Abstract
Biosorption of uranyl ions from aqueous solution by Saccharomyces cerevisiae was studied in a batch system. The influence of contact time, initial pH, temperature and initial concentration was investigated. The optimal conditions were found to be 3.5 h of contact time and pH = 4.5. Temperature had no significant effect on adsorption. The uptake of uranyl ions was relatively fast and 85 % of the sorption was completed within 10 min. The experimental data were well fitted with Langmuir isotherm model and pseudo-second order kinetic model. According to this kinetic model, the sorption capacity and the rate constant were 0.455 mmol UO2 2+/g dry biomass and 1.89 g mmol−1 min−1, respectively. The Langmuir isotherm indicated high affinity and capacity of the adsorbent for uranyl biosorption with the maximum loading of 0.477 mmol UO2 2+/g dry weight.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of the environment with toxic heavy metals is spreading throughout the world along with industrial progress. The commonly used treatment methods to remove heavy metals from wastewaters include chemical precipitation, ion exchange, reverse osmosis and membrane processes. However, these methods are not effective when the heavy metal concentrations in the wastewater are low [1]. The technologies are also expensive when a very low concentration of heavy metals in the treated water is required [2]. Biosorption, the uptake of heavy metals by non-living biomass, has gained increased credibility during recent years, as it offers a technically feasible and economical approach. Several biological materials have been investigated for heavy metal removal includes bacteria, yeasts, algae and fungi [3–7].
Uranium is one of the most seriously threatening heavy metals. It is naturally present in all environmental media at low concentrations [8]. Uranium species are both toxic and radiotoxic for humans and the contamination of this hazardous metal poses a threat in some surface and groundwater. So for this reason the removal of uranium from water and wastewater streams using natural and synthetic sorbents is a subject of continuously increasing importance [9–11].
In this work, the sorption of uranyl by Saccharomyces cerevisiae from aqueous solutions was investigated in a batch technique. The effect of pH, temperature, contact time and initial concentration on uranyl adsorption was studied. Two important isotherm models (Langmuir and Freundlich) and the pseudo-second-order kinetic model were applied to describe the uranyl adsorption.
Experimental
The strain of S. cerevisiae PTCC 5052 was obtained from the Persian Type Culture Collection, Tehran, Iran. The culture medium contained 120 g L−1 glucose (d(+)-glucose monohydrate, Carl Roth), 4 g L−1 yeast extract (Merck), 1 g L−1 NH4Cl (Scharlau), 5 g L−1 MgSO4·7H2O (Carl Roth) and 1 g L−1 KH2PO4 (Scharlau) [9]. Yeast S. cerevisiae was grown in 200 mL sterile liquid media for 24 h on a rotary shaker at 30 °C. The growth temperature affects the adsorption capacity of the sorbent [12]. The biomass was harvested by centrifugation and washed twice with sterile distilled water and then dried in an oven at 60 °C for 24 h. The dry biomass was ground and stored for sorption studies.
Uranyl solutions were prepared by diluting 1 mM stock solution of UO2 2+ prepared by dissolving 0.251 g of UO2(NO3)2·6H2O (Merck) in 500 mL of distilled water. The initial pH of each solution was adjusted to the required value with 1 N HNO3 or 1 N NaOH.
All biosorption experiments were carried out in a batch system, using 250 mL Erlenmeyer flasks. To each flask the same amount of biosorbent (0.02 g) was added and was mixed with 50 mL uranyl solution. The flasks were agitated on a rotary shaker at 200 rpm. After shaking, the biomass was separated by centrifugation and the uranyl concentration of the supernatant solution was determined using an Optima 7300 DV Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES). In each set of experiment, samples without biomass were used as controls.
The biosorption equilibrium uptake (q, mmol cation/g biomass dry wt.) for each sample was calculated by the following equation:
where C 0 and C e are the initial and equilibrium concentrations of uranyl ion in solution (mM) respectively, V is the volume of the solution (L), and M is the weight of the dry biosorbent used (g) [13].
Kinetic study of biosorption
In order to determine a suitable contact time for the sorption equilibrium experiments, the sorption studies were carried out over a time interval (0–390 min). Uranyl sorption kinetics was studied at 25 °C and pH = 4.5. The initial concentration of UO2 2+ in the solution was 0.2 mM.
Effect of solution pH
The optimal pH for uranyl removal was determined by measuring of uranyl uptake from 0.02 mM solutions over a range of pH values from 3.5 to 5.5, at 25 °C with 3.5 h of contact time.
Effect of temperature
In order to examine the effect of temperature on the uranyl biosorptive uptake, the experimental sets were tested at four different temperatures of 20, 25, 30 and 40 °C.
Effect of initial uranyl concentration
Experiments were carried out at different initial UO2 2+ concentrations ranging from 0.02 to 1 mM at pH = 4.5, T = 25 °C with 3.5 h of contact time.
Results and discussion
The mechanism of biosorption is complex, mainly ion exchange, chelation, adsorption by physical forces, entrapment in inter and intrafibrillar capillaries and spaces of the structural polysaccharide network as a result of the concentration gradient and diffusion through cell walls and membranes [14].
The mechanism of biosorption was based on the interactions between metal ions and the functional groups on the cell wall surface of the biomass. It was known that S. cerevisiae contains reasonable amount of protein and amino acids like histidine, which serve as a matrix of –COOH and –NH2 groups, which in turn take part in binding of metal ion. Carboxyl groups, associated with cell wall surface components, are considered to be among the main groups of cell membrane responsible for metal binding. Hence it can be concluded that non-living S. cerevisiae biomass can be used as an effective biosorbent for uranyl removal [12, 15].
Figure 1, shows the adsorption capacity of the adsorbent for UO2 2+ ions by S. cerevisiae versus contact time. The studied biomass exhibited a rapid cation uptake, 85 % of equilibrium was reached within 10 min and the process saturates after 3.5 h. The rapid cation uptake has been suggested as being essential for any good biosorbent as it allows short solution-sorbent contact time.
In order to modeling the sorption rate of uranyl ion, the pseudo-second order rate equation was applied. Assuming the sorption capacity of uranyl by the biomass is proportional to the number of active sites occupied on the sorbent, the pseudo-second order rate equation is given by:
where q eq and q t are the amount of metal sorbed per unit weight of the sorbent at equilibrium and at any time t, respectively (mmol g−1) and k is the rate constant of pseudo-second order sorption (g mmol−1 min−1).
Integrating the Eq. 2 for the boundary conditions for t = 0, q t = 0 gives;
The intercept of the linearized pseudo-second order rate equation gives the second order rate constant, k [16].
The linearized form of the pseudo-second order model for contact times of 390 min is given in Fig. 2. The correlation coefficient for the linear plot of t/tq.q versus time was 1. The values of q eq and k are tabulated in Table 1, the theoretical value of q eq is in good agreement with the experimental data. Therefore, the pseudo-second order kinetic model provided a good correlation for the biosorption of uranyl ions.
pH is one of the most important physical parameters that influence the biosorption process [17]. In the present investigation, biosorption of uranyl ions was studied in solutions with pH ranging from 3.5 to 5.5 at 0.2 mM (UO2 2+) at 25 °C. Figure 3, represents the effect of initial pH on the removal of uranyl ions by S. cerevisiae biomass. It is evident from the figure that the sorption rate increased with increase in pH value up to 4.5 and then declined with further increase in pH. This implies that uranyl removal is pH dependant and is maximal at pH 4.5. Studies on uranium sorption by different kinds of adsorbents, for example U(VI) sorption on ACSD,Footnote 1 U(VI) removal by CNABFootnote 2 and olive cake showed the similar adsorption behavior in the defined pH range. The difference between optimal pH was related to the difference of adsorbents negative surface charges [18–20]. for example the optimum pH for the removal of uranium by olive cake was found about pH 7.5. This value is higher than corresponding values given in the literature for uranium adsorption on microorganisms and algae, indicating that, in the case of olive cake, surface groups of weaker acidity (e.g., phenolic groups) play an important role in uranium adsorption on the surface [20].
The pH dependence of metal biosorption can largely be related to type and ionic state of the functional groups present on the biosorbent and the type of metal species present in the solution [17]. Metal sequestering by different parts of the cell can occur via various processes; complexation, chelation, coordination, ion exchange, micro precipitation and reduction. It has been suggested by many researchers that ion exchange is neither the sole nor the main mechanism for metal biosorption [21]. The ion exchange mechanism for uranyl ions binding to the biomass is complicated by the fact that the uranyl cation UO2 2+ is hydrolyzed in aqueous solutions within the range of the sorption system pH. Portioning of the hydrolyzed uranyl species depends on the solution pH and on the total uranyl concentration in the solution. In the range of acidic to near neutral pH values, four major hydrolyzed complex ions, UO2 2+, (UO2)2(OH) 2+2 , UO2OH+, (UO2)3(OH) +5 and a dissolved solid Schoepite (4UO3·H2O), uranyl hydroxide, exist in the solution. The hydrolysis equilibrium constants are pK = 5.8 for UO2OH+, pK = 5.62 for (UO2)2(OH) 2+2 and pK = 15.63 for (UO2)3(OH) +5 [22]. According to the results obtained by experiment, pH 4.5 seems to be optimal for uranyl biosorption. The dominant species of uranium at the pH 4.5 are UO2 2+ and (UO2)2(OH) 2+2 [9].
The hydrolyzed species can obviously be adsorbed better than the free hydrated ions. They could replace protons on separate binding sites in the biomass through ion exchange process [23].
At low pH values, the cell wall ligands will be closely associated with hydronium ions (H3O+), which will restrict uranyl ions access to ligands as a result of repulsive forces; this repulsion becomes stronger with a decrease in pH [24]. In other words, at low pH some binding sites are not available to the divalent UO2 2+. As the pH increases, more ligands will be exposed, i.e. carrying negative charges, with the subsequent attraction of metallic ions with positive charges and adsorption to the cell surface. On the other hand, the non-ion dissolved solid Schoepite starts appearing in the solution when the pH level is high. At too high pH values, the uranium sorption is hindered by the decrease in ion concentration [25].
Figure 4, illustrates the effect of temperature on sorption capacity. This profile indicates that temperature has no significant effect on uranyl sorption capacity. Similar result was also reported for the biosorption of U(VI) onto fungus Aspergillus fumigates [26].
However, temperature increase resulted in a very small q decrease. It is suggested that high temperatures might affect the integrity of the cell membranes and hinder compartmentalization of metal ions, leading to reduced uptake levels [27].
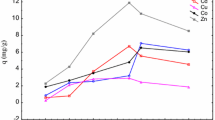
Uranyl ions sorption was studied at initial concentrations ranging from 0.02 to 1.0 mM and equilibrium sorption capacity of the sorbent was calculated. Figure 5, shows the biosorption isotherm at 25 °C. The experimental results showed that the uranyl uptake (q) increases rapidly with the increased equilibrium ion concentration. At low concentrations biosorbent sites take up the available metal ions more quickly. However, when the uranyl ion concentration reaches a certain level, the upward trend of sorption capacity becomes slower. The analysis of the isotherm data is important to develop an equation, which accurately represents the results and could be used for design purposes. In order to investigate the sorption isotherm, two important models were analyzed, the Langmuir, and the Freundlich. The Langmuir sorption isotherm is perhaps the best known of all isotherms describing sorption [28]. This is based on an assumption that the sorption occurs at specific homogeneous sites within the adsorbent. The equation is:
where q e is the amount of uranyl adsorbed (mmol g−1), C e is the equilibrium concentration of uranyl in solution (mM), q max is the monolayer sorption capacity (mmol g−1) and b is the constant related to the free energy of sorption (mM−1).
The constants q max and b are the characteristics of the Langmuir equation and can be determined from a linearized form of Eq. 4, represented by:
The Freundlich isotherm [29] is an empirical equation employed to describe heterogeneous systems. The Freundlich equation is expressed by Eq. 6:
and the Eq. 6 may be linearized by taking logarithms:
where K and n are Freundlich sorption isotherm constants, indicative of the extent of the sorption and the degree of nonlinearity between solution concentration and sorption, respectively.
Figures 6 and 7 represent the fitted linear curves for Langmuir and Freundlich models using Eqs. 5 and 7, respectively. The parameters obtained from the two isotherms are given in Table 2. The average percentage errors (Ω %) between the experimental values and the predicted values using the Langmuir and Freundlich models for the entire data set were 14.9 and 19.9 %, respectively. The correlation coefficients (R 2) of Langmuir and Freundlich models were 0.992 and 0.836, respectively. The fitness of the experimental data to the linear form of Langmuir model can be seen in Fig. 6 as it is also evident from R 2 = 0.992. The adsorption process was not well fitted to the linear form of Freundlich isotherm (Fig. 7). Moreover, Based on the low correlation coefficient (R 2 = 0.836), it could be concluded that the Freundlich model cannot be used to describe UO2 2+ adsorption by the biosorbent. The high correlation coefficient obtained from Langmuir model indicates that high affinity between biosorbent surface and uranyl ions plays the major role in the adsorption mechanism and monolayer binding of UO2 2+ happens on to the adsorbent homogenous surface.
Conclusions
Dry biomass of S. cerevisiae is capable of removing uranyl ions from aqueous solution especially in very dilute solutions. The batch sorption process is found to be depended on pH, contact time and initial uranyl concentration. The optimum biosorption was seen at pH = 4.5 and 3.5 h of contact time. The temperature had no significant effect on the uranyl sorption. The equilibrium data fitted well to the Langmuir isotherm model with an appropriate R 2 value. This is suggesting monolayer adsorption on a homogeneous surface. Also, the time-dependent UO2 2+ sorption data were exceptionally well-described by pseudo-second-order model (R 2 > 0.999).
Thus, it can be concluded that the S. cerevisiae dry biomass can be used as an excellent sorbent for the removal of uranyl ions.
Notes
Alginate coated CaSO4.2H2O sepiolite and calcined diatomite earth.
Chitosan coated natural attapulgite beads.
References
Patterson JW (1985) Industrial wastewater treatment technology. Butterworth, Boston
Veglio F, Beolcini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216
Zouboulis AI, Matis KA, Loukidou MX, Sebesta F (2003) Metal biosorption by PAN-immobilized fungal biomass in simulated wastewaters. Colloids Surf A 212:185–196
Kefala MI, Zouboulis AI, Matis KA (1999) Biosorption of cadmium ions by actinomyces and separation by flotation. Environ Pollut 104:283–293
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300
Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39:909–916
Peterson J, MacDonell M, Haroun L, Monette F (2007) Radiological and chemical fact sheets to support health risk analyses for contaminated areas. Argonne National Laboratory Environmental Science Division, Lemont
Sarri S, Misaelides P, Papanikolaou M, Zamboulis D (2009) Uranium removal from acidic aqueous solutions by Saccharomyces cerevisiae, Debaryomyces hansenii, Kluyveromyces marxianus and Candida colliculosa. J Radioanal Nucl Chem 279:709–711
White SK (1983) Removing uranium by current municipal water treatment processes. J Am Water Works Assoc 75:374
Laul JC (1992) Natural radionuclides in groundwaters. J Radioanal Nucl Chem 156:235–242
Anagnostopoulos VA, Bekatorou A, Symeopoulos BD (2011) Contribution to interpretation of metal uptake dependence upon the growth phase of microorganisms. The case of uranium (VI) uptake by common yeasts, cultivated at different temperatures, with or without aeration. J Radioanal Nucl Chem 287:665–671
Volesky B (2003) Sorption and biosorption. BV Sorbex Inc., Montreal
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Dhankhar R, Hooda A, Solanki R, Sainger PA (2011) Saccharomyces cerevisiae: a potential biosorbent for biosorption of uranium. Int J Eng Sci Technol 3:5397–5407
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Tripathi M, Mishra SS, Tripathi VR, Garg SK (2011) Predictive approach for simultaneous biosorption of hexavalent chromium and pentachlorophenol degradation by Bacillus cereus RMLAU1. Afr J Biotechnol 10:6052–6061
Donat R, Cilgi GK, Aytas S, Cetisli H (2009) Thermodynamic parameters and sorption of U(VI) on ACSD. J Radioanal Nucl Chem 279:271–280
Pang C, Liu Y, Cao X, Hua R, Wang C, Li C (2010) Adsorptive removal of uranium from aqueous solution using chitosan-coated attapulgite. J Radioanal Nucl Chem 286:185–193
Konstantinou M, Pashalidis I (2007) Adsorption of hexavalent uranium on biomass by-product. J Radioanal Nucl Chem 273:549–552
Brady JM, Tobin JM (1995) Binding of hard and soft metal ions to Rhizopus arrhizus biomass. Enzyme Microb Technol 17:791–796
Base CF, Mesmer RE (1976) The hydrolysis of cations. Wiley, New York
Collins YE, Stotzky G (1992) Heavy metals alter the electrokinetic properties of bacteria, yeasts, and clay materials. Appl Environ Microbiol 58:1592–1600
Zhou JL (1999) Zn biosorption by Rhizopus arrhizus and other fungi. Appl Microbiol Biotechnol 51:686–693
Khani MH, Keshtkar AR, Meysami B, Firouz Zarea M, Jalali R (2006) Biosorption of uranium from aqueous solutions by nonliving biomass of marine algae Cystoseira indica. Electron J Biotechnol 9:100–106
Bhainsa KC, D’Souza SF (1999) Biosorption of uranium(VI) by Aspergillus fumigatus. Biotechnol Tech 13:695–699
Brady D, Duncan JR (1994) Bioaccumulation of metal cations by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 41:149–154
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faghihian, H., Peyvandi, S. Adsorption isotherm for uranyl biosorption by Saccharomyces cerevisiae biomass. J Radioanal Nucl Chem 293, 463–468 (2012). https://doi.org/10.1007/s10967-012-1814-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1814-y