Abstract
The present investigation entails the biosorption studies of radiotoxic Strontium (90Sr), from aqueous medium employing dry cow dung powder (DCP) as an indigenous, inexpensive and, eco-friendly material without any pre or post treatments. The Batch experiments were conducted employing 90Sr(II) as a tracer and the effect of various process parameters such as optimum pH, temperature, amount of resin, time of equilibration, agitation speed and concentration of metal ions have been studied. The kinetic studies were carried out employing various models but the best fitting model was Lagergren pseudo-second order model with high correlation coefficient R 2 value of 0.999 and cation exchange capacity of DCP was found to be 9.00 mg/g. The thermodynamic parameters for biosorption were evaluated as ΔG° = −5.560 kJ/mol, ΔH° = −6.396 kJ/mol and ΔS° = 22.889 J/mol K, which indicated spontaneous and exothermic process with high affinity of Sr(II) for DCP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the domain of toxic radionuclide persisting in our environment, 90Sr is considered as one of the most hazardous fission product due to its long physical half-life of 29 years [1] and its inevitable presence in the water, soil and food chain. The anthropogenic activities such as nuclear weapon testing, reprocessing of liquid spent fuel, etc. are major source of 90Sr in our environment. Strontium is referred as a bone seeker as it imitates the calcium in the human body and increases the risk of bone cancer, leukemia, etc. [2]. On the contrary, if managed scientifically, it has also been proved to be aiding to mankind. 90Sr has been used as a power source for radioisotope thermoelectric generators (RTGs), as well as used in cancer therapy and in forensic sciences [3]. Hence, there is a great necessity to adapt a methodology which can be employed for the eco-friendly removal of 90Sr from the aqueous system with a view of reprocessing the same and to reap out its aforementioned benefits.
In the field of radionuclide research, some of the well established processes such as chemical precipitation, membrane process, liquid extraction, and ion exchange [4, 5] have been applied as a tool for the removal of this metal ion. These all methods are not considered to be greener due to some of their shortcomings such as incomplete metal ion removal, high requirement of energy and reagents, generation of toxic sludge or other waste materials which in turn require treatments for their cautious disposal. Eventually, it adds on to the cost, time and feasibility of the entire procedure.
The greener and cleaner approach of biosorption is a sure solution to this situation. Biosorption phenomenon is acquiring strong footage due to its mechanism based on non-directed physico-chemical interactions that occur between metal species and dead biomass. Biosorption deals with both living biomass as well as non-living aggregates of biomaterial. But biosorption by dead biomass is often faster [6], since only passive cell wall based binding transport, into the cell takes place.
Literature survey reveals that in the domain of natural adsorbent, materials such as Rhytidiadelphus squarrous [7], magnetically modified yeast cells [8], Azolla filiculoides [9], brucite [10], clay minerals [11], Oscillatoria homogenea cynobacterium [12], Cystoseira indica brown alga [13], seeds of Ocimum basilicum [14], Pakistani coal [15], etc. have been utilized for Sr(II) removal. These naturally available materials require some degree of physical and chemical enhancement so as to optimize, which add on to the economy of entire adsorption process. The HA has been successfully extracted by authors from DCP and this piece of work has been published in the International Journal [16]. Also, biosorption with living media leads to biological and chemical sludge due to growing biomass and nutrients. Hence, for the efficient biosorption method, economy of environmental remediation dictates that the biomass must come from nature or even has to be a waste material.
Materials and methods
Adsorbent
DCP (100 mesh) was provided by Keshav Shrushti, Research Centre on Cow product (Thane, India) and due precautions were taken to avoid the contaminations. DCP is naturally available bio-organic, complex, polymorphic fecal matter. It is enriched with minerals, carbohydrates, fats, proteins, bile pigments, aliphatic–aromatic species such as ‘humic acid’ and many functional groups such as carboxyl, phenols, quinols, amide, etc. which enhances its adsorption properties.
Characterization of DCP
Cow dung powder was dried properly before its utilization, so as to prevent its oxidation by acid due to presence of mixture of alcohols if any. The absence of alcohol is also supported by FTIR analysis of DCP, which is devoid of any characteristic band of alcohol. The integrity of DCP before and after the adsorption was studied by measuring the mesh size and was found to be same; indicating during adsorption process there was no physical attrition of resin. All the characterization techniques have been carried out at Indian Institute of Technology, IIT, Powai, Mumbai. The DCP has been characterized using XRF technique for its quantitative as well as qualitative elemental composition and for the complete elemental assay, complimentary to XRF technique, C, H, N, S, O technique, has also been obtained as detailed in Table 1.
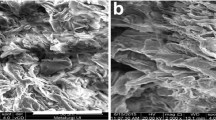
The SEM pattern of DCP clearly reveals the surface texture and porosity of the DCP. The porous morphology of DCP is responsible for the easier diffusion during adsorption process. For the confirmation of biosorption process EDAX (Energy dispersive X-ray analysis) spectrum of DCP, after and before the adsorption of Sr(II) have been studied. The EDAX spectrum of DCP before and after biosorption of Sr(II) (Fig. 1a, b), indicated that Si, K, Ca, Ti, Mn, Fe, Zn are naive metal ion of the matrix and after biosorption of Sr(II) on DCP, Sr(II) was present along with all other natural elements indicating that ion exchange is not the mechanism for Sr(II) adsorption on DCP.
Adsorbate
All the chemicals used were of analytical grade. The stock solution of Sr(II) 1 mg/mL was prepared using SrCl2 and distilled water. All the solutions were standardized by standard analytical methods [17].
Tracer technique
It is the radio analytical technique in which micro amount of radioactive isotope is added to a system in order to trace or monitor [18] the chemical reaction of a certain element in the system. In comparison to classical approach, tracer technique offers some unique advantage such as, high sensitivity, non-destructive pattern, freedom from reagent blank, etc. The Radiotracer 90Sr(II) (beta-emitter) was procured from BRIT (Board of Radiation and Isotope Technology, Mumbai, India).
Batch equilibration mode
A known amount of DCP was mixed with 10 mL of solution containing radiotracer and 1 mg/mL solution of carrier. The pH was adjusted using dil. HCl and NaHCO3 as per the requirement. The resultant aliquot was equilibrated for 10 min with mechanical stirrer and was then centrifuged. After separating supernatant, adsorbent was washed with 5 mL of distilled water and activity present in supernatant was measured using end window type Geiger–Muller Counter (PEA GCS 101P) in conjugation with a decade scalar, timer and a high voltage unit, for the beta-emitter.
The effect of different experimental parameters such as pH (from 1 to 10), metal ion concentration (0.5–20 mg/mL), contact time (0–30 min), agitation speed (0–5,000 rpm), amount of adsorbent (50–650 mg), temperature (283–363 K), have been studied so as to optimize the parameters for developing efficient adsorption process. The kinetic and thermodynamic studies have also been carried out so as to optimize the system under study for the removal of Sr(II) ions from aqueous medium. All experimental data were measured in triplicate and percentage sorption was calculated using formula given below:
where A(i) = activity taken, A(f) = total activity in supernatant.
Results and discussion
All the aforementioned parameters were comprehensively studied for the optimization of the system. The results revealed that having 10 min of contact time, at 4,000 rpm of agitation speed, at the optimum pH of 6, 350 mg of DCP can effectively remove Sr(II) up to 85–90%.
Effect of pH
DCP being heterogeneous adsorbent possess positively charged site owing to some proteins, enzymes and negatively charged sites due to presence of some acidic groups. Hence, on varying the pH of a solution, the overall surface charge of DCP could be modified so as to get maximum adsorption. The adsorptive behavior of Sr(II) ions on DCP might be explained on the basis of electrostatic force of attraction between the oppositely charged adsorbate and adsorbent. On varying the pH from 1 to 10 as shown in Fig. 2, it was observed that, the optimum pH for maximum adsorption of Sr(II) on DCP was at pH 6. At low pH, adsorption became unfavorable due to electrostatic repulsion between the positive charged surface, in excess of H+ ions, and Sr(II) ions, resulting in the decrease in the adsorption. The neutral range of pH favored the adsorption of Sr(II) ions on the DCP which was indicative from the figure.
On further increase in pH, the adsorption was found to decrease, which may be due to preference of Sr(II) ions to form a stable complex of SrCO3 then to get adsorbed on DCP surface. It was observed that the maximum sorption of Sr(II)on DCP was obtained at pH 6.
Effect of amount of adsorbent
Adsorption being surface phenomenon, the extent of adsorption is directly proportional to the surface area available. Increasing the adsorbent amount, increase in adsorption percentage was observed. After certain dose of adsorbent, maximum adsorption sets in after which the percentage adsorption was almost constant. This suggests that after optimum dose, number of ions bound to adsorbent and the number of free ions remained constant even with further addition of the adsorbent. It may be due to partial aggregation of active adsorbent sites [19]. Our results suggest that optimum amount of DCP for Sr(II) was 350 mg.
Effect of contact time
To optimize contact time for the experiment, keeping all other parameters constant, contact time was varied from 0 to 30 min. The results reveals that as contact time increased, percentage adsorption also increased, but after some time, it gradually approached a constant value, denoting attainment of equilibrium. Further increase in contact time, did not increase adsorption due to desorption of ions on the available adsorption sites of adsorbent.
Effect of metal ion concentration
Metal ion concentration was varied from 0.5 to 20 mg. As shown in Fig. 3, percentage adsorption decreased with increase in metal ion concentration. In case of low metal ion concentration, the ratio of number of moles of metal ions to the available surface area of adsorbent was lower and subsequently the fractional adsorption becomes independent of metal ion concentration [20]. On increasing the concentration of Sr(II) ions, the metal ion concentration becomes higher than that of the sorbent binding sites, resulting in decreased sorption percentage. This study reveals that 350 mg of DCP can absorb 20 mg/mL of Sr(II) up to 50%. Also, on increasing the amount of DCP, the removal of Sr(II) can be increased even at higher metal ion concentration.
Effect of temperature
In the process of biosorption, temperature plays a vital role and these processes are normally exothermic. Hence, the extent of adsorption generally increases with decrease in temperature. For this purpose the temperature was varied between 283 and 363 K. Our results reveal that the adsorption of metal ions increases at lower temperature and were found to be maximum at the room temperature range. On further increasing the temperature, percentage adsorption was decreased and it may be due to desorption caused by an increase in the available thermal energy. Also, higher temperature induces mobility of adsorbate, eventually causing desorption, due to weakening of adsorptive forces between the active sites of adsorbent and adsorbate species and also between the adjacent molecules of the adsorbed phase [21]. Hence, the temperature ranges, i.e. 293–303 K favors the adsorption rate of the system under study.
Effect of agitation speed
This experiment proved that speed of agitation has important role in the process of biosorption. All agitation speeds were found to have a positive impact on the system. Increasing the speed at rate of 500 rpm, percentage adsorption showed markedly high values. This is because agitation facilitates proper contact between the metal ions in solution [22] and the binding sites and thereby promotes effective transfer of adsorbate ions to the adsorbent sites. The agitation speed of 4,000 rpm was standardized.
Thermodynamic parameters
Thermodynamic considerations are necessary to conclude whether the process is spontaneous or not. The Gibb’s free energy change, ΔG° is an indication of spontaneity of chemical reactions. The free energy of biosorption reaction can also be evaluated by considering the biosorption equilibrium constant K a which is given by the following equation:
Where, ΔG° is the standard free energy change (J), R is the universal gas constant = 8.314 J/mol K, and T is absolute temperature (K). The free energy change for the temperature range of 293–308 K using Eq. 1 has been evaluated and has obtained the negative values of ΔG° for the entire range as shown in Table 2. Any reaction if occurs spontaneously at a given temperature, its ΔG° is always a negative quantity. The negative value also confirms the feasibility and the spontaneous nature of biosorption process of Sr(II) on DCP with ΔG 308° = −5.560 kJ/mol.
A plot of ln K a, versus temperature 1/T × 103, was found to be linear, Fig. 4. The values of ∆H° and ∆S° were determined from the slope and intercept of the plot. The enthalpy change ∆H°, and the entropy change ∆S°, for the biosorption process were deduced from the graph to be −6.396 kJ/mol and 22.889 J/mol K, respectively. The negative value of enthalpy suggests the exothermic reaction. The positive value of entropy change suggest the increase in the randomness at the solid/liquid interface reflecting affinity of DCP for the Sr(II) ions.
Kinetic parameter
The kinetic adsorption data could be processed to understand the dynamics of the biosorption reaction in terms of the order of the rate constant. The different kinetic models such as first order, second order, pseudo-first order, pseudo-second order and the intraparticle diffusion have been studied. But among these models, best fitting model was Lagergren pseudo-second order model. The correlation coefficients have R 2 values close to one as shown in Table 3, indicating the applicability of pseudo-second order model to the present system. The kinetic data was treated with the Lagergren pseudo-second order kinetic model [23]. It is generally expressed as follows:
where q e and q t is adsorption capacity at equilibrium and at time t, respectively (mg/g) and k 2 is the second order rate constant of adsorption (g/mg min). Integrating Eq. (1) for the boundary conditions q = 0 to q = q t at t = 0 to t = t is to obtain the following equation:
The plot of t/q t versus t should show a linear relationship if the second order kinetics is applicable and same is evident from the graph. The equilibrium rate constant, k 2 and equilibrium capacity or adsorption capacity or cation exchanged capacity, q e were determined from the slope and intercept of the line in Fig. 5. The applicability of this model suggested that biosorption of elements under study, on DCP was based on chemical reaction, between metals and active sites of the biosorbent.
Conclusions
A simple and eco-friendly method for the utilization of DCP as an effective green adsorbent material for the removal of radiotoxic 90Sr from aqueous medium has been developed. Being naturally and easily available, DCP can be employed without any pre or post treatment. Hence, it has an edge over other processed natural and synthetic adsorbent considering their production cost, time, and energy efficiency.
References
Argonne National Laboratory, EVS Human Health Fact Sheet, Nov 2006
Luo H, Dai S, Bonnesen P (2004) Anal Chem 76(10):2773–2779
Horwitz E, Chiarizia R, Dietz M (1992) Sol Extr Ion Exch 10(2):313–336
Yu L, Chakraboty S, Basu J (2006) Sep Purif Technol 50(3):336–341
Maresova J, Pipiska M, Rozloznik M, Hornik M, Remenarova L, Augustin J (2010) Desalination. doi:10.1016/j.desal.2010.08.014
Jia Y, Hub Y, Tiana Q, Shaoa Lib J, Safarikovac M, Safarik I (2010) Sep Sci Technol 45:1499–1504
Mashkani S, Ghazvini M (2009) Bioresour Technol 100(6):1915–1921
Bochkarev G, Pushkareva G (2009) J Mining Sci 45(3):290–295
Petrova A, Flower A, Krip I, Shimchuk T, Petrushka I (2008) Radiochemistry 50(5):434–438
Dabbagh R, Ghafourian H, Bhagvand A, Nabi G, Riahi H, Ahmadi Faghih M (2007) J Radioanal Nucl Chem 272(1):53–59
Dabbagh R, Ghafourian H, Bhagvand A, Nabi G, Riahi H (2007) J Environ Sci Tech 33
Chakraborty D, Maji S, Bandopadhyay A, Basu S (2007) Bioresour Technol 98(15):2949–2952
Shaukat M, Sarwar M, Qadeer R (2005) J Radioanal Nucl Chem 265:73–79
Bagla H, Barot N (2009) Green Chem Lett Rev 2(4):217–221
Vogel I (1975) Textbook of quantitative inorganic analysis, 3rd edn. Longman Green & Co., London
Lieser K (2001) Nuclear & radiochemistry: fundamentals and applications, 2nd edn. Wiley, Germany
Waranusantigul P, Pokethitiyook P, Krutrachue M, Upatham E (2003) Environ Pollut 125:385–392
Saiffudin N, Raziah A (2007) J Appl Sci Res 3(12):2091–2099
Singh K (2005) J Indian Chem Soc 82:342–346
Ahalya N, Kanamadi RD, Ramchandra T (2005) Electron J Biotech 8(3):323–393
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 24:1–39
Acknowledgments
We thank Gemmological Institute of India, Mumbai, for providing EDAX facility. We are also thankful to Dr. Raju Apte, Head of Gaushala, Keshav Shrushti, Thane, for providing DCP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barot, N.S., Bagla, H.K. Biosorption of radiotoxic 90Sr by green adsorbent: dry cow dung powder. J Radioanal Nucl Chem 294, 81–86 (2012). https://doi.org/10.1007/s10967-011-1539-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1539-3