Abstract
An analytical technique was developed to determine the age of uranium particles. After the chemical separation of uranium and thorium, the 230Th/234U ratio was measured using single-collector inductively coupled plasma mass spectrometry and a 233U-based reference material comprising a certain amount of 229Th as a progeny nuclide of 233U. The results allowed us to determine the purification age of two certified materials, i.e., U-850 and U-100, which was in good agreement with the reference purification age (61 y). Moreover, the age of a single U-850 particle was determined with a difference of − 28 to 2 years from the reference date.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The analysis of safeguards environmental samples has been adopted by the International Atomic Energy Agency (IAEA) for the detection of undeclared nuclear activities, where isotopic and quantitative analyses of nuclear materials have been performed in environmental swipe samples collected during IAEA safeguards inspections [1, 2]. Determining the age of nuclear materials can reveal important and detailed data on nuclear activity, as it can provide hints about their origin and history. To date, several studies have focused on determining the age of nuclear material for nuclear safeguards and forensics purposes using the 230Th–234U or 231 Pa–235U chronometer for uranium [3,4,5,6,7,8,9,10,11,12] and the 241Am–241Pu or 236U–240Pu chronometer for plutonium [13,14,15,16,17].
The nuclear materials contained in safeguards environmental swipe samples collected from nuclear facilities are expected to be found in small quantities in the form of micron-sized particles. Therefore, the age dating in the field of swipe sample analysis for safeguards should focus mainly on individual nuclear particles. In previous studies, we have determined the age of plutonium for single plutonium or uranium–plutonium mixed particles [18,19,20]. However, the determination of the age of uranium, which is one of the most interesting elements in safeguards, is more challenging compared to plutonium, because the number of daughter atoms grown from their parent nuclide is small due to the long half-lives of 234U and 235U compared to those of 240Pu and 241Pu. Several studies have reported the age determination of micron-sized particles consisting of uranium oxides. Pointurier et al. [7] determined the age (40 years) of 1%-enriched uranium samples of a few micrograms (10–6 g) by spiking of 229Th and 233U reference solutions, chemical separation using anion-exchange columns, and determination of the 230Th/234U ratio by combination of inductively coupled plasma mass spectrometry (ICP–MS) and thermal ionization mass spectrometry measurements. They determined 6–25 fg of 230Th in their analysis. Meanwhile, further ultra-trace analytical technique should be used for the IAEA safeguards environmental samples to determine the age of micron-sized uranium particles. Fauré et al. [10] determined the age of uranium oxide particles (0.8–2.8 μm in diameter) of U-100, U-850, U-900, and U-970 by secondary ion mass spectrometry (SIMS). They directly measured 230Th/234U ratio in a particle which may contain sub-fg of 230Th. Szakal et al. [21] determined the purification age of individual uranium micron-sized particles by large geometry secondary ion mass spectrometry (LG–SIMS). In many research works on the U–Th age determination, the 230Th/234U ratio was measured by SIMS/LG–SIMS, or isotope dilution method for determining uranium and thorium in a sample separately. In our laboratory, a batch of 233U spike solution have been stored for determining the total mass of uranium in the IAEA safeguards environmental samples by the isotope dilution method. It was found that our batch of the CRM 111-A was contaminated with trace plutonium, and the uranium in this CRM 111-A was chemically purified in 2004. Almost all other elements, including the long-lived thorium isotopes (229Th and 230Th) were also chemically eliminated by this purification. The progeny nuclides of the purified 233U, including 229Th were growing in equilibrium in this bottle. This aged 233U solution was naturally spiked with 229Th, whose 229Th/233U atomic ratio is calculable, and let us come up with this idea to simply determine the purification age of a single uranium particle by adding this 229Th–233U spike to the samples.
Herein, we demonstrated a method for determining the age of uranium particles using the 229Th–233U spike solution. Uranium oxide particles with known purification age were used, while the 230Th–234U chronometers along with the chemical separation of femtograms (fg) of thorium from uranium were applied for the accurate determination of the purification age. This analytical technique could be further applied to individual uranium particles collected from safeguards environmental swipe samples.
Experimental
Materials and reagents
Two types of uranium certified reference materials (CRM), U-850 (85% enriched 235U, 0.6437% 234U) and U-100 (10% enriched 235U, 0.0676% 234U) as U3O8 now distributed by New Brunswick Laboratory (NBL), USA, whose uranium purification was completed on December 31, 1957 and January 8, 1959 [6], respectively, were used for the analysis of this study. The atomic ratios of 234U and 230Th in the uranium samples were determined by spiking a CRM 111-A solution (99.4911% enriched 233U, NBL, USA) stored in our laboratory, where uranium was chemically purified on August 5, 2004 by anion-exchange chromatography. The feed CRM 111-A (807 μg of 233U) was dissolved in 2 mL of 10 M HCl–0.1 M HNO3, and loaded on an anion-exchange column (inner diameter: 3.5 mm, length 130 mm, volume: 1.25 mL). Then, 6.4 mL of 10 M HCl–0.1 M HNO3, 7 mL of 10 M HCl, and 14.2 mL of 10 M HCl–0.1 M HI were eluted in order to remove trace plutonium as a contaminant. The thorium (Th(IV)) was not retained on the anion-exchange resins, and eluted to the waste fractions. The amount of remaining 229Th in the purified CRM 111-A were negligible to determine the uranium purification age; less than 6 × 10–9 of 229Th/233U atomic ratio, which was estimated from the analytical results of similar experiments of thorium and uranium separation. On this date, the progeny nuclides, including 229Th, were also removed from the CRM. The amount of 229Th increased as a decay product of 233U, and the calculated atomic ratio of 229Th/233U in the spike solution as of September 2019 was 6.56 × 10–5. The decay-corrected 229Th/233U, 234U/233U, and 230Th/229Th ratios of CRM 111-A were in good agreement with the experimental results measured by ICP–MS within the uncertainty. Thus, the calculated atomic ratio was used to determine 230Th, which is the 234U progeny nuclide produced in uranium particles.
Highly pure HCl and HNO3 (TAMAPURE AA-100 and/or AA-10, TAMA Chemicals Co. Ltd., Japan) were used for all chemical treatments. Water was deionized (resistivity: 18.2 MΩ cm) with the Milli-Q system (Millipore Corp., USA), while quartz glass beakers and columns and small PFA jars (capacity: 7 mL) were purified before their use by soaking in HNO3 (analytical grade) and rinsing with Milli-Q water.
Sample preparation
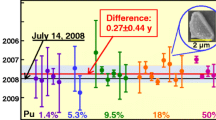
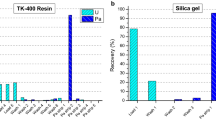
Sixteen and twelve samples with varying number of particles were prepared for the CRM U-850 and U-100, respectively. In particular, 2–210 particles with a diameter of 3–17 μm (equivalent to 0.7–126 ng of the total uranium content) were included in U-850, whereas 23–375 particles with a diameter of 2–15 μm (equivalent to 9.9–356 ng of the total uranium content) were included in U-100. Five sets of single U-100 particles (diameter: 6–20 μm) ranging between 0.9 and 16.3 ng and six sets of single U-850 particles (diameter: 8–10 μm) ranging between 0.9 and 3.4 ng were also prepared. The total uranium amount in each sample was calculated by the isotope dilution mass spectrometry method based on the measured 234U/233U isotopic ratio, the CRM isotopic abundance, and the CRM 111-A amount added to each sample. The CRM uranium oxide particles were collected and transferred to a 5 × 5 mm piece of silicon wafer (Semitec Co. Ltd., Japan) using a manipulator attached to a scanning electron microscope (SEM, JSM-7800F, JEOL Ltd., Japan) [22]. There was no electrostatic charging during the particle transfer due to using a glass-needle coated with gold. Figure 1 shows a typical SEM image of a U-850 particle. The silicon wafer loaded with the uranium particles was then placed into a small PFA jar, and 1.8 mL of 8 M HNO3 and 0.2 mL of 6 M HCl were poured into the jar. The PFA jar lid was tightly screwed and the jar was heated on a hot plate at 180 °C for 7 h to completely dissolve the uranium particles. After removing the silicon wafer, 0.22 mL of the CRM 111-A solution (2.3 ng-U mL–1), in which 0.5 ng of 233U is containing, was spiked into this acid-digested solution in the PFA jar, followed by evaporation to dryness. The dried residue was dissolved again using 0.7 mL of 15 M HNO3 to achieve a more efficient mixing of the contents, and the solution was evaporated to dryness. The dried residue was dissolved again in 0.7 mL of 9.5 M HCl to form the corresponding hydrochlorides and the resulting solution was evaporated again to eliminate the nitrate ions. The final dried residue was dissolved in 0.7 mL of 8.5 M HCl. Then, thorium and uranium contained in the solution were chemically separated by anion-exchange chromatography using an anion-exchange resin (MCI GEL, CA08Y, Mitsubishi Chemical Corporation, Japan) packed into a quartz glass column (inner diameter: 2 mm, length: 16 mm, volume: 0.05 mL). The feed solution was loaded onto the column and thorium and uranium were sequentially eluted using 0.7 mL of 8.5 M HCl and 0.5 mL of 0.2 M HCl, respectively. The obtained thorium and uranium fractions were then evaporated to dryness and redissolved in 0.3 mL of 15 M HNO3. After further evaporation to dryness, the residues were dissolved in 7 mL of 0.81 M HNO3 and these solutions were used for the ICP–MS measurements. All operations, including the sample preparation and the ICP–MS measurements, were performed in clean rooms (ISO Class 6) at the Clean Laboratory for Environmental Analysis and Research (CLEAR) of the Japan Atomic Energy Agency (JAEA) [23]. Moreover, to evaluate the performance of the single anion-exchange separation method, the decontamination factor and the recovery yield were estimated by separating five aliquots of a solution containing 270 ng of the CRM U-850 and 6 ng of the CRM 111-A and measuring the intensity of the 229Th and 233U signals in the spikes.
Instrumentation and purification age calculation
The isotope ratios of thorium and uranium were measured using an ICP–MS instrument (ELEMENT-2, Thermo Fischer Scientific Inc., USA) under the conditions shown in Table 1. Specifically, the thorium isotope ratios were measured using the ICP–MS instrument attached to a desolvation inlet system (APEX-Q, Elemental Scientific Inc., USA). This inlet system enhanced the sensitivity by a factor of three. The mass bias was corrected by measuring the isotope ratio of the CRM U-015 (NBL, USA) solution and using the following equation:
where Rt is the certified isotope ratio, Rm is the measured isotope ratio, ΔM is the mass difference, and C is the mass discriminator factor. In addition, the uranium age (t) was determined using Eq. (2):
where R represents the 230Th/234U ratio in the sample and λU234 and λTh230 are the decay constants of 234U and 230Th, respectively, calculated based on the half-life of 234U (2.455 (6) × 105 y [24]) and 230Th (7.54 (3) × 104 y [25]), respectively.
The 230Th/234U ratio (R) was in turn determined based on Eq. (3):
where (230Th/229Th)Meas. and (234U/233U)Meas. are the isotope ratios of 230Th/229Th and 234U/233U measured by ICP–MS in the thorium and uranium fractions, respectively. Moreover, the ratios (230Th/229Th)Sp., (234U/233U)Sp., and (229Th/233U)Sp. represent the ratios in the CRM 111-A spike calculated from the 233U and 234U abundance data based on the CRM-111A reference sheet. The radioactive decay and growth after the uranium purification were also corrected. Considering that the CRM 111-A contains 0.1847 atomic% of 234U and, that 230Th is growing as a progeny nuclide of 234U, the amount of 234U, and 230Th impurities in the CRM 111-A spike were estimated by calculating the radioactive decay and growth. The calculated amount of the 234U and 230Th impurities in the spike were subtracted from the measured values, which in turn facilitated the accurate age determination of uranium. The atomic ratio of 229Th/233U in the CRM 111-A, (229Th/233U)Sp., was calculated from the radioactive decay of 233U and growth of 229Th, assuming that no 229Th was present in the purified 233U spike. In addition, the combined uncertainty was estimated taking into account all uncertainties in the ICP–MS intensities, mass bias correction, certified values of the reference materials, and half-life values of each nuclide to comply with the principles described in the Guide to the Expression of Uncertainty in Measurement (GUM) [26].
Results and discussion
As shown in Table 2, the measured ratios of 229Th/233U, 234U/233U, and 230Th/229Th of the CRM 111-A were in good agreement with the corresponding calculated ratios within one standard deviation of each ICP–MS measurement. This result indicated that the uranium–thorium decay chain (i.e., 233U–229Th and 234U–230Th) was closed in this system. Thus, the calculated values for the 229Th/233U, 234U/233U, and 230Th/229Th ratios of the CRM 111-A were used to further calculate the 230Th/234U ratio using Eq. (3). Based on Table 3, uranium and thorium were separated with a high chemical recovery yield, which was > 97% for uranium and 88–96% for thorium, while the decontamination factor of uranium in the thorium fraction was greater than 2 × 106. These results suggested that uranium and thorium were sufficiently separated and recovered in each fraction, thus allowing the precise determination of the purification age.
The whole process blanks and detection limits determined for the investigated nuclides are shown in Table 4. Based on the three-sigma criterion, the detection limits of the target nuclides at these interested masses were determined using three times the standard deviation of the process blank count rate (cps) under the analytical conditions, 7 mL of the solution volume, and sensitivity (12.8 cps ppq–1 for 229Th and 230Th and 13.4 cps ppq–1 for 233U and 234U). The process blanks in the entire analysis were estimated to be 0.04 ng of natural uranium and 1 fg of 230Th. The detection limit for 230Th was calculated to be 1.3 fg, and was in line with the results of Pointurier et al., who have reported that the instrument detection limit for 230Th is 1 fg by measuring a diluted 2% HNO3 solution commonly used for the instrument rinsing [7].
Furthermore, according to Table 5, the determined purification ages for the U-850 particles agreed with the reference age (61 y) within the expanded uncertainty at the 95% confidence level. In the case of the U-850 particles, the difference between the determined ages and the reference purification age ranged between − 9.8 to 12 y and was 0.8 y in average, except for samples No. 4 and No. 5, where significantly low chemical recovery yields (~ 27% each) were obtained. Especially when the total amount of uranium particles analyzed was higher than 10 ng (No. 11–16); the determined age showed a difference of only three years from the reference age. However, according to Table 6, the precision of the age estimation for the U-100 particles was lower than that for the U-850 particles due to the ultra-trace contents of 230Th and 234U. As shown in Table 6, when more than 50 ng of U-100 particles were analyzed (No. 20–28), the difference from the reference date ranged between − 7.7 to 1.5 y. Consequently, this trace analytical method proved to be available for the determination of the purification age of uranium particles in uranium–thorium samples.
The contribution of uncertainty (uncertainty budgets) to the age determination of the representative samples was also investigated. As shown in Table 7, the 230Th/229Th ratio (0.202 ± 0.013) was the main contributor to the uncertainty for the U-850 particles, whereas both the 230Th/229Th (91–95%) and 234U/233U (5–8%) ratios contributed to the uncertainty for the U-100 particles. Moreover, Williams and Gaffney performed an age determination analysis using large amounts (of mg scale) of U-100 and reported that the contribution of the 230Th/229Th ratio measurement to the overall uncertainty was 55.19% [6]. Thus, we concluded that the 230Th/229Th ratio measurement uncertainty significantly contributes to the uncertainty of the purification age only when the sample amount is low. Therefore, the 230Th/229Th ratio should be measured with high accuracy to achieve a small uncertainty in the age determination when small sample amounts, such as particles, are used.
The purification age of single U-850 and U-100 particles was also determined (Table 8). For the U-850 particles, the difference from the reference purification age ranged between − 28 to 2 y, which was in good agreement with the actual age, although the uncertainties were large. In contrast, only the purification age of the U-100 particles No. 38 and No. 39, whose total uranium weight was more than 14 ng, could be determined. Figure 2 shows the relationship between the difference from the reference purification date and (a)the total uranium amount, (b)the 234U amount, and (c)the 230Th amount in the U-850 and the U-100 particles. Figure 2(a) shows the measured purification date of the U-100 particles whose total uranium amount is less than 14 ng was older than the reference date, but the difference was not found in the case of the U-850. By changing the horizontal axis of this figure to the 234U amount (Fig. 2b) and 230Th (Fig. 2c), the systematic error of the purification date can be identified. The systematic error was obviously confirmed in the case of less than 3 pg of 234U and 0.5 fg of 230Th in the measured U-100 particles. In the case of the U-850 particles, the all measured date were consistent with the reference date within the uncertainty. The all U-850 particles contained enough 234U and 230Th amount more than the critical values, which were estimated from the analytical results of U-100 particles. The purification date of the U-100 particles gradually decreased (older) as the 234U and the 230Th amount decreased. The cause of this systematic difference can be clarified by discussing the accuracy of 234U and 230Th amount in the analyzed uranium particle. The 234U amount in a particle of sample ID 35 listed in Table 8, which sample was shown the largest difference from the reference purification date, was estimated to be 0.6 pg (234U atomic abundance: 0.0676%). This amount is enough to obtain the 234U/233U isotopic ratio with high accuracy and good precision because several hundred counts were measured in the ICP–MS measurement. Whereas, the 230Th amount of sample ID 35 was approximately 0.1 fg. In this case, around 0.2 cps of the 230Th intensity can be expected in the ICP–MS measurement. This intensity is extremely lower than that of the 230Th process blank (1.9 cps) listed in Table 4. As shown in the Table 7, accuracy and uncertainty of the measured 230Th/229Th ratio mainly contributed to the whole uncertainty and the lower limit of determination of the purification age in this analytical technique. Figure 3 shows the relationship between the 230Th/234U ratio of the U-100 particles (Table 8) calculated from Eq. (3) and the total uranium amount analyzed in this study. The 230Th/234U ratio (i.e., the purification age) in a single U-100 particle gradually increased as the total uranium amount decreased. Moreover, the three curves in Fig. 3 indicated that the gradual increase in the 230Th/234U atomic ratio in a U-100 particle was due to the contamination of ultra-trace 230Th as a process blank. The determined 230Th/234U ratio was dotted ranging in the 1–2-fg curvature region, indicating that 230Th contamination as a process blank from 1 to 2 fg randomly occured during the age determination analysis run of these U-100 paticles. This systematic error of the variation between 1 and 2 fg implied that the 230Th process blank should be carefully controlled and accurately corrected at the fg level to accurately determine the purification age of a single low-enriched uranium particle. Figure 3 shows 230Th contamination of 1–2 fg may happen during the age determination analysis run, including the chemical separation, the sample preparation of ICP–MS measurement. However, many of the particles containing 230Th of approximately 1 fg were good agreement with the reference purification date within the uncertainty as shown in Fig. 2c. Consequently, the practical small particle-size limit for being able to analyze an age determination with the method in this work considered to be the particle containing 230Th of 1 fg, which particle size were, then, estimated to be 5.9 µm in diameter for the U-850, and 12.6 µm in diameter for the U-100, respectively (in case of the age 61 y, and the form of spherical shape U3O8). The precise measurement of the isotope ratios using a multi-collector ICP–MS (MC–ICP–MS) and LG–SIMS would also contribute to the effective determination of the purification age.
Conclusions
The purification age of a high-enriched (1 ng) and a lower-enriched single uranium particles (tens ng) with micron-sized was accurately determined with the isotope dilution method using a 229Th–223U mixed spike. This mixed uranium spike was readily prepared by leaving a chemically purified 233U spike solution to stand for a long time, thus increasing the 229Th amount emerging as a progeny nuclide of 233U. The results indicated that the main factor contributing to the whole uncertainty in the results was the uncertainty in the 230Th/229Th ratio measurement in the chemically separated thorium fraction. Thus, it was strongly suggested that the thorium isotopes should be accurately analyzed at the sub-fg to fg level, and that the 230Th process blank should be carefully controlled at the sub-fg level. MC–ICP–MS will be thus applied as a powerful tool to achieve this ultra-trace analysis and the analytical results will be reported in the near future.
References
Donohue DL (1998) Strengthening IAEA safeguards through environmental sampling and analysis. J Alloys Compd 271–273:11–18
Donohue DL (2002) Strengthened nuclear safeguards. Anal Chem 74:28A-35A
Wallenius M, Morgenstern A, Apostolidis C, Mayer K (2002) Determination of the age of highly enriched uranium. Anal Bio Chem 374:379–384
LaMont SP, Hall G (2005) Uranium age determination by measuring the 230Th/234U ratio. J Radioanal Nucl Chem 264:423–427
Varga Z, Surányi G (2007) Production date determination of uranium-oxide materials by inductively coupled plasma mass spectrometry. Anal Chim Acta 599:16–23
Williams RW, Gaffney AM (2011) 230Th–234U model ages of some uranium standard reference materials. In: Proceedings of Radiochim Acta 1:31–35 (Oak Ridge National Laboratory: DOE/K25 Archives)
Pointurier F, Hubert A, Roger G (2013) A method for dating small amounts of uranium. J Radioanal Nucl Chem 296:593–598
Varga Z, Mayer K, Bonamici CE, Hubert A, Hutcheon I, Kinman W, Kristo M, Pointurier F, Spencer K, Stanley F, Steiner R, Tandon L, Williams R (2015) Validation of reference materials for uranium radiochronometry in the frame of nuclear forensic investigations. Appl Radi Isot 102:81–86
Gaffney AM, Hubert A, Kinman WS, Magara M, Okubo A, Pointurier F, Schorzman KC, Steiner RE, Williams RW (2016) Round-robin 230Th–234U age dating of bulk uranium for nuclear forensics. J Radioanal Nucl Chem 307:2055–2060
Fauré AL, Dalger T (2017) Age dating of individual micrometer-sized uranium particles by secondary ion mass spectrometry: an additional fingerprint for nuclear safeguards purposes. Anal Chem 89:6663–6669
Varga Z, Venchiarutti C, Nicholl A, Krajkó J, Jakopič R, Mayer K, Richter S, Aregbe Y (2016) IRMM-1000a and IRMM-1000b uranium reference materials certified for the production date. Part I: methodology, preparation and target characteristics. J Radioanal Nucl Chem 307:1077–1085
Venchiarutti C, Varga Z, Richter S, Nicholl A, Krajko J, Jakopič R, Mayer K, Aregbe Y (2016) IRMM-1000a and IRMM-1000b: uranium reference materials certified for the production date based on the 230Th/234U radiochronometer. Part II: certification. J Radioanal Nucl Chem 308:105–111
Wallenius M, Mayer K (2000) Age determination of plutonium material in nuclear forensics by thermal ionization mass spectrometry. Fresenius J Anal Chem 366:234–238
Wallenius M, Tamborini G, Koch L (2001) The “Age” of plutonium particles. Radiochim Acta 89:55–58
Chen Y, Chang ZY, Zhao YG, Zhang JL, Li JH, Shu FJ (2009) Studies on the age determination of trace plutonium. J Radioanal Nucl Chem 281:675–678
Shinonaga T, Donohue D, Ciurapinski A, Klose D (2009) Age determination of single plutonium particles after chemical separation. Spectrochim Acta B 64:95–98
Mayer K, Wallenius M, Varga Z (2013) Nuclear forensics Science: correlating measurable material parameters to the history of nuclear material. Chem Rev 113:884–900
Miyamoto Y, Esaka F, Suzuki D, Magara M (2013) Precies age determination of a single plutonium particle using inductively coupled plasma mass spectrometer. Radiochim Acta 101:745–748
Esaka F, Suzuki D, Miyamoto Y, Magara M (2015) Plutonium age determination from 240Pu/236U ratios in individual particles by ICP-MS without prior chemical separation. Microchem J 118:69–72
Miyamoto Y, Suzuki D, Esaka F, Magara M (2015) Accurate purification age determination of individual uranium-plutonium mixed particles. Anal Bioanal Chem 407:7165–7173
Szakal C, Simons DS, Fassett JD, Fahey AJ (2019) Advances in age-dating of individual uranium particles by large geometry secondary ion mass spectrometry. Analyst 144:4219–4232
Esaka F, Esaka KT, Lee CG, Magara M, Sakurai S, Usuda S, Watanabe K (2007) Particle isolation for analysis of uranium minor isotopes in individual particles by secondary ion mass spectrometry. Talanta 71:1011–1015
Usuda S, Yasuda K, Kokubu YS, Esaka F, Lee CG, Magara M, Sakurai S, Watanabe K, Hirayama F, Fukuyama H, Esaka KT, Iguchi K, Miyamoto Y, Chai JY (2006) Challenge to ultra-trace analytical techniques of nuclear materials in environmental samples for safeguards at JAERI: methodologies for physical and chemical form estimation. Int J Environ Anal Chem 86:663–675
Browne E, Tuli JK (2007) Nuclear data sheets for A = 234. Nucl Data Sheets 108:681–772
Browne E, Tuli JK (2012) Nuclear data sheets for A = 230. Nucl Data Sheets 113:2113–2185
Usuda S, Magara M, Esaka F, Yasuda K, Kokubu YS, Lee CG, Miyamoto Y, Suzuki D, Inagawa J, Sakurai S, Murata F (2010) QA/QC activities and estimation of uncertainty for ultra-trace analysis of uranium and plutonium in safeguards environmental samples. J Nucl Radiochem Sci 11:A5–A9
Acknowledgements
This work was financially supported by the Nuclear Regulation Authority, Japan. The authors would like to thank Mr. T. Watanabe and Mr. D. Endo for the chemical treatments, Mr. T. Onodera and Mr. H. Matsuyama for the sample preparation, and Mr. Y. Takahashi for the ICP–MS measurements. The valuable comments of reviewers are appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, D., Tomita, R., Tomita, J. et al. Age determination analysis of a single uranium particle for safeguards. J Radioanal Nucl Chem 328, 103–111 (2021). https://doi.org/10.1007/s10967-021-07626-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07626-x