Abstract
Age of individual uranium–plutonium (U/Pu) mixed particles with various U/Pu atomic ratios (1–70) were determined by inductively coupled plasma mass spectrometry. Micron-sized particles were prepared from U and Pu certified reference materials. The Pu reference was stored for 4–6 years since the last purification (July 14, 2008). The Pu purification age was obtained from the 241Am/241Pu ratio which was calculated from the product of three measured ratios of Pu and Am isotopes in the eluted fractions. These ratios were measured by a high-resolution inductively coupled plasma mass spectrometer equipped with a desolvation system. Femto-gram to pico-gram quantities of Am, U, and Pu in a sample solution were sequentially separated on a small anion-exchange column. The 241Am/241Pu ratio was accurately determined by spiking pure 243Am into the sample solution. The average determined age for the particles for the five independent U/Pu ratios was in good agreement with the expected age with high accuracy (difference age 0.27 years) and high precision (standard deviation 0.44 years). The described analytical technique can serve as an effective tool for nuclear safeguards and environmental radiochemistry.

Young (4‒6 y) Pu purification age of individual U/Pu mixed micron-sized reference particles for the five independent U/Pu ratios (1‒70) were determined with 0.27±0.44 y difference from the expected age. Sub pico-gram quantities of Am, U and Pu were sequentially separated a small column, and their isotope ratios were accurately measured using an ICP-MS by applying the 243Am spiking technique to the analysis and correcting the impurity and the contaminations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The terms age determination, age dating, and chronometry are commonly used in geology, cosmochemistry, and archeology to refer to the period of mineral formation and material productions. The age determination technique is based on the continuous increase of progeny nuclides after the last chemical separation of parent nuclide. The time (age) can be calculated from the measured atomic ratio between the parent nuclide and its derived progeny using the radioactive decay equation. Neutron capture reaction of uranium (U) produces the anthropogenic actinide plutonium (Pu), whose progeny nuclide 241Am grows from its parent 241Pu. Therefore, the Pu age, defined as the time elapsed since the last chemical separation (purification), can be obtained by measuring the 241Am/241Pu ratio. Various analytical techniques have been employed for the age determination of Pu samples [1–11]. Isotopic ratio analysis and mass determination of nuclear material in environmental samples collected by the International Atomic Energy Agency (IAEA) safeguard inspectors at nuclear facilities and provide important insights on the activities [12, 13]. In particular, the Pu age can reveal undeclared activities. Recently, we reported the determination of the purification age of young (3.9 years) individual micron-sized Pu particles based on the analysis of the 241Am/241Pu ratio using inductively coupled plasma mass spectrometry (ICP-MS) combined with anion-exchange chromatography [1]. The accuracy and precision of the Pu age were then improved by addition of an 243Am spike to the sample solutions (7.1–105 days and 0.16–0.5 years, respectively). U-and-Pu mixed oxide (MOX) fuel is used in light water reactors for reducing fuel cycle costs. MOX particles can be found in plutonium fuel-fabrication facilities. Determination of the purification age and characterization of U/Pu mixed particles are of utmost importance for nuclear safeguards and forensics. However, a Pu-age determination technique for MOX particles has yet to be developed. MOX particles with U/Pu ratios in the range 1–70 were prepared [14], and feasibility of the isotope ratio measurements of U and Pu in individual particle by ICP-MS and secondary ion mass spectrometry (SIMS) were examined [15, 16]. In order to determine the Pu age of MOX particle accurately and precisely, femto-gram quantities of Pu and Am have to be separated from pico-gram quantities of U. In the study presented herein, Am, U, and Pu were chemically separated by a single anion-exchange column and using pure high-grade inorganic acids as eluents. The age was determined using the 243Am-spiking technique [1]. The performance of this method for age determination of individual U/Pu mixed particles was evaluated by comparison with reference particles.
Materials and methods
Sample preparation
U/Pu mixed particles were prepared from the certified reference materials CRM U-010 (New Brunswick Laboratory (NBL), USA) and SRM 947 (National Bureau of Standards (NBS), USA), respectively. The certified Pu material was purified by anion-exchange chromatography on July 14, 2008 to remove Am. 241Am was not detected in the purified solution by alpha spectrometry. Solutions with U/Pu atom ratios of 1, 4.6, 9.5, 18, and 70 were prepared by mixing U and Pu solutions. U/Pu mixed particles (particle size = 2–5 μm) were then produced. Scanning electron microscope (SEM) image of a representative U/Pu mixed particle, particle production, and measured U/Pu isotope ratios were previously described [14]. A silicon disk (25 mm diameter) containing U/Pu particles was introduced in a SEM instrument, and the individual particles were transferred onto a separate Si chip with a dimension of 5 mm × 5 mm (Semitec Co. Ltd., Japan) using a glass needle attached to a manipulator. A new batch of U/Pu mixed particles was transferred from the same stock as those measured in the previous works [14–16], and the isotope ratios of this new batch were measured independently of the previous works in order to determine the Pu purification age in this work.
Chemical separation
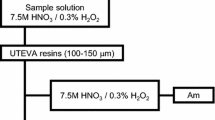
Each Si chip containing a single U/Pu particle was placed into an 8-mL Teflon centrifuge tube, and 2 mL of deionized Milli-Q water was added. After ultrasonication for 5 min, the Si chip was removed, the suspension was dried and the particles were dissolved in a mixture of HF (1 mL, 21 M) and HNO3 (1 mL, 15 M). This dissolution process was repeated twice and the solvent was fully evaporated. The evaporation residue was dissolved in 3 mL of a 15 M HNO3 solution. Figure 1 shows the anion-exchange separation procedure. Each sample solution was spiked with 0.1 mL of 243Am solution of 5.3 pg g−1 (Oak Ridge National Laboratory (ORNL), USA). The 243Am spike solution was first purified using anion-exchange columns to remove impurities (U, Pu, and 244Cm). As of January 22, 2010, 241Am/243Am atomic ratios of 241Am impurity (∆241Am) were (1.85 ± 0.03 (k = 1)) × 10−4 which was measured by alpha spectrometry. The 243Am-spiked samples were fully evaporated and were then dissolved in 3 mL of an 8 M HNO3 solution. One milliliter of this solution, called Fraction N, was used for the ICP-MS measurement of 243Am/239Pu ratios. The remaining portion of each sample was fully evaporated and dissolved in 1 M HCl solution. A NH2OH·HCl solution (30 μL, 3 M, guaranteed reagent (GR) grade; 99.0 % purity, Merck Co., Germany) was then added to reduce Pu to Pu3+. Finally, the evaporated residue was dissolved in 1.6 mL of a 9 M HCl–0.2 M HNO3 solution. This feed solution was served to separate Am, U, and Pu by anion-exchange chromatography. The anion-exchange resin (MCI GEL CA08P (particle size = 75–150 μm, strongly basic anion-exchange resins, 8 % cross linkage, Cl− form, base material of styrene–divinyl benzene (DVB) copolymer), Mitsubishi Chemical Corporation, Japan) was packed into a polyethylene column (3.9 mm inner diameter, 40 mm length, 0.48 mL volume). After loading the feed solution, the elements were separated using the following eluents: 3.2 mL of 9 M HCl–0.1 M HNO3 solution, 20 mL of 8 M HNO3–0.01 M HF solution, and 6 mL of 0.5 M HCl–0.01 M HF solution, for the elution of Am, U, and Pu, respectively. The elution fractions of Am and Pu, called fraction A, and fraction P, respectively, were fully evaporated, and 3 mL of 0.81 M HNO3 solutions were prepared for ICP-MS analysis. All treatments were carried out in clean rooms (ISO class 5 and 6) at the Clean Laboratory for Environmental Analysis and Research (CLEAR) of Japan Atomic Energy Agency (JAEA) [17, 18], except for the preparation of U/Pu mixed particles from the standard solutions. Water was deionized and purified (resistivity = 18.2 MΩ cm) with the Milli-Q system (Millipore Corp., USA). Highly pure grades of HCl, HNO3, and HF (TAMA-Pure AA-10 or AA-100 grade, TAMA Chemicals Co. Ltd., Japan) were used without purification for all chemical treatments. All labware, including beakers and bottles, was made of Teflon (PTFE and PFA) and was immersed in and rinsed with highly pure acids before use.
Instrumentation
Isotope ratios were measured by a high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS), (ELEMENT-1, Thermo Fischer Scientific Inc., Germany) in electric scanning (E-scan) mode. A PFA MicroFlow nebulizer (ES-2002, self-aspiration rate = 100 μL min−1, Elemental Scientific Inc. (ESI), USA) was used. In order to measure precise isotope ratios, a desolvating inlet system (APEX-Q, ESI, USA) was used for sample introduction to the ICP-MS. This system consists of a heated cyclonic spray chamber and a Peltier cooled condenser, allowing highly efficient sample introduction. Using this desolvation system, the sensitivity was improved by a factor 6. From the results of evaluation tests using a Pu isotope standard solution (SRM-947) of 5.14 pg g−1, 10,600 and 1700 cps ppt−1 were measured with and without desolvation, respectively. In addition, the sensitivity was found to vary between the elements of interest. Measurements obtained with the APEX-Q system had higher sensitivity towards Am than towards Pu. An 243Am/239Pu mixed reference solution (243Am/239Pu atomic ratio = 1.268) was prepared from the 243Am and Pu (SRM-947) standard solutions, and the difference was corrected by measuring the 243Am/239Pu ratio of this reference solution before commencing the sample measurements. In the study present herein, the relative sensitivity factor of Am/Pu was calculated to be 1.01–1.02. Mass bias was corrected by measuring a certified U isotopic standard solution (NBL, CRM U015; 51.83 pg mL−1 U) both before and after each batch of measurements. The mass bias factor, C was calculated using the following power function presented as Eq. (1):
where R t is the certified 235U/238U ratio (0.015565) of the U standard solution, R m is the average measured 235U/238U ratio, ∆m is the mass difference, and C is the mass discrimination factor. The counting statistics of the measured U isotope ratio was 0.2–0.5 %, and the standard deviation of the average U isotope ratio (R m) was approximately 0.5 %. For measurement of the 239Pu isotope ratios, including 241Pu/239Pu, 243Am/239Pu, and 241Am/239Pu, the interference of 238UH on 239Pu was corrected using the 238UH/238U ratio (2 × 10−5). The correction value was measured using a U isotopic standard CRM U0002 (NBL, USA) solution (235U/238U = 1.755 × 10−4, 100 pg mL−1 U). The precision and accuracy of measured Pu isotope ratios were evaluated in a previous study [19]. In addition, the relative standard deviations of Pu isotope ratios were calculated to be 1.6, 5.2, and 3.9 % for 240Pu/239Pu, 241Pu/239Pu, and 242Pu/239Pu, respectively. Furthermore, the difference in measured isotope ratios from the certified values was within these standard deviations. The operating conditions for the ICP-MS are listed in Table 1. The overall uncertainties were estimated using the uncertainty contributions arising from the measurement variability, mass bias correction, and the certified value of the reference material, according to the principles described in the Guide to the Expansion of Uncertainty in Measurements (GUM) [18]. The variability of isotope ratios contributed to >99 % of the overall uncertainty.
Results and discussion
Separation performance
Prior to age determination, the performance of the sequential anion-exchange separation technique was evaluated using a mixed spike solution (243Am 9.4 pg, 242Pu 11 pg, and 233U 200 pg). Recovery yields and decontamination factors are listed in Table 2. The elements of interest were clearly separated, with a decontamination factor greater than 3900, and a final recovery of >97 % yield. It was confirmed that due to its excellent separation performance, this simple separation technique could be applied to prepare analytical samples for isotope ratio measurements of femto-gram quantities of Am and Pu.
Age determination
The Pu purification age was determined according to Eq. (2):
where λPu and λAm are the decay constants for 241Pu and 241Am, respectively, and t is the Pu age. The half-lives of 241Pu (14.325 ± 0.024 (k = 2) y [20]) and 241Am (432.6 ± 0.6 (k = 1) y [21]) were used for this calculation. As shown in Eq. (3), calculation of the 241Am/241Pu atomic ratio is based on the value of three atomic ratios:
where the subscript letters, N, A, and P indicate the fractions described previously in the Materials and Methods section, and S is the relative sensitivity for the atomic ratio of Am/Pu (1.01–1.02).
Correction of impurity and contamination
The measured 241Am/243Am ratio in an Am fraction was corrected for the 241Am impurity (∆241Am) in the 243Am spike and the contamination of Pu (241Pu) in the Am fraction, as shown in Eq. (4):
The ∆241Am/243Am ratio of the 243Am spike, (∆241Am/243Am)spike was found to be 1.85 × 10−4 in a previous measurement. In a number of samples, 241Pu contamination in Am fractions was detected. The 241Pu/243Am ratio in the Am fractions was calculated from the quotient of (241Pu/239Pu)P and (243Am/239Pu)A in order to evaluate the Pu contamination level, and the ratios are listed in Table 3. Generally, the detected values were in the range of 10−6–10−4, except in the case of largely contaminated samples (sample nos. 11 and 17). These levels of contamination led to a 0.01–1 % systematic error in the (241Am/243Am)A values, and a 0.06–0.1 years systematic error for the purification period. In the exceptional case of sample no. 17, a high 241Pu/243Am ratio (0.0063) was detected because of unexpectedly high Pu contamination in the Am fraction. This high ratio contributed to 14 % of the 241Am/243Am ratio (0.0446) in the Am fraction. This contamination may therefore result in a systematic error of 0.63 years in the purification period. By subtracting this 241Pu contamination in the Am fraction from the 241Am/243Am ratio using the correction equation, Eq. (4), the accurate age (difference from the reference age = −0.17 years) was successfully obtained. For the 241Am impurity in the 243Am spike, the large systematic error of 0.5–0.9 years for the purification period in all samples was subtracted using the correction equation. Thus, the 241Am impurity in an 243Am spike will result in more serious systematic errors in the purification period than 241Pu contamination. For accurate determination of the purification period, it is therefore important to measure the ratio of the 241Am impurity to 243Am in a spike (∆241Am/243Am) and/or use an elementally and isotopically pure 243Am spike. In this correction process, the counting statistics of the measured (241Am/243Am)A ratio was found to be the main component of the overall uncertainty. The uncertainty of this measured Am isotope ratio thus contributed to >98 % of the overall uncertainty of corrected 241Am/243Am ratio. In the case of high Pu contamination in an Am fraction, such as sample no. 17, the counting statistics of (241Pu/239Pu)P contributed to 1.7 % uncertainty of the corrected 241Am/243Am ratio. The uncertainty of the 241Am impurity ratio (standard deviation = 1.6 %) also contributed to that of the corrected Am isotope ratio, but the proportion was negligibly small (<6 ppm).
Purification age
The determined Pu age is depicted in Fig. 2 as the difference from the reference date. In addition, Table 4 shows the determined dates for the U/Pu mixed particles with various U/Pu ratios, along with the difference between the determined date and the reference date (July 14, 2008), and the determined period at the end of chemical separation. It should be noted that the average determined age for the particles for the five independent U/Pu ratios agreed within the difference of ±0.4 years and the standard variation (k = 1) of 0.5 years. For all particles, the average and standard deviation (k = 1) of the differences from the reference date were found to be 0.27 and 0.44 years, respectively. This high accuracy and precision of the measured Pu purification ages demonstrates that the purification date of mixed (U/Pu) nuclear fuels purified within several years can be determined independently of the U/Pu ratio by analysis of a single micron-sized particle in an inspection sample.
Pu age of individual U/Pu mixed particles with various U/Pu ratios, calculated as the difference from the reference date (July 14, 2008). The error bars indicate the combined uncertainty (k = 1). The horizontal solid and dashed lines indicate, respectively, the average and the standard deviation for each group of U/Pu ratio. The red line and grey area indicate, respectively, the average and the standard deviation for the overall data
Uncertainty budget
The determined isotope ratios of each fraction and the 241Am/241Pu ratios calculated using Eq. (3) are listed in Table 3 along with the relative standard deviations. The uncertainty of the 241Am/241Pu ratio was estimated by combining the standard deviations of all isotope ratios, including the 241Am impurity in the 243Am spike, and the 241Pu contamination. The main part of the 241Am/241Pu uncertainty stems from uncertainties in the 241Am/243Am and 241Pu/239Pu ratios. The uncertainty of 243Am/239Pu in the non-separated fraction (N fraction) was found to be 0.39–3.1 %, and this component contributed to 0.3–5 % of the overall uncertainty. The uncertainty budget of the 241Am/241Pu ratio was calculated from the median uncertainties of the three isotope ratios. In the particle with a U/Pu ratio of 70, the overall uncertainty was composed of 84 % 241Pu/239Pu uncertainty, 15 % 241Am/243Am uncertainty, and 0.3 % 243Am/239Pu uncertainty. In the case of the particle with a U/Pu ratio of 18 and 1.0, the contribution of the 241Am/243Am uncertainty increased to 78 % and 74 %, respectively. In addition, contribution of the 241Pu/239Pu uncertainty to the overall uncertainty decreased to 22 % and 23 %, respectively. In the case of the particles with a U/Pu ratio of 9.5 and 4.6, the contribution of 241Am/243Am uncertainty was 38 and 60 %, respectively. Figure 3 shows the variations in the median relative uncertainties of 241Am/241Pu, (241Am/243Am)A, (241Pu/239Pu)P, and (243Am/239Pu)N with respect to the percentage abundance of Pu. The uncertainties of the (241Am/243Am)A and (241Pu/239Pu)P ratios were found to sharply increase with decreasing percentage abundance of Pu. The (241Am/243Am)A ratio of a particle with U/Pu = 70 (1.4 % of Pu) could not be determined using the 400-scans-per-replicate set for the ICP-MS measurement, because of the low counting statistics of the 241Am signal. When the number of scans was increased to 500 to emphasize the 241Am signal, the uncertainty of the Am isotope ratio was found to be 3–10 %. However, this alternation in the settings was not effective in reducing the uncertainty for the (241Pu/239Pu)P ratio. The relative uncertainty of the Pu isotope ratio decreased inversely to the square root of 239Pu intensity for all samples. This correlation is in line with the relationship between the number of events and the standard deviation in a normal distribution, taking into account the pulse-counting statistics of the ICP-MS measurement system. Thus, it is clear that the main contributor to the overall uncertainties for the Pu isotope ratio is the counting statistics of the Pu fraction measurement. For more precise age determination of particles with low Pu abundance, the uncertainties in Pu ratio sensitivity must be reduced by enhancing the Pu counting sensitivity using ICP-MS auxiliary components, such as high transmission skimmer cone and sensitive detectors. The study presented herein is based on the assumption that following Pu purification, no 241Am isotope is present in the particle. However, it is possible that a practical particle contains 241Am isotopes as purification impurities or nuclear fuel additives [22], thus producing a systematic error in the Pu age analysis. The development of novel techniques to correct this error will be reported elsewhere in due course.
Conclusions
We herein report an analytical technique for the accurate and precise Pu age determination for individual U/Pu mixed micron-sized particles with various U/Pu ratios (1–70). The purification date determined for an individual particle, which is prepared from U and Pu reference materials and is purified on July 14, 2008, was in good agreement with the reference value (uncertainty of 0.44 years, accuracy of 0.27 years). The combination of 243Am spike addition and sequential anion-exchange separation of Am, U, and Pu by a single column ensures exact measurements. For measurement of accurate purification period, it is essential to correct the systematic errors arising from 241Am impurity in the 243Am spike and 241Pu contamination in the Am fraction. This high performance analytical technique can provide precise information on the history of nuclear fuel, and it can therefore serve as important tool for fingerprinting of environmental samples in nuclear safeguards and forensics. Increased precision and accuracy of age measurement for particles with low Pu content are possible using additional instrumental components that enhance sensitivity.
References
Miyamoto Y, Esaka F, Suzuki D, Magara M (2013) Precise age determination of a single plutonium particle using inductively coupled plasma mass spectrometer. Radiochim Acta 101:745–748
Wallenius M, Tamborini G, Koch L (2001) The “age” of plutonium particles. Radiochim Acta 89:55–58
Nygren U, Ramebäck H, Nilsson C (2007) Age determination of plutonium using inductively coupled plasma mass spectrometry. J Radioanal Nucl Chem 272:45–51
Alvarado JAC, Chawla F, Froidevaux P (2011) Determining 241Pu in environmental samples: case studies in alpine soils. Radiochim Acta 99:121–129
Wallenius M, Mayer K (2000) Age determination of plutonium material in nuclear forensics by thermal ionisation mass spectrometry. Fresenius J Anal Chem 266:234–238
Wallenius M, Peerani P, Koch L (2011) Origin determination of plutonium material in nuclear forensics. J Radioanal Nucl Chem 99:121–129
Chen Y, Chang ZY, Zhao YG, Zhang JL, Li JH, Shu FJ (2009) Studies on the age determination of trace plutonium. J Radioanal Nucl Chem 281:675–678
Zhang HT, Zhu FR, Xu J, Dai YH, Li DM, Yi XW, Zhang LX, Zhao YG (2008) Age determination of plutonium material by alpha spectrometry and thermal ionization mass spectrometry. Radiochim Acta 96:327–331
Shinonaga T, Donohue D, Ciurapinski A, Klose D (2009) Age determination of single plutonium particles after chemical separation. Spectrochim Acta B 64:95–98
Spencer KJ, Tandon L, Gallimore D, Xu N, Kuhn K, Walker L, Townsend L (2009) Refinement of Pu parent-daughter isotopic and concentration analysis for forensic (dating) purposes. J Radioanal Nucl Chem 282:549–554
Sturn M, Richter S, Aregbe Y, Willum R, Mialle S, Mayer K, Prdraska T (2014) Evaluation of chronometers in plutonium age determination for nuclear forensics: what if the ‘Pu/U clocks’ do not much? J Radioanal Nucl Chem 302:399–411
Donohue DL (1998) Strengthening IAEA safeguards through environmental sampling and analysis. J Alloys Compd 11:271–273
Mayer K, Wallenius M, Varga Z (2013) Nuclear forensic science: correlating measureable material parameters to the history of nuclear material. Chem Rev 113:884–900
Suzuki D, Esaka F, Miyamoto Y, Magara M (2015) Direct isotope ratio analysis of individual uranium-plutonium mixed particles with various U/Pu ratios by thermal ionization mass spectrometry. Appl Radiat Isot 96:52–56
Esaka F, Magara M, Suzuki D, Miyamoto Y, Lee CG, Kimura T (2011) Feasibility study of isotope ratio analysis of individual uranium-plutonium mixed oxide particles with SIMS and ICP-MS. Mass Spectrom Lett 2:80–83
Esaka F, Suzuki D, Miyamoto Y, Magara M (2015) Determination of plutonium isotope ratios in individual uranium-plutonium mixed particles with inductively coupled plasma mass spectrometry. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4113-6
Usuda S, Yasuda K, Kokubu YS, Esaka F, Lee CG, Magara M, Sakurai S, Watanabe K, Hirayama F, Fukuyama H, Esaka KT, Iguchi K, Miyamoto Y, Chai JY (2006) Challenge to ultra-trace analytical techniques of nuclear materials in environmental samples for safeguards at JAERI: methodologies for physical and chemical form estimation. Int J Environ Anal Chem 86:663–675
Usuda S, Magara M, Esaka F, Yasuda K, Kokubu YS, Lee CG, Miyamoto Y, Suzuki D, Inagawa J, Sakurai S, Murata F (2010) QA/QC activities and estimation of uncertainty for ultra-trace analysis of uranium and plutonium in safeguards environmental samples. J Nucl Radiochem Sci 11:A5–A9, http://www.radiochem.org/j-online.html
Esaka F, Magara M, Suzuki D, Miyamoto Y, Lee CG, Kimura T (2010) Isotope ratio analysis of individual sub-micrometer plutonium particles with inductively coupled plasma mass spectrometry. Talanta 83:569–573
Wellum R, Verbruggen A, Kessel R (2009) A new evaluation of the half-life of 241Pu. J Anal At Spectrom 24:801–807
Martin MJ (2005) Nuclear data sheets for A = 241. Nucl Data Sheets 106:89–158
Yoshimochi H, Nemoto M, Mondo K, Koyama S, Namekawa T (2004) Fabrication technology for MOX fuel containing AmO2 by an in-cell remote process. J Nucl Sci Technol 41:850–856
Acknowledgments
This work was financially supported by the Nuclear Regulation Authority, Japan. The authors would like to thank Mr. N. Kohno for the particle production, Mr. T. Onodera and Ms. R. Usui for the chemical treatments, and Mr. Y. Takahashi for the ICP-MS measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyamoto, Y., Suzuki, D., Esaka, F. et al. Accurate purification age determination of individual uranium–plutonium mixed particles. Anal Bioanal Chem 407, 7165–7173 (2015). https://doi.org/10.1007/s00216-015-8880-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8880-2