee HJ, Hwang IK. Effect of drying in antioxidant activity and changes of ascorbic acid and color by different drying and storage in Korean red pepper (Capsicum annuum, L.). Int J Food Sci Technol. 2006;41:90–5.
Tannenbaum SR, Young VR. Vitamins and minerals. In: Fennema OR, editor. Food chemistry. New York: Elsevier; 1985. p. 488–93.
Miranda M, Maureira H, Rodriguez K, Vega-Gálvez A. Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of aloe vera (Aloe Barbadensis Miller) gel. J Food Eng. 2009;91(2):297–304.
Ramesh MN, Wolf W, Tevini D, Jung G. Influence of processing parameters on the drying of spice paprika. J Food Eng. 2001;49:63–72.
Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Lei S, Zhang X, Zeng X. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem. 2013;20:1182–7.
Baslar M, Ertugay MF. The effect of ultrasound and photosonication treatment on polyphenoloxidase (PPO) activity, total phenolic component and color of apple juice. Int J Food Sci Technol. 2013;48:886–92.
Lewis WK. The rate of drying of solid materials”. Ind Eng Chem. 1921;13:427–32.
Henderson SM, Pabis S. Grain drying theory. I. Temperature effect on drying coefficient. J Agric Engrg Res. 1961;6:169–74.
Henderson SM. Progress in developing the thin layer drying equation. Trans ASAE. 1974;17:1167–72.
Wang CY, Singh RP. A single layer drying equation for rough rice. ASAE. 1978;78:3001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tekin, Z.H., Baslar, M. The effect of ultrasound-assisted vacuum drying on the drying rate and quality of red peppers. J Therm Anal Calorim 132, 1131–1143 (2018). https://doi.org/10.1007/s10973-018-6991-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6991-7