Abstract
In this study, the Saccharomyces cerevisiae (S. cerevisiae) was modified by γ-ray. The RNA-seq results reflect that the high γ-ray energies could change some gene fragments, such as deletion, recombination, and mutation. The biosorption of strontium ions (Sr2+) to different types of S. cerevisiae (S. cerevisiae (K-0), modified S. cerevisiae (Y-7), and non-living S. cerevisiae (H-K)) from the simulated high-level liquid waste (S-HLLW) was assessed at different experimental conditions. The sorption experimental results show that, under an appropriate condition, γ-ray radiation can enhance its biosorption capacity slightly of Sr2+ to S. cerevisiae. The maximum metal uptake and efficiency of Y-7 under S-HLLW were 11.656 mg g−1 and 37.91% at 32 h (wet weight), respectively. They decreased to 9.46 mg g−1 and 30.76% under radiation conditions. SEM-EDX and TEM analysis indicates that Sr2+ was adsorbed both on the cellular surface and the inner parts of the cells. Our experimental results fit well to the Langmuir and Freundlich model isotherms (r2 > 0.94), and the maximum biosorption capacity values reached qmax > 24.74 mg g−1 at 32 °C. Negative values of ΔG0 and positive values of ΔH0 were observed, indicating the spontaneous and endothermic nature of Sr2+ biosorption on modified S. cerevisiae. The biosorption kinetics follow a pseudo-second-order equation at 32 °C (r2 > 0.94). The desorption efficiency of Sr2+ adsorbed onto Y-7 was 7.65 ± 0.52%, 76.51 ± 2.13%, and 65.62 ± 2.42% by deionized water, 1 M HCl, and 0.1 M EDTA-Na, respectively. However, they were lower than H-K (18.82, 83.32, and 73.32%). Our findings demonstrate that living S. cerevisiae (Y-7) is a promising sorbent material for the treatment of radioactive process streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With a worldwide increased demand for power generation, nuclear power development has presented new solutions to address energy shortage. For a sustainable development of nuclear energy, the safety management of high-level liquid waste (HLLW) has become one of the most important research topics (Shozugawa et al. 2012). Among the fission products present in HLLW, strontium isotope such as 90Sr or 89Sr is the most hazardous and radioactive contaminant in the environment, which causes a wide range of environmental pollution and exists as a serious threat to human health (Bailly du Bois et al. 2012). In fact, the purified strontium can be reused as radiation and heat sources in the field of industry. To minimize the long-term radiological risk and facilitate the management of HLLW, developing effective approaches to separate and recycle strontium is highly desirable.
The conventional methods employed for recycling strontium ion from HLLW include precipitation (Luo et al. 2004; Nilchi et al. 2009), ion exchange (Dabbagh et al. 2007; Ho et al. 1996), solvent extraction, chromatography, and the membrane separation. Those methods require high cost but give low radiation stability (Chen and Wang 2008; Eccles 1995). On the other hand, economic biomaterials have demonstrated the advantages of good selectivity, low cost, and easy implementation for wastewater treatment, ever since the first report on biosorption (Clarke P H. 1985). In particular, microorganisms are able to decrease the concentration of radionuclides solution from milligrams per liter to micrograms per liter level (environmentally relevant level), which is highly efficient and environment-friendly (Farooq et al. 2010; Sud et al. 2008).
Saccharomyces cerevisiae is an inexpensive and eco-friendly biomaterial for heavy metal removal from wastewater, which is readily available from the source of biomass. Our previous study showed that the uptake of strontium by living S. cerevisiae reached 92% under laboratory conditions, and the biosorption capacity can be enhanced using denatured yeast cells (Qiu et al. 2017). The living S. cerevisiae can adsorb up to 26.12 mg g−1 uranium in the initial 600 mg L−1 (Liu et al. 2010). Compared to yeast, the other biosorbents were of less concern. The removal ratios of strontium ions were 44 and 39% for Rhizopus arrhizus and Penicillium chrysogenum, respectively (Marešová et al. 2011). The algae showed higher biosorption capacity approximately 220 mg g−1 for strontium ions after special treatment (Tu et al. 2015). Moreover, living S. cerevisiae is an ideal model organism to investigate the interactions of the metal-microbe at the molecular level (Wang et al. 2017). Therefore, living S. cerevisiae is a promising biological adsorbent for metal removal.
The biosorption of strontium by growing or living microorganism involves three processes: an initial rapid, passive adsorption followed by a much slower active bioaccumulation process, and further sorption stage. The first and last stages are metabolism-independent processes, while the second step is metabolism-dependent (Wang et al. 2017). The majority of studies on the bioaccumulation of heavy metal ions by microorganisms only consider the final concentration of metal ions, and a comprehensive study on the interaction between strontium ion and other radionuclides during the process of biosorption is still lacking. The HLLW has many hazardous components including toxic metals, other potentially damaging radionuclides/radiation, extreme pH ranges, and other chemical factors (e.g., high salinity, solvents, etc). Furthermore, although the radiation resistance is a critical factor that can determine whether S. cerevisiae can be used as a biosorbent for removing radioactive nuclear waste (Parab et al. 2016), no study has been conducted on the effect of γ-ray radiation on the biosorption of Sr2+ to S. cerevisiae. Thus, it is timely important to conduct a comprehensive study on the adsorption and bioaccumulation processes from HLLW by living S. cerevisiae, as well as the underlying mechanisms, which will promote the application of these techniques in wastewater treatment.
In this work, we carried out an investigation of the radiation effects on the S. cerevisiae. The strontium biosorption capacity of different S. cerevisiae was compared under S-HLLW condition, from which we investigated the strontium biosorption conditions of living S. cerevisiae. Based on these experimental results, we further determined the Sr2+ biosorption isotherm and kinetic model. The biosorption mechanism was also investigated through comparison of the desorption efficiencies of the living and non-living cells.
Materials and methods
Yeast and reagents
In our previous studies, we had used cyclic irradiation method to culturing an irradiated S. cerevisiae: K-4000 and Y-7 (Qiu et al. 2017). In this paper, we investigated the reason why S. cerevisiae can survive after continuous exposure to irradiation and observed whether it can still survive under S-HLLW conditions.
RNA extraction, library preparation, and RNA-seq
After K-4000 and K-0 reached the beginning of the exponential growth phase, the total RNA was extracted by TRIZOL reagent (Invitrogen, San Diego, USA) following the manufacturer’s protocol. Then, purified using oligo (dT) magnetic beads. The integrity of the RNA was verified with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA) and lodged in Basepair Technology Company (Suzhou, China). The paired-end library preparation and sequencing was achieved by using a TruSeq RNA sample preparation kit (Illumina Inc., San Diego, USA), respectively. The libraries were sequenced by HiSeqTM 2000 device (Illumina).
Biosorption experiments
The components of S-HLLW are listed in Table 1 (Izumida T 1990; R.L. Smith 1997; Zhang A 2006). To simulate the environment of real HLLW (Kubota M 1980), all batch sorption experiments were carried out with the initial conditions of pH = 3–4, C0 = 615 mg L−1, T was room temperature (25–32 °C), and the initial biomass was 25 g L−1 (wet weight). The different S. cerevisiae strains (K-0, Y-7, H-K) were observed for sorption capacity, respectively (Dai et al. 2014). (H-K: The cell was killed by heating, 100 °C for 10 min.) The experiments were conducted in duplicate, and control experiments without biosorbents were performed.
To investigate the effects of environmental factors on biosorption capacity, batch experiments were studied in 100-mL Erlenmeyer flasks. Cell suspensions were mixed well by shaking and incubated on a rotary shaker (120 rpm). A sample of the suspension liquid (5 mL) was taken and centrifuged (4000 rpm; 10 min; 4 °C) to obtain concentrated samples for atomic absorption spectrometry (AAS). The percentage adsorption R (%) and adsorption capacity q t (mg g−1) were determined at different experimental conditions, respectively.
Metal uptake (q t ) was determined as follows:
C t was the residual Sr2+ concentration at any time (mg L−1), C 0 was initial Sr2+ concentration (mg L−1), m was adsorbent dosage (g), and V was the volume of the experimental system.
Effect of radiation
Sorption experiments under radiation environment (RE) studies were performed at the same conditions by different S. cerevisiae (Y-7, K-00, H-K). The S. cerevisiae (20 g L−1) was suspended in S-HLLW and the samples of suspension liquid were separated from the solution by centrifugation (4000 rpm, 10 min, 4 °C) after 32 h. The total dose was 4000 Gy and the dose was 2.08 Gy min−1.
Effect of S. cerevisiae (Y-7) concentration
Similar batch experiments were carried out (mentioned in 2.3). The initial biomass (Y-7) was studied from 10 to 250 mg (wet weight). The samples of suspension liquid were taken out and determined at the time of 32 h, respectively.
Effect of temperature
The sorption experiments were carried out with the initial conditions of pH = 3–4, C0 = 615 mg L−1, and Cm = 20 g L−1 at different temperatures of 10, 20, 27, 32, 38, and 40 °C. The samples of suspension liquid were taken out and determined at the time of 32 h, respectively.
Effect of contact time
Similar batch experiments were carried out (mentioned in 2.3) and the biomass (Y-7) was 20 g L−1 at different times of 4, 8, 12, 16, 20, 24, 30, and 36 h, respectively.
The staining experiment after biosorption process
We used staining experiment to detect the survival of S. cerevisiae after biosorption.
The staining experiments under S-HLLW
Sorption experiments with different S. cerevisiae (Y-7, K-0) were carried out and the samples of S. cerevisiae were separated from the solution by centrifugation (4000 rpm, 10 min, 4 °C) at different times of 14, 24, 30, and 36 h. The S. cerevisiae was washed (by PBS for 3 times) and stained (with methyl blue), then observed by high power microscope (Olympus CX22, Japan).
The staining experiment under RE + S-HLLW
The S. cerevisiae was separated from the samples of suspension liquid of 2.3.2. The staining experiment was the same as 2.4.1.
Characterization
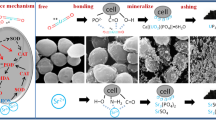
The cellular localization of strontium complexes formed by cells of Y-7 was performed by using transmission electron microscopy (TEM) (JEM-1011 JEOL Japan) and energy dispersive X-ray spectroscopy (EDX) for elemental information analyses. Cells of Y-7 and strontium-adsorbed were treated under the optimal conditions, fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at 4 °C for 4 h, and washed three times with the same phosphate buffer. The cell pellets were treated with 1% OsO4 for 30 min at 4 °C, and then dehydrated through a graded ethanol (70, 80, 90, and 95%) series for 10 min. The dehydrated samples were embedded in epoxy resins and sectioned into ultra-thin specimens. Thin sections were supported on copper grids and examined after staining with lead citrate.
The isotherm biosorption and kinetic studies of Sr2+ to Y-7
Isotherm biosorption studies
Biosorption equilibrium was described two isotherm models: the Langmuir and Freundlich models. The Langmuir model is the most commonly used isotherm for sorption, and it assumes that a reversible sorption process occurs on a homogeneous surface forming a monolayer sorption without interactions between the adsorbed species, in addition to uniform energies of the sorption onto the surface, and is expressed as the following equation:
q e is the metal uptake capacity (mg g−1) and C e is the equilibrium concentration of metal ions in the solution (mg L−1); q m (mg g−1) is the biosorption capacity when the surface is covered with metal ions completely (maximum biosorption capacity); and b is a constant that represents the affinity between the biosorbent and the metal ion. (Ho et al. 1996).
The Freundlich isotherm model considers the heterogeneity of the surface and multilayer biosorption to the binding sites located on the surface of the biosorbent. It is empirically expressed as follows:
KF (Lm g−1) is the Freundlich constant indicating biosorbent capacity and n is the Freundlich adsorption constant known as biosorbent intensity. The model parameters of Freundlich isotherm can be determined from plotting lnqe versus lnCe. (Ho et al. 1996).
Kinetic studies
In order to investigate the biosorption mechanism of heavy metals, pseudo-second order was used to fit the kinetic experimental data according to Ho’s method. The sorption data was analyzed in terms of a pseudo-second-order mechanism:
t = 0; q t = 0; the integral form:
K2 is the second order rate constant (g/mg h) and q t is the amount of biosorption at time “t” (mg g−1); q e is adsorption Sr2+ concentration on the adsorbent at equilibrium (mg g−1).
The studies of thermodynamic parameters
In environmental engineering practice, both energy and entropy factors must be considered in order to determine what processes will spontaneously occur. The Gibbs free energy change ΔG0 is the fundamental criterion of spontaneity. Reactions occur spontaneously at a given temperature if ΔG0 < 0. The biosorption process of metal ions can be summarized by the following reversible process, which represents a heterogeneous equilibrium. The apparent equilibrium constant (\( {K}_c^{\prime } \)) of the biosorption is defined as:
Cad, e adsorption Sr2+ concentration on the adsorbent at equilibrium (mg L−1).
The value of\( {K}_c^{\prime } \) in the lowest experimental concentration can be obtained. The \( {K}_c^{\prime } \)value is used in the following equation to determine the Gibbs free energy of biosorption:
The enthalpy (ΔH0) and entropy (ΔS0) can be obtained from the slope and intercept of a van’s Hoff equation of ΔG0 versus T:
Desorption experiments
After biosorption experiment conducted as described above, the bacterial suspension was centrifuged and the biomass was resuspended in one of three desorption reagents: deionized water, 1.0 mol L−1 HCl (AR grade, Sigma, USA) and 0.1 mol L−1 EDTA-Na2 (AR grade, Sigma, USA). The suspension was kept a shaker for 24 h (32 °C, 160 rpm) and then centrifuged at 10000 rpm for 5 min. The Sr2+ concentration in the supernatant was analyzed by AAS (Persee TA5-990, China). The desorption efficiency (nd) of Sr2+was calculated as follows:
where C d was the concentration of strontium in the desorption solution (mg Sr/L); V d is the volume of solution for desorption (mL); m a is the adsorbed amount of strontium (mg); m d is the desorbed amount of strontium from the cell (mg).
Results and discussion
Differentially expressed genes (DEGs) analysis
Through our pretreatment, we finally got a total of 30 million valid RNA-sep data. The data quantity is 23.8 G and the average length is 126 bp. The sample data is more than 5 G and it could meet the needs of analysis (Chepelev et al. 2009).
In order to analyze the expressed genes after irradiation, the filtered sequence was screened and assembled. Those results were compared to the standard database and screened the differential expression genes through statistical comparison (Fig. 1) (Anders and Huber 2010). (cufflinks: t test, fold change test, p value.) From Table S.1, we found that only 613 of the total unigene appeared to be differentially transcribed between K-0 and K-4000. For all of those DEGs, 316 were up- and 297 were down-regulated in K-4000.
Gene ontology analysis
For the further understanding of functions and metabolic pathways involved for these stages-specific genes and differentially expressed genes, GO analysis and pathway enrichment analysis were performed (Wang et al. 2010).
From the results (Table S.2), there were 970 genes could be noted in Go database and total of DEGs were 357. Two hundred thirteen DEGs were attributed to molecular function (59.67%), 90 DEGs belonged to biological process (25.21%), and the rest of DEGs were about cellular component (15.12%).
From the results (Table S.3), there were 451 genes could be noted in pathway base and the total of DEGs were 130. Forty DGEs were attributed to metabolic pathway (30.77%); the DEGs belonged to signaling pathways are 7 (5.38%) and the rest of DEGs (63.85%) were about genetic information.
Our RNA-seq results reflected that under the pressure of radiation, some genes could be evolved. The DEGs could be classified for three main categories, “cellular component,” “molecular function,” and “biological process” category, respectively. The most important components (Table 2) were “developmental maturation” (Go:0021700, etc) and “cell death” (Go:0022415, etc) subgroups of the “biological process” category and the “endoribonuclease activity activity (Go:0016891, etc)”, “cellular assembly (Go:0019069, etc)” of subgroups of the “molecular function”. From the results of pathway significant enrichment analysis (Table S.3), most of the DEGs belonged to “genetic information” (2.7.7.49, 2.7.7.7, etc). This phenomenon indicated that the “deletion” (sce03040), “recombination” (2.7.7.49), and other events of gene fragments were the critical factor that helped the modified S. cerevisiae adapt to the radiation environment (Bahieldin et al. 2015).
Yeast strains S. cerevisiae (K-4000) were modified from radioactive environment; genome of radioactive (K-4000) and non-radioactive (K-0) were evaluated. Information related to the radiation resistance of some yeasts has been reported upon; their D10 values fall in the range 0.1–0.5 k Gy, the absolute lethal dose of S. cerevisiae is about 3.5 k Gy (Yaochuan Wang 1994). Previous studies had also showed that the proportion of primary radiation damage would increase after repeated exposures and the cells were not able to recover (Petin et al. 2014). However, in this study, we found that radiation resistance could be induced by gradient recycle irradiation and the modified yeast (K-4000) had a higher radiation resistance than reference yeast strain that has been reported (Deák and Beuchat 1996; Li et al. 2015). Furthermore, K-4000 could keep living under irradiation environment. Perhaps microorganisms have developed mechanisms of resistance that may lead to the selection of resistant variants that can tolerate radiation (Petin and Kim 2014).
Biosorption studies
Biosorption by S. cerevisiae
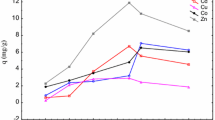
In environmental engineering practice, both energy and entropy factors must be considered in order to determine what processes will spontaneously occur. In our previous study, we had tested the resistance to radiation and strontium of Y-7, respectively (Qiu et al. 2017). Here, we tested the sorption capacity of Y-7 under HLLW or RE conditions. The results (Fig. 2) showed that the bioremoval of strontium ions from simulated wastewater was different compared to that from a simple solution (Wang et al. 2013b). In the S-HLLW, the hydrolysis condition, redox reactions, and precipitation are strongly influenced by those heavy metal, and conversely, strongly influence the biosorption availability. The highest metal uptake and efficiency were 11.656 mg g−1 and 37.91% at 32 h by Y-7. A similar result was obtained for Dicratetia inornata and Platymonas subcordiformis, which can adsorp strontium ion concentrations greater than 1.44 mmol L−1(Mei et al. 2004). The D. inornata biosorption capacity was severely inhibited when the initial strontium concentration reached 5.76 mmol L−1 (approximately 500 mg L−1) (Liu et al. 2008).
The different adsorption rates of H-K, K-0, and Y-7 may due to their biosorption mechanisms being different (Liu et al. 2014). From that, we inferred that the dead S. cerevisiae might adsorb Sr2+ only owing to electrostatic interaction caused by the functional groups of the cell surface including carboxyl, phenyl, hydroxyl, amino, etc. (Wang et al. 2013b). Those results suggest that strontium by living S. cerevisiae (Y-7) in HLLW is feasible and could be an efficient means for treating nuclear wastewater. Figure 2 also presents the biosorption efficiency of Sr2+ by Y-7 and other strains at 4000 Gy with S-HLLW, respectively. From the results, the sorption capacity of Y-7 improved when compared with the other S. cerevisiae. The uptake and biosorption efficiency of Sr2+ by Y-7 strain was 9.46 mg g−1 and 30.76% at 32 h, respectively. However, compared with the results of no-RE, the uptake and efficiency of Y-7 had decreased in the radiation environment.
Effect of S. cerevisiae (Y-7) concentration
Biosorption experiments were performed with using solutions containing 10–25 g L−1 biomass for 32 h. From Fig. 3, the biosorption efficiency of Sr2+ by Y-7 increased and the sorption capacity decreased with increasing of initial biomass. The maximum sorption rate was found about 37.125% at 250 mg, with the sorption capacity results, the most favorable initial concentration range was 20 g L−1. When increasing the initial biomass after 20 g L−1, the uptakes of Sr2+ would not increase. This appears to be because of the increase the number of biomass competing for the available binding sites in the S-HLLW and also due to the saturated binding sites for complexation of Sr2+ higher concentration levels. (Tan Y et al. 2017).
Effect of contact time
The uptake of Sr2+ by Y-7 was studied in the time range of 4–40 h. From Fig. 4, it showed that strontium biosorption was closely related to contact time. The maximum uptakes were found about 10.05 mg g−1 at 30 h and the theoretical maximum should be about 32 h. The biosorption rate increased quickly in the first 12 h and then gradually came up to 32.68%. It suggested that the activity sites of Sr2+ would increase in the S-HLLW at longer time, which could provide more the effective occupation degree of Sr2+ at the active sites of Y-7. After 30 h, those active sites might be saturated and from our straining experiment, the cells begin to die which means the living cells cannot adsorb Sr anymore (Chen and Wang 2016). This could explain why the maximum biosorption efficiency was observed at 32 h.
Effect of temperature
From our previous studies, the biosorption experiments were performed with using solution containing 10–40 °C. From Fig. 5, the maximum uptake was found about 11.5 mg g−1 at 32 h at 32 °C. In addition, the activity of Sr2+ increased in the aqueous phase at higher temperature, which may improve the effective occupation degree of Sr2+ at the active sites of yeast cells. But at higher temperature (> 32 °C), Sr2+ might not interact with the binding sites may be because of the result of self-regulation by Y-7. It was the same as our previous reported (Qiu et al. 2017).
Those results suggested that factors that affected biosorption include ion mixing, time, temperature, pretreatment, and biomass quantity. The optimal conditions for strontium biosorption for living S. cerevisiae were cell concentration 20 g L−1, contacting time 32 h, and temperature 32 °C. Some results were different from traditional studies; this is probably because the S. cerevisiae was living, when the conditions increased, the biosorption efficiency increased rapidly and then remained. For the biosorption system of living cells, each living cell is not utilized to adsorb strontium ions. (Coexistent metal ions mainly affect the biosorption of strontium ions from wastewater.) With the increased of biomass concentration, there were many strontium ions for every cell to absorb and the live cell system always stayed in the excess strontium ions state. But in this system, the biomass concentration was constant, it reflected that for living cells, the saturated binding sites were limited (Liu et al. 2014). The contacting time result suggested that when living cells was in a new culture environment that it broke the ionic equilibrium. Thus, additional time was necessary for the cell to regenerate its balance and survive in such an environment. The result was similarly reported in other studies (Gipps and Coller 1980).
Some studies had shown that the maximum adsorption or bioaccumulation quality of strontium by a non-living or living microorganism in the traditional adsorption method varies from 4.05 to 7.35 mg g−1 (Hu et al. 2017; Nie et al. 2016). In this study, we found that maximum strontium biosorption efficiency and quality of living S. cerevisiae under HLLW were 37.91% and 11.656 mg g−1. Those results are higher than the adsorption quantity reported in previous studies (Marešová et al. 2011; Weerasekara et al. 2013), but also those results indicate that radiation and the toxicity of heavy meals could decrease the sorption capacity. Therefore, it is necessary to study the behavior of these biosorbents for removing the potential radionuclides as a multicomponent system.
Results of staining experiment
In this study, we used methylene blue to verify whether the S. cerevisiae survived after biosorption. For living cells, their cell membranes are integrity which can exclude the methylene blue into the cell; if the cell loses its activity, it can increase the permeability of cell membrane, so methylene blue can enter the cell and bind the nucleic acid, making it become blue. Figure S.4 reflected the survival of S. cerevisiae after biosorption. Y-7 could remain active for 24 h in simulated condition (Fig. S.4(A)), but some dead cells were observed when the sorption time prolonged 32 h. For K-0 (Fig. S.4(B)), the staining result reflected that it could not remain active for 12 h and most of the cells were already dead when the sorption time prolonged 24 h. After biosorption for 32 h under RE, Y-7 retained its activity but K-0 was dead (Fig. S.5).
Results of TEM and EDX
In our previous studies, we used TEM-EDX to analyze the elemental information after biosorption. But from the result (S.7), we could find that despite the emergence of strontium, there are still too many energy peaks of other heavy metals. In order to avoid the error caused by other heavy metals in the EDX test, we decided to use SEM-EDX to the analysis of Y-7 after biosorption.
As shown in Fig. S.7(A), before biosorption, it was obvious that we can observe some organelles like vacuole, mitochondria, and nucleus. After biosorption, we only could observe part of vacuole and mitochondria from Fig. S.7(B), but it was obvious that the cell internal structure changed significantly and irreversible damage formed. From EDX results, before biosorption, it was not detected by TEM-EDX for Y-7 (Fig. S.9). From SEM-EDX (Fig. S.8), strontium was detected in one area (upper, spot1) after biosorption. Combined with the desorption results, those findings indicated that strontium ions entered into Y-7 by some mechanism, and were not merely fixed on the cell surface. As reported before (Lan et al. 2014; Merroun et al. 2003), metal binding to anionic functional groups or micro precipitated on the cell surface are general mechanisms. In response to metal pollution, microorganisms immobilize the toxic (heavy) metals using extracellular materials and prevent them into the cells. However, in this study, we had exposed the tolerance of irradiated S.cerevisiae (K-4000) and our previous studies had showed that Y-7 could survive after continuous exposure to strontium and continue to grow under culture conditions. Thus, we could find that after biosorption, the volume of vacuole had decreased and the cytoplasm was destroyed, this might because that the heavy metal entered into the cell. Therefore, we speculate that living microorganism biosorption can occur by metabolism-dependent and metabolism-independent processes (Kapoor and Viraraghavan 1995; Tomioka et al. 1998). The adsorption mechanism must be very complicated; it includes physical adsorption; chemisorption, and biological accumulation (Robalds et al. 2016).
Equilibrium of strontium biosorption to Y-7
To analyze the effect of pretreatment and HLLW on the isotherm biosorption of Sr2+ to Y-7, two isotherms of the Langmuir and Freundlich models were discussed in the present study. The non-linearized Langmuir and Freundlich isotherms of Sr2+ are shown Fig. 6, the experimental data were analyzed by non-linear regression analysis, and then, the isotherm constants and correlation coefficients are given in Table 3. From those results, it can be seen that the coefficient of determination (r2) decreased in the following order: Langmuir > Freundlich models and it should be noted that in Langmuir results, the qe was found smaller to be smaller than qmax (24.74 mg L−1) indicating that the biosorption of Sr2+ to Y-7 is occurred by a monolayer-type adsorption in which the surface of the microorganism was not fully covered.
Kinetics of strontium biosorption by Y-7
The correlation coefficients (r2) for the pseudo-second-order kinetic model at different initial Sr2+ concentrations were found to be more than 0.9 (Table 3). It indicated that the biosorption of Sr2+ ions to Y-7 follows a pseudo-second-order model. This suggests that chemical biosorption involves valence forces through the sharing or exchange of electrons between the biosorbents. However, it should be noted that biosorption of Sr2+ to Y-7 under S-HLLW is not a rapid process (Fig. 7), this is probably due to the other metal ions in the conditions which may decrease the biosorption capacity of Sr2+. The adsorption may be the rate-limiting step of biosorption process (Liu et al. 2014).
Thermodynamic parameters
The values of the Gibbs free energy change (ΔG0), enthalpy change (ΔH0), and entropy change (ΔS0) for the biosorption process were obtained from Eqs. The negative value (Table 5) of ΔG0 confirms the feasibility of the process and the spontaneous nature of biosorption of Sr2+ to Y-7. The ΔG0 values indicate the degree of spontaneity of the adsorption process, where more negative values reflect a more energetically favorable adsorption process. The values (Table 4) of ΔH0 the biosorption of Sr2+ to Y-7 were positive which indicated that the biosorption processes are endothermic and the positive of ΔS0 reflected the increase of randomness at the solid/solution interface and the good affinity of Sr2+ for Y-7. The thermodynamic results showed that all the biosorption reactions were spontaneous process and the temperature increasing were favorable for the biosorption of Sr2+ to Y-7 for the experimental conditions considered.
Desorption results
Several mechanisms responsible for the binding of metal ions to bacterial cells can be quantitatively assessed using appropriate desorption reagents. Desorption of the adsorbed strontium on Y-7 was investigated in the presence of deionized water, HCl, and EDTA-Na2 in solution. As depicted in Table 5, the HCl and EATA-Na are high-efficiency desorbing agents, capable of recovering 60.62 and 76.51% of strontium adsorbed by Y-7 and H-K for 0.1 M EDTA-Na2, respectively. The desorption efficiency of strontium adsorbed onto Y-7 and H-K was 76.51 and 83.32% by 1 M HCl. However, the desorption efficiency was only 7.65 and 18.82% for Y-7 and H-K by deionized water, respectively. When metal ions come into contact with bacterial cells, some ions enter into the cell wall mesh structure and are adsorbed by physical entrapment. This part is bound weakly and can be desorbed by water (Wang et al. 2013a), it could be called “microprecipitation” (Robalds et al. 2016). Another fraction that complexes with cell wall functional groups such as carboxyl and phosphate groups can be desorbed by EDTA but not by water, this part include chemisorption (Robalds et al. 2016). Also, the adsorbed strontium can be desorbed from various biomaterials by H+, because it includes not only the functional group, but also interaction (redox) and ion exchange (Mg2+, Ca2+) (Wang et al. 2013a), and this part could be attributed as “electrostatic attraction” (Robalds et al. 2016).
Also, from the results, we could observe that the desorption rate of H-K was much higher than that of Y-7. This result was similar to previous studies (Chen and Wang 2016; Wang et al. 2013a) and it indicated that the adsorption of non-living cells mainly concentrated on the cell surface, for living cell strontium was considered to be accumulated inside the cell. Considering cost, safety, and efficiency, HCl was suggested to be applied for strontium recovery from Y-7.
Conclusion
Strontium sorption by S. cerevisiae was increased after pre-exposure to irradiation. The significant changes observed in RNA-seq results and the structural degradation of biomass may be due to the radiolysis from the marginal increment of the sorption capacity of S. cerevisiae towards strontium.
The biosorption of strontium to living cells (Y-7) from S-HLLW is a complicated process controlled by many environment variables, including time, cell concentrations, and temperature. The biosorption capacity of living cells (Y-7) under S-HLLW conditions could reach 11.656 mg g−1. From our biosorption process analysis, the strontium ions were firstly adsorbed to the surface of cells, and then moved into the S. cerevisiae across the cell membrane, which were not merely fixed on the cell surface. We propose that the absorption of strontium by living S. cerevisiae has two steps: the first step is passive biosorption, during which strontium ions are adsorbed to the cell surface due to chemical and physical sorption. The second step is the penetration of the cell membrane into cytoplasm or on organelle, which can be regarded as initiative biosorption. Initiative biosorption may depend on energy and is related to the metal transport in the living cells. Our work indicates that living S. cerevisiae (Y-7), as a sorbent material, is promising for applications in the treatment of radioactive process streams.
References
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Bahieldin A, Atef A, Sabir JSM, Gadalla NO, Edris S, Alzohairy AM, Radhwan NA, Baeshen MN, Ramadan AM, Eissa HF, Hassan SM, Baeshen NA, Abuzinadah O, al-Kordy MA, el-Domyati FM, Jansen RK (2015) RNA-Seq analysis of the wild barley (H. spontaneum) leaf transcriptome under salt stress. C R Biol 338(5):285–297
Bailly du Bois P, Laguionie P, Boust D, Korsakissok I, Didier D, Fiévet B (2012) Estimation of marine source-term following Fukushima Dai-ichi accident. J Environ Radioactiv 114(12):2–9
Chen C, Wang J (2008) Removal of Pb2+, ag+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass. J Hazard Mater 151(1):65–70
Chen C, Wang J (2016) Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J Environ Radioactiv 162:134–145
Chepelev I, Wei G, Tang Q, Zhao K (2009) Detection of single nucleotide variations in expressed exons of the human genome using RNA-Seq. Nucleic Acids Res 37(16):e106–e106
Clarke PH (1985) The scientific study of bacteria, 1780–1980. In: The scientific study of bacteria, 1780–1980. Springer, Berlin
Dabbagh R, Ghafourian H, Baghvand A, Nabi G, Riahi H, Ahmadi Faghih M (2007) Bioaccumulation and biosorption of stable strontium and 90Sr by Oscillatoria homogenea cyanobacterium. J Radioanal Nucl Ch 272(1):53–59
Dai Q, Zhang W, Dong F, Yulian Z, Wu X (2014) Effect of γ-ray radiation on the biosorption of strontium ions to baker’s yeast. Chem Eng J 249:226–235
Deák T, Beuchat LR (1996) Handbook of food spoilage yeasts. CRC Press, Boca Raton
Eccles H (1995) Removal of heavy metals from effluent streams—why select a biological process? Int Biodeter Biodegr 35(1–3):5–16
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101(14):5043–5053
Gipps J, Coller B (1980) Effect of physical and culture conditions on uptake of cadmium by Chlorella pyrenoidosa. Mar Freshw Res 31(6):747–755
Ho Y, Wase DJ, Forster C (1996) Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ Technol 17(1):71–77
Hu W, Dong F, Yang G, Peng X, Huang X, Liu M, Zhang J (2017) Synergistic interface behavior of strontium adsorption using mixed microorganisms. Environ Sci Pollut Res Int 12:1–10
Izumida TKF (1990) Precipitates formation behavior in simulated high level liquid waste of fuel reprocessing. J Nucl Sci Technol 27(3):267–274
Kapoor A, Viraraghavan T (1995) Fungal biosorption—an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53(3):195–206
Kubota MFT (1980) Formation of precipitate in high-level liquid waste from nuclear fuel reprocessing. J Nucl Sci Technol 17(10):783–790
Lan T, Feng Y, Liao J, Li X, Ding C, Zhang D, Yang J, Zeng J, Yang Y, Tang J, Liu N (2014) Biosorption behavior and mechanism of cesium-137 on Rhodosporidium fluviale strain UA2 isolated from cesium solution. J Environ Radioactiv 134:6–13
Li CC, Chung HP, Wen HW, Chang CT, Wang YT, Chou FI (2015) The radiation resistance and cobalt biosorption activity of yeast strains isolated from the Lanyu low-level radioactive waste repository in Taiwan. J Environ Radioactiv 146:80–87
Liu J, Sheng H, Xu Y, Feng K (2008) Effect of Fe on the growth of Scenedesmus quadricauda. Environ Pollut Control 8:61–64
Liu M, Dong F, Yan X, Zeng W, Hou L, Pang X (2010) Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions. Bioresour Technol 101(22):8573–8580
Liu M, Dong F, Kang W, Sun S, Wei H, Zhang W, Nie X, Guo Y, Huang T, Liu Y (2014) Biosorption of strontium from simulated nuclear wastewater by Scenedesmus spinosus under culture conditions: adsorption and bioaccumulation processes and models. International Int J Env Res Pub He 11(6):6099–6118
Luo H, Dai S, Bonnesen PV (2004) Solvent extraction of Sr2+ and Cs+ based on room-temperature ionic liquids containing monoaza-substituted crown ethers. Anal Chem 76(10):2773–2779
Marešová J, Pipíška M, Rozložník M, Horník M, Remenárová L, Augustín J (2011) Cobalt and strontium sorption by moss biosorbent: modeling of single and binary metal systems. Desalination 266(1):134–141
Mei LI, Jin XU, Liu ZL, Jun XU (2004) Strontium stress on marine microalgae dicrateria inornata growth and antioxidant enzymes activities. Oceano Limnol Sin 35:467–472
Merroun M, Chekroun KB, Arias J, Gonzalez-Munoz M (2003) Lanthanum fixation by Myxococcus xanthus: cellular location and extracellular polysaccharide observation. Chemosphere 52(1):113–120
Nie X, Dong F, Liu M, Sun S, Yang G, Zhang W, Qin Y, Ma J, Huang R, Gong J (2016) Removel of uranium from aqueous solutions by Spirodela Punctata as the mechanism of biomineralization. Procedia Environ Sci 31:382–391
Nilchi A, Hadjmohammadi MR, Garmarodi SR, Saberi R (2009) Studies on the adsorption behavior of trace amounts of 90Sr2+, 140La3+, 60Co2+, Ni2+and Zr4+ cations on synthesized inorganic ion exchangers. J Hazard Mater 167(1–3):531–535
Parab H, Devi PSR, Shenoy N, Kumar SD, Bhardwaj YK, Reddy AVR (2016) Gamma irradiation stability studies of coir pith: a lignocellulosic biosorbent for strontium. J Radioanal Nucl Chem 308(1):323–328
Petin VG, Kim JK (2014) Survival and recovery of yeast cells after combined treatment with ionizing radiation and heat. Radiat Res 161(1):56–63
Petin VG, Evstratova ES, Kim JK (2014) Radiosensitivity, liquid-holding recovery and relative biological effectiveness of densely-ionizing radiation after repeated irradiation of yeast cells. Mutat Res-Gen Tox En 771(9):37–42
Qiu L, Feng J, Dai Y, Chang S (2017) Biosorption of the strontium ion by irradiated Saccharomyces cerevisiae under culture conditions. J Environ Radioactiv 172:52–62
Robalds A, Naja GM, Klavins M (2016) Highlighting inconsistencies regarding metal biosorption. J Hazard Mater 304:553–556
Shozugawa K, Nogawa N, Matsuo M (2012) Deposition of fission and activation products after the Fukushima Dai-ichi nuclear power plant accident. Environ Pollut 163(4):243–247
R.L. Smith J, P. Atmaji, Y. Hakuta z, M. Kawaguchi, T. Adschiri, K. Arai (1997) Recovery of metals from simulated high-level liquid waste with hydrothermal crystallization. J Supercrit Fluid 11(1):103–114
Sud D, Mahajan G, Kaur M (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—a review. Bioresour Technol 99(14):6017–6027
Tan Y, Feng J, Qiu L, Zhao Z, Zhang X, Zhang H (2017) The adsorption of Sr(II) and Cs(I) ions by irradiated Saccharomyces cerevisiae. J Radioanal Nucl Ch 314(3):2271–2280
Tomioka N, Tanaka K, Uchiyama H, Yagi O, Kokufuta E (1998) Recovery of 137Cs by a bioaccumulation system using Rhodococcus erythropolis CS98. J Biosci Bioeng 85(6):604–608
Tu Y-J, You C-F, Chen Y-R, Huang C-P, Huang Y-H (2015) Application of recycled iron oxide for adsorptive removal of strontium. J Taiwan Inst Chem E 53:92–97
Wang L, Feng Z, Wang X, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26(1):136–138
Wang L, Wan C, Lee D-J, Tay J-H, Chen X, Liu X, Zhang Y (2013) Adsorption–desorption of strontium from waters using aerobic granules. J Taiwan Inst Chem E 44:454–457
Wang T, Zheng X, Wang X, Lu X, Shen Y (2017) Different biosorption mechanisms of Uranium(VI) by live and heat-killed Saccharomyces cerevisiae under environmentally relevant conditions. J Environ Radioactiv 167:92–99
Weerasekara NA, Choo KH, Choi SJ (2013) Metal oxide enhanced microfiltration for the selective removal of Co and Sr ions from nuclear laundry wastewater. J Membrane Sci 447(22):87–95
Yaochuan Wang XG (1994) Lethal dose of Escherichia coli, Salmonella enteritidis, Staphylococcus aureus and Saccharomyces cerevisiae. Nucl Agric Bull 15:171–174
Zhang AKE, Kumagai M (2006) Removal of Pd(II), Zr(IV), Sr(II), Fe(III), and Mo(VI) from simulated high level liquid waste by extraction chromatography utilizing the macroporous silica-based polymeric materials. Sep Purif Technol 50(1):35–44
Funding
Sponsored by the Natural Science Foundation of Jiangsu Province (SBK2014041829), the graduate student innovation fund of Nanjing University of Aeronautics and Astronautics (kfjj201444), the environmental protection scientific research subject in Jiangsu province (Grant No. 2016003), the A Project Fund by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the National Natural Science Foundation of China (Grant No. 11705089).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Georg Steinhauser
Rights and permissions
About this article
Cite this article
Qiu, L., Feng, J., Dai, Y. et al. Biosorption of strontium ions from simulated high-level liquid waste by living Saccharomyces cerevisiae. Environ Sci Pollut Res 25, 17194–17206 (2018). https://doi.org/10.1007/s11356-018-1662-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1662-6