Abstract
In this paper, the effects of three coexisting ion systems (Cs, Sr), (Cr, Sr) and (Cs, Cr, Sr) on the adsorption of Sr(II) by irradiated Saccharomyces cerevisiae in solution were investigated. The three systems generally inhibited the adsorption of Sr(II) by yeast. The effects of several environmental factors on yeast adsorption of Sr(II) in coexisting ionic solutions were determined. Three adsorption models, Langmuir, Freundlich and Linear, were used to fit the experimental data. FTIR results showed that the mechanism by which Cs(I) and Cr(III) inhibited the adsorption of Sr(II) was related to the functional groups on the cell wall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a kind of competitive clean energy, nuclear energy can replace fossil fuels on a large scale, and it can assist in realizing energy structure adjustment and sustainable development. But new opportunities also bring new challenges. We need to further protect human health and protect the environment from these nuclear products and their related wastes [1, 2]. The Fukushima nuclear accident occurred on March 11, 2011. The radionuclides produced in this accident caused serious pollution to the marine environment, and it was also one of the largest nuclear accidents in human history. A large number of radioactive pollutants, including 134Cs, 137Cs, 90Sr, 14C, etc. are released into the ocean [3]. The Japanese government announced in April this year that the radioactive wastewater generated after the nuclear accident would be discharged into the ocean, making the treatment of radioactive wastewater a major problem. For radioactive waste liquid, the treatment method is usually to reduce the volume of radioactive waste liquid by traditional methods, solidify the radionuclides as much as possible, and then cut and seal the filtered solid waste[4]. Studies have shown that compared with traditional treatment methods, biosorption has the advantages of low cost, high efficiency, minimization of chemical sludge and biological sludge, renewable biosorbents, and recyclable pollutants [5]. Some scholars have studied the biosorption of the main radionuclides 233U, 241Am, 144Ce, 137Cs, and 90Sr in radioactive waste, and found that biosorption is a more suitable technology for removing radionuclides in the water environment [6, 7]. Therefore, it has been favored by more and more scholars in the past ten years [8,9,10,11].
Strontium (90Sr, half-life of 28.78 years) is an important component of radioactive wastewater and one of the most dangerous radioactive pollutants in the environment [12, 13]. The metabolism mode of strontium in the body is similar to that of calcium, which can cause severe damage to the bone marrow hematopoietic tissue and induce aplastic anemia and leukemia[14]. As an engineered strain, Saccharomyces cerevisiae has been well applied in wastewater treatment [15]. We selected Saccharomyces cerevisiae previously induced by cyclic irradiation and high strontium concentrations as the biosorbent [16].
Since there is rarely only one toxic metal in wastewater, the research involving the influence of two or more coexisting ions on adsorption has more important significance [17]. Studies have shown that coexisting ions will compete with target ions, reducing the adsorption capacity of target ions and reducing the biosorption capacity of the adsorbent [18,19,20]. In our previous study, Cs(I) can antagonize the adsorption of strontium ions by Saccharomyces cerevisiae [21]. Cs(I) and Cr(III) are important components in low- and medium-level radioactive waste. Studying their influence mechanism on the adsorption of strontium ions by irradiated Saccharomyces cerevisiae in a more complex ternary ion system will help lay the foundation for their application in the treatment of radioactive wastewater. Different pH and initial concentrations of coexisting ions in the waste liquid will affect the adsorption capacity of the adsorbent in the presence of ions. Studying different pH and initial concentrations of coexisting ions will also reveal the biosorption behavior in the case of the coexistence of ions [22, 23]. Fourier transform infrared spectroscopy can be used to identify the effect of functional groups on macromolecules and further reveal the mechanism of competitive adsorption [18].
Therefore, the coexisting ions Cs(I) and Cr(III) are selected in this paper, and different pH and initial concentrations of coexisting ions are set. Binary and ternary coexisting ion systems (Cs, Sr), (Cr, Sr), (Cs, Cr, Sr) are set. The effects of Cs(I) and Cr(III) on the adsorption of strontium ions by irradiated Saccharomyces cerevisiae were observed, and the mechanism of their effect on strontium adsorption was deeply studied by FTIR analysis.
Materials and methods
Yeast and reagents
Saccharomyces cerevisiae (CICC 30,225) cells were obtained from the China Center of Industrial Culture Collection (CICC). In our previous studies [16], we had used the cyclic irradiation method to culturing an irradiated Saccharomyces cerevisiae: Y-7. In this paper, we will use Y-7 as living biosorbents and do our further researches. Yeast were cultured in liquid media containing glucose (50.0 g), yeast extract (0.5 g), Na2HPO4 (0.5 g), (NH4)2SO4 (1.0 g), urea (1.0 g), and 1500 ml distilled water. The strontium solution was prepared using Sr(NO3)2. Adjust the pH value of the adsorption system with 1.0 mol L−1 HCl and NaOH solution. Use Sr(NO3)2, CsCl, and Cr(NO3)3·9H2O to prepare a culture medium containing Sr(II), Cs(I), and Cr(III). All chemicals were of analytical grade unless otherwise stated.
Biosorption experiments
We selected the coexisting ion concentration gradients of 100 mg L−1, 200 mg L−1, and 300 mg L−1 to study the influence of Cr(III) and Cs(I) on the adsorption of strontium ions by Y-7. We have studied the adsorption conditions of Y-7 in the early stage, and the results show that the best adsorption conditions for Sr(II) adsorption by living body Y-7 are pH = 7, the adsorption time is 30 h, and the adsorption temperature is 32 ℃ [16].
The effect of coexisting metal ions at different concentrations on the adsorption of strontium on Y-7 at pH = 7 was studied. Choose the Cs(I) concentration as 100 mg L−1, 200 mg L−1, 300 mg L−1, and the Sr(II) concentration as 50 mg L−1, 100 mg L−1, 250 mg L−1, 300 mg L−1, 400 mg L−1, 600 mg L−1, 1000 mg L−1, three systems (100Cs, Sr), (200Cs, Sr), (300Cs, Sr) coexisting ion culture medium were obtained. The configuration of the binary coexisting ion system (Cr, Sr) solution and the ternary coexisting ion system (Cs, Cr, Sr) solution are the same as above, and the coexisting culture medium: (100Cr, Sr), (200Cr, Sr), (300Cr, Sr) and (100Cs, 100Cr, Sr), (200Cs, 200Cr, Sr), (300Cs, 300Cr, Sr). The effect of coexisting metal ions at the same concentration under different pH conditions on the adsorption of strontium on Y-7 was studied. The pH is 3, 4, 5, 6, 7, and 8, respectively. The concentrations of Cs(I) and Cr(III) were both 200 mg L−1, and coexisting ion culture solutions (200Cs, Sr), (200Cr, Sr) and (200Cs, 200Cr, Sr) were obtained.
The logarithmic phase Y-7 (100 mg dry weight) was added to 10 mL of liquid medium containing different ion concentrations, and continue shaking culture at 150 rpm for 30 h at 32 °C. The supernatant obtained after centrifugation was used to measure its strontium ion concentration using an atomic absorption flame spectrophotometer (AAS).
The adsorption amount q is calculated using the following formula.
q is the adsorption capacity of Y-7 to adsorb strontium (mg g−1), C0 is the initial concentration of strontium ions in the culture solution (mg L−1), Ce is the equilibrium concentration of strontium ions in the culture solution (mg L−1), V is the volume of the adsorption solution during the experiment (L), m is the dry weight of Y-7 (g).
Isothermal biosorption
Appropriate models play an important role in analyzing experimental data and understanding the mechanism of the adsorption process. Models can also help predict operating conditions and optimize processes [24, 25]. This paper uses Langmuir, Freundlich, and Linear models to fit and analyze the research results.
The Langmuir model is the most commonly used adsorption isotherm. It is assumed that the adsorption capacity of the solid phase material is the same everywhere on the surface, and the single-layer adsorption is completely independent before the adsorbed material. Its expression is:
qe is the adsorption amount of strontium by Y-7 (mg g−1), Ce is the equilibrium concentration of strontium ions in the solution (mg L−1), qm is the maximum biosorption amount of strontium by Y-7 (mg g−1), kL is the affinity constant of Langmuir.
The Freundlich isotherm does not indicate the limited absorption capacity of the adsorbent, so it can only be reasonably applied in the low to medium concentration range. Its expression is:
KF (Lm g−1) is a parameter of the amount of biosorption, n is the Freundlich adsorption constant.
The linear model assumes that the adsorbent is a fluid or semi-fluid substance without a fixed form, and the adsorption sites are also infinite. Its expression is:
Kp is the linear distribution coefficient.
To compare the degree of fit of Langmuir, Freundlich, and Linear models to the fitting results, use the following formula to calculate the adjustment coefficient R2adj.
N is the number of experimental data points, m is the number of fitting model parameters.
Fourier transformed infrared spectrometry(FTIR)
The bacteria after adsorption of each coexisting ion system under the conditions of strontium ion concentration of 250 mg L−1 and pH = 7 were used for FTIR analysis. In the three coexisting ion systems, the concentrations of Cs(I) and Cr(III) are both 100 mg L−1, 200 mg L−1, and 300 mg L−1. After the cell sample is cultured to the logarithmic phase, vacuum dry for 6 h to reduce the H2O and CO2 on the surface. After being evenly mixed with KBr, use a tablet press to press it into tablets. The flake sample was used to check the group composition on the surface of the cell sample at 900–4000 cm−1 using a Fourier infrared spectrometer (NEXUS87, USA).
Results and discussion
Effect of different coexisting metal ions on the adsorption of strontium ions by Y-7 at the same pH
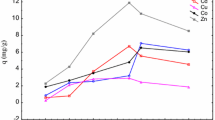
The effects of binary and ternary coexisting ion systems on the adsorption of strontium ions by Y-7 were studied. The results show (Figs. 1, 2 and 3) Cs, Cr and (Cs, Cr) inhibit Y-7 from adsorbing strontium ions when the PH is 7, and the degree of inhibition is (Cs, Cr) > Cr > Cs. The inhibition degree of Cs, Cr and (Cs, Cr) concentration on adsorption was 300 mg L−1 > 200 mg L−1 > 100 mg L−1, 100 mg L−1 > 200 mg L−1 > 300 mg L−1, 100 mg L−1 > 200 mg L−1 > 300 mg L−1. The results show that the effect of coexisting ion concentration on the adsorption of (Cs, Sr) system is opposite to that of (Cr, Sr) and (Cs, Cr, Sr) system, which may be due to the different inhibition modes of Cs(I) and Cr(III) on the adsorption of strontium ions by Y-7.
Langmuir, Freundlich and Linear models were used to fit the strontium adsorption behavior of Y-7. The calculation results of R2adj show (Tables 1, 2 and 3) that the R2adj value of the Langmuir model is generally greater than the R2adj value of the Freundlich and Linear models under the same conditions. In this experiment, the Langmuir model has the best fitting effect, and the Linear model has the worst effect. It is suggested that the inhibitory effect of coexisting ions on the adsorption of strontium on Y-7 may be mainly due to the competition for adsorption sites on the bacterial surface, and the adsorption sites on the Y-7 surface are limited [18, 26].
Compare the adsorption capacity of Y-7 on strontium ions with different concentrations of Cs, Cr and (Cs, Cr) at different initial concentrations of strontium ions (Fig. 4). From the different initial concentrations of strontium ions, the ratio of the concentration of coexisting ions to the initial concentration of strontium ions was introduced, and the results showed that the inhibitory effect of coexisting ions on Sr(II) adsorption was related to the ratio of ion concentration. For Cs, the larger the ratio, the stronger the inhibitory effect; for Cr and (Cs, Cr), the smaller the ratio, the stronger the inhibitory effect (Table 4). It is speculated that both Cs(I) and Cr(III) will compete with Sr(II) for adsorption, which will reduce the number of strontium ions adsorbed by Y-7 [27]. As the concentration of Cs(I) increases, the amount of Cs(I) that competes with Sr(II) for adsorption increases, thus enhancing the ability of Cs(I) to compete for adsorption sites. When Sr(II) coexists with Cs(I) and Cr(III), the inhibitory effect of low-concentration coexisting ions is the strongest. But with the increase of the concentration of coexisting ions, Cr(OH)3 precipitates and the inhibitory effect is weakened.
Effect of coexisting metal ions at different pH on the adsorption of strontium ions by Y-7
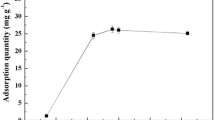
Y-7 has the highest adsorption capacity for strontium ions in the single-ion system when the pH is 7 (Fig. 5). In the single-ion system of Y-7 adsorbing strontium ions, when pH > 7, the adsorption capacity decreases with the increase of pH. When pH < 7, the adsorption capacity increases with the increase of pH. (Cs, Sr), (Cr, Sr) and (Cs, Cr, Sr) three systems inhibit Y-7 from adsorbing strontium ions in acidic or alkaline environments and have different effects on adsorption under different acid–base conditions. In the (Cs, Sr) system, when pH > 7, the inhibitory effect of Cs increases with the increase of pH. When pH < 7, the inhibitory effect of Cs weakens with the increase of pH. In the (Cr, Sr) system, when pH > 7 or pH < 7, the adsorption capacity decreases with the increase of pH, and the inhibitory effect of Cr increases with the increase of pH. In the (Cs, Cr, Sr) system, when pH > 7, the adsorption capacity increases with the increase of pH, and the inhibitory effect of (Cs, Cr) decreases with the increase of pH. The inhibition was greater at pH 3 and 6, but decreased at pH 4 and 5.
The Langmuir adsorption isotherm of strontium ion on Y-7 was fitted for the single ion system of strontium ion and 200 mg L−1 (Cs, Sr), (Cr, Sr) and (Cs, Cr, Sr) at different pH values. R2 of the three systems is mostly in the range of 0.7–0.9 (Table 5). Therefore, under the conditions of different pH and coexisting ions, it is mainly monolayer adsorption.
Yeast adsorbs metal ions when the pH is low, the metal ions will compete with a large number of hydronium ions (H3O+) in the water for adsorption sites to reduce the adsorption rate of metal ions. At higher pH, the negative charge on the cell surface increases and more metal cations can be bound. But if the pH is too high, the activity of biological macromolecules is inhibited. Moreover, heavy metal ions are easily hydrolyzed into oxides and hydroxides, resulting in insoluble solids.
Cs(I) will not precipitate under different pH values, nor will it form insoluble complexes with common chemicals (such as sulfates and halides) [28]. Cr(III) is an intermediate transition metal ion, and it is dominated by cations at pH < 4 and hydrolyzes and forms complexes with hydroxyl groups at pH > 4. The adsorption of Cs(I) is related to the electrostatic attraction between the cell surface and Cs(I). Low pH will increase the electrostatic repulsion on the cell surface and reduce the amount of Cs(I) adsorption. As the pH increases, the negative charge on the cell surface increases, and the amount of Cs(I) adsorption increases. However, it reaches the maximum value at pH = 6, and a too-high pH will inactivate the groups on the surface of yeast cells and affect the adsorption of Cs(I) [29]. The adsorption rate of the strain on Cr(III) has a wide pH range. When the pH is in the range of 4–7, the adsorption capacity increases with the increase of pH [30].
In the (Cs, Sr) system, although Cs(I) will increase its adsorption to the cell surface as the pH increases, at the same time the adsorption capacity of strontium ions on Y-7 is also improved. Therefore, the results of this study show that when pH > 7, the ability of Cs(I) to compete for adsorption sites increases with the increase of pH. When pH < 7, the competitive ability of Cs(I) for adsorption sites decreases with the increase of pH. In the (Cr, Sr) system, the inhibitory effect of Cr(III) increases with the increase of pH, so the competitive adsorption capacity of Cr(III) increases with the increase of pH. In the (Cs, Cr, Sr) system, because Cs(I) and Cr(III) have different abilities to adsorb strontium on Y-7 under different pH conditions, there is no obvious rule. However, compared with the (Cr, Sr) system, the addition of Cs(I) will weaken the inhibitory effect of Cr(III) on Sr(II) adsorption.
FTIR spectrum analysis
We conducted infrared spectroscopy to examine the effect of coexisting ions on the functional groups in Y-7 and further analyzed the mechanism of coexisting ions affecting the adsorption of strontium ions. Representative spectral results are shown in Fig. 6 and Table 6.
Y-7 infrared spectra of different coexisting systems after adsorption equilibrium (Sr(II) concentration is 250 mg L−1). A: A1- blank, A2- Sr(II) 250 mg L−1; B: B1- Cs(I) 100 mg L−1, B2- Cs(I) 200 mg L−1, B3- Cs(I) 300 mg L−1; C: C1- Cr(III) 100 mg L−1, C2- Cr(III) 200 mg L−1, C3- Cr(III) 300 mg L−1; D: D1-(Cs, Cr) 100 mg L−1, D2- (Cs, Cr) 200 mg L−1, D3- (Cs, Cr) 300 mg L−1;
I: The peak at 3407 cm−1 belongs to the associated -OH and N–H stretching vibration peaks[31]. After Y-7 adsorbed Sr(II) in the Sr single-ion system, its absorption peak at 3407 cm-1 did not shift significantly, indicating that Sr did not bind to this functional group. The peak at 1650 cm−1 is the amide group (O = CN-H) I band, which is the stretching vibration peak of C = O[32]. After Y-7 adsorbed Sr(II), the peak at 1650 cm−1 did not shift significantly, indicating that there was no Sr binding to the functional group. The peak at 1383 cm−1 is the amide III band, which is the C-N stretching vibration peak[32]. After Y-7 adsorbed Sr(II) in the single-ion system, there was no obvious peak near 1382 cm−1.
II The peak at 1056 cm−1 is the stretching vibration peak of C–OH in the polysaccharide or the stretching vibration peak of P-O-C. In the single ion system, Y-7 adsorbed Sr(II) from 1056 to 1082 cm−1, indicating that this group participated in the adsorption of Sr(II). In the (Cs, Sr) binary system, the concentration of Cs(I) added is 100 mg L−1, 200 mg L-1, and 300 mg L−1. The peaks at 1056 cm−1 all move to 1080 cm−1, but the intensity of the peak changes with the concentration. It shows that in a binary system, the concentration of the group that binds to the part of Cs(I) will affect the strength of the group. In the (Cr, Sr) binary system, the concentration of Cr(III) added is 100 mg L-1, 200 mg L−1, and 300 mg L−1. The peaks at 1056 cm−1 all moved to 1033 cm−1, and the intensity of the peak changes with the change of concentration. It shows that the group will bind to a certain amount of Cr(III), and the concentration of Cr(III) will affect the strength of the group. The changing trend of the (Cs, Cr, Sr) ternary system is not significantly different from (Cr, Sr), but the peak intensity is weakened. Through the above analysis, it is found that C–OH and P-O-C are involved in the combination of Sr(II)[16]. When Cs(I) and Cr(III) are added separately, the coexisting ions compete for the adsorption sites of the group, and their concentration affects the bonding strength of the group.
III: The peak at 671 cm−1 is the absorption frequency region of some heavy atom stretching vibration peaks and some deformation vibrations[33]. After Y-7 adsorbed Sr(II) in the single ion system, 671 cm−1 shifted to 610 cm−1. In the (Cs, Sr) binary system, adding different concentrations of Cs(I) does not significantly shift the peak, but the intensity of the peak changes with the concentration. In the (Cr, Sr) binary system, when the concentration of Cr(III) reaches 300 mg L−1, it shifts to 603 cm−1, and the intensity of the peak changes with the concentration. In the (Cs, Cr, Sr) system, when the concentration of (Cs, Cr) reaches 200 mg L−1, it shifts to 602 cm−1, and then the concentration increases to 300 mg L−1 without any significant shift. This shows that there is a group that binds to Sr(II), but it is not clear what kind of atom the bond forms. When Cr(III) and Cs(I) are added separately, the concentration of coexisting ions will affect the adsorption of the group to Sr(II), and even Cr(III) will compete for the adsorption site at high concentrations. However, when Cr(III) and Cs(I) coexist, Cr(III) and Cs(I) may interact and affect the combination of Cr(III) and this group.
FTIR results showed that in different coexisting ion systems, the presence of coexisting ions caused the peak positions of functional groups (-OH, N–H, C = O, C–OH and P-O-C) on the yeast cell wall to shift. Cr(III) and Cs(I) will compete with the adsorption site of the Sr(II) binding group, which weakens the binding strength of the group and Sr(II). The difference in the concentration of coexisting ions will affect the strength of the interaction between metal ions and functional groups. Yeast’s adsorption of multiple metal ions makes the changes in functional groups more complicated.
Conclusion
The coexisting ions Cs, Cr and (Cs, Cr) all inhibited Y-7 from adsorbing strontium ions, and the degree of inhibition was (Cs, Cr) > Cr > Cs. The inhibitory effect of coexisting ions on the adsorption of strontium is related to the ratio of the concentration of coexisting ions to the initial concentration of strontium ions. Changing the pH of the adsorption environment shows that the coexisting ions have different effects on Y-7's adsorption of strontium ions under different acid–base conditions. The Langmuir model has the best fitting effect on adsorption, suggesting that under the influence of coexisting ions, the adsorption of strontium ions in living body Y-7 is mainly monolayer adsorption. FTIR results showed that the coexisting ions shifted the peak positions of the functional groups (C–OH and P-O-C) on the yeast cell wall, and the results were related to the types of coexisting ions and the concentration of coexisting ions.
References
Hu Q-H, Weng J-Q, Wang J (2010) Sources of anthropogenic radionuclides in the environment: a review. J Environ Radioact 101:426–437. https://doi.org/10.1016/j.jenvrad.2008.08.004
Tanha MR, Hanafiah M, Khalid F, Storai M, Hoeschen C (2019) Current status of radioactive waste management in Afghanistan. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06974-z
Men W, Deng F, He J, Yu W, Wang F, Li Y, Lin F, Lin J, Lin L, Zhang Y, Yu X (2017) Radioactive impacts on nekton species in the Northwest Pacific and humans more than one year after the Fukushima nuclear accident. Ecotoxicol Environ Saf 144:601–610. https://doi.org/10.1016/j.ecoenv.2017.06.042
Liu H, Wang J (2013) Treatment of radioactive wastewater using direct contact membrane distillation. J Hazard Mater 261C:307–315. https://doi.org/10.1016/j.jhazmat.2013.07.045
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300
Parekh NR, Poskitt J, Dodd BA, Potter E, Sanchez A (2008) Soil microorganisms determine the sorption of radionuclides within organic soil systems. J Environ Radioact 99:841–852. https://doi.org/10.1016/j.jenvrad.2007.10.017
Sen K, Sinha P, Lahiri S (2008) Immobilization of long-lived radionuclides 152,154Eu by selective bioaccumulation in Saccharomyces cerevisiae from a synthetic mixture of 152,154Eu, 137Cs and 60Co. Biochem Eng J 40:363–367. https://doi.org/10.1016/j.bej.2008.01.005
Zinicovscaia I, Safonov A, Zelenina D, Ershova Y, Boldyrev K (2020) Evaluation of biosorption and bioaccumulation capacity of cyanobacteria Arthrospira (spirulina) platensis for radionuclides. Algal Res 51:102075. https://doi.org/10.1016/j.algal.2020.102075
Hu J, Lv Y, Cui W, Chen W, Li S (2019) Study on treatment of uranium-containing wastewater by biosorption. IOP Conf. Series: Earth and Environ Sci 330:032029. https://doi.org/10.1088/1755-1315/330/3/032029
Kim T, Hong J, Park HM, Lee U, Lee S-Y (2020) Decontamination of low-level contaminated water from radioactive cesium and cobalt using microalgae. J Radioanal Nucl Chem 323:1–6. https://doi.org/10.1007/s10967-019-07008-4
Fomina M, Gadd G (2014) Biosorption: current perspectives on concept, definition and application. Biores Technol. https://doi.org/10.1016/j.biortech.2013.12.102
Niedrée B, Berns A, Vereecken H, Burauel P (2012) Do Chernobyl-like contaminations with (137)Cs and (90)Sr affect the microbial community, the fungal biomass and the composition of soil organic matter in soil? J Environ Radioact 118C:21–29. https://doi.org/10.1016/j.jenvrad.2012.11.007
Bhatt N, Bagla H (2011) Biosorption of radiotoxic 90Sr by green adsorbent: dry cow dung powder. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-011-1539-3
Shin J, Lee Y-G, Kwak J, Kim S, Lee S-H, Park Y, Lee S-D, Chon K (2021) Adsorption of radioactive strontium by pristine and magnetic biochars derived from spent coffee grounds. J Environ Chem Eng 9:105119. https://doi.org/10.1016/j.jece.2021.105119
Faghihian H, Peyvandi S (2012) Adsorption isotherm for uranyl biosorption by Saccharomyces cerevisiae biomass. J Radioanal Nucl Chem 293:463–468. https://doi.org/10.1007/s10967-012-1814-y
Qiu L, Feng J, Dai Y, Chang S (2017) Biosorption of the strontium ion by irradiated Saccharomyces cerevisiae under culture conditions. J Environ Radioact 172:52–62. https://doi.org/10.1016/j.jenvrad.2017.03.007
Dai S, Yang S, Zhou D (2012) The influence of coexisting ions on adsorption-flotation of Pb2+ in water by gordona amarae. Adv Mater Res 433–440:183–187. https://doi.org/10.4028/www.scientific.net/AMR.433-440.183
Ni B-J, Huang Q-S, Wang C, Ni T-Y, Sun J, Wei W (2018) Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.12.053
Deng l, Zhu X, Su Y, Su H, Wang X (2008) Biosorption and desorption of Cd2+ from wastewater by dehydrated shreds of Cladophora fascicularis. Chin J Oceanol Limnol 26:45–49. https://doi.org/10.1007/s00343-008-0045-0
Imran M, Anwar K, Akram M, Shah G, Ahmad I, Shah N, Khan ZUH, Rashid M, Akhtar M, Ahmad S, Nawaz M, Schotting R (2019) Biosorption of Pb(II) from contaminated water onto Moringa oleifera biomass: kinetics and equilibrium studies. Int J Phytorem 21:1–13. https://doi.org/10.1080/15226514.2019.1566880
Tan Y, Feng J, Qiu L, Zhao Z, Zhang X, Zhang H (2017) The adsorption of Sr(II) and Cs(I) ions by irradiated Saccharomyces cerevisiae. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5598-y
Aranda-García E, Chávez-Camarillo G, Cristiani-Urbina E (2020) Effect of Ionic strength and coexisting ions on the biosorption of Divalent nickel by the acorn shell of the oak quercus crassipes Humb. & Bonpl. Processes 8:1229. https://doi.org/10.3390/pr8101229
Huang Y, Li M, Yang Y, Zeng Q, Praburaman L, Hu L, Zhong H, He Z (2020) Sulfobacillus thermosulfidooxidans: an acidophile isolated from acid hot spring for the biosorption of heavy metal ions. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-020-02669-1
Limousin G, Gaudet J-P, Charlet L, Szenknect S, Barthès V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275. https://doi.org/10.1016/j.apgeochem.2006.09.010
Ahalya N, Ramachandra T, Kanamadi R (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Zinicovscaia I, Yushin N, Abdusamadzoda D, Grozdov D, Shvetsova M (2020) Efficient removal of metals from synthetic and real galvanic zinc-containing effluents by Brewer’s yeast saccharomyces cerevisiae. Materials 13:3624. https://doi.org/10.3390/ma13163624
Göksungur MY, Uren S, Güvenç U (2005) Biosorption of cadmium and lead ions by ethanol treated waste Baker’s yeast biomass. Biores Technol 96:103–109. https://doi.org/10.1016/j.biortech.2003.04.002
Wang J, Zhuang S (2019) Removal of cesium ions from aqueous solutions using various separation technologies. Rev Environ Sci Bio/Technol 18:231–269
Ngwenya N, Chirwa E (2010) Single and binary component sorption of the fission products Sr2+, Cs+ and Co2+ from aqueous solutions onto sulphate reducing bacteria. Miner Eng 23:463–470. https://doi.org/10.1016/j.mineng.2009.11.006
Blázquez G, Hernáinz F, Calero M, Tenorio G (2009) The effect of pH on the biosorption of Cr (III) and Cr (VI) with olive stone. Chem Eng J 148:473–479. https://doi.org/10.1016/j.cej.2008.09.026
Schmitt J, Flemming H-C (1998) FTIR spectroscopy in microbial and material analysis. Int Biodeterior Biodegrad 41:1–11. https://doi.org/10.1016/S0964-8305(98)80002-4
Qiu L, Feng J, Dai Y, Chang S (2018) Biosorption of strontium ions from simulated high-level liquid waste by living Saccharomyces cerevisiae. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-1662-6
Kuppusamy V, Palanivelu K, Manickam V (2006) Biosorption of Copper(II) and Cobalt(II) from Aqueous solution by crab shell particles. Biores Technol 97:1411–1419. https://doi.org/10.1016/j.biortech.2005.07.001
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No.11705089) and Natural Science Foundation of Jiangsu Province 331 (SBK201404182).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, J., Wang, W., Zhao, X. et al. Effect of Cs(I) and Cr(III) on the adsorption of strontium ion by living irradiated Saccharomyces cerevisiae. J Radioanal Nucl Chem 331, 3093–3105 (2022). https://doi.org/10.1007/s10967-022-08356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08356-4