Abstract
Nondestructive method has been used to determine the age, activity, isotopic composition of uranium, and total uranium ore concentration (UOC) for the El-Sella site at the Egyptian Eastern Desert. This method is based on measuring gamma rays emitted from different uranium isotopes using a highly sensitive hyperpure germanium detector (HP-Ge) with 50% relative efficiency. In the framework of forensic analysis to study the radioactivity level, there are essential factors that have been determined: identification of the present radioactive isotopes, calculation of their specific activities, enrichment percentage of 235U, and uranium age dating. Therefore, this study provides all information that is important to illustrate the environmental radioactivity in the selected site; in addition, the enrichment percentage and age of uranium isotopes are presented. The results show that El-Sella site has low lifetime age values, high radioactivity levels, high uranium concentration, and is naturally enriched in 235U. These results are tabulated, discussed, and compared to the finding from the recently published national and international research with respect to the radioactivity levels and limits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Radio-chronometry is an essential factor that must be determined in the course of forensic analysis and nuclear material investigation. Within this framework, the material age, the date of production of nuclear materials, the time that passed since these nuclear materials have been chemically separated from their daughter nuclides, and the process that caused elemental or isotopic fractionation will be determined [1].
Three parent/daughter relationships can be used for determining the age of uranium samples, namely: 234U/230Th, 235U/231Pa, and 236U/232Th; the latter one is valid only for irradiated and reprocessed uranium, as 236U is not a naturally occurring isotope. In addition, the 235U/231Pa ratio is very high because of the great difference between the half-lifes of 235U (7.038 × 108 years) and 231Pa (32760 years); therefore, chemical separation of 235U and 231Pa should be performed, which is a limitation for the direct measurement. Thus, the most convenient ratio for the age analysis is the 234U/230Th ratio, which is determined by specific geological and environmental events or chemical processes where the disequilibrium between 234U and 230Th takes place [2]. The secular equilibrium will occur after up to seven half-lives of 230Th [3]. Eventually, the thorium decay rate will be equal to the rate of uranium decay in which the thorium is produced. This phenomenon will allow the dating up to 500 000 years of age [4].
The first step in determining the age of a sample is to identify the isotopic composition, the risk assessment, the activity, and the enrichment of this sample according to their characteristic gamma lines. These analyses are usually performed using a high-resolution gamma-ray spectrometer (HRGS) equipped in most cases with a high-purity germanium (HP-Ge) detector [5], which is used for determining the characteristics of nuclear and radioactive materials [6]. The efficiency and energy calibration of the detector is performed to ensure precise and accurate results. It is known that each radioactive element has its gamma rays, which are emitted with specific energies and intensities [6]. Therefore, 238U can be identified from the energy lines of its daughters 234Th (63.29 keV) and 234Pa (1001.03 and 766.36 keV), whereas 235U can be identified from its own energy lines (143.7, 163.3, 185.7, and 205.3 keV) [7].
The natural enrichment percentage of uranium in the environmental samples is 0.72%, except the locations near uranium enrichment facilities, which mostly use UF6 gas in uranium separation technologies. These technologies lead to the release of enriched uranium traces into the environment [8]. The uranium concentration varies with the location where it has been collected. For example, the uranium concentration is approximately 4 ppm when uranium is mixed with granite, which covers 60% of the earth’s crust [9]. However, there are uranium-rich areas in Egypt like Gattar mountains which contain uranium in the range from 1000 to 5000 ppm [10].
This investigation is focused on the analysis of highly radioactive samples that have been collected from El-Sella site of Egypt. We performed a set of measurements to calculate the age, uranium isotopic composition, total uranium concentration, enrichment, and activity and to gain the knowledge about the radioactive nature of this selected site.
EXPERIMENTAL
Sample collection and preparation. The radioactive granite rocks were collected from the El-Sella site in the Egyptian Eastern Desert. These rocks were prepared and measured in the laboratory of the Nuclear Engineering Department of the Military Technical College through a series of steps, where they were crushed, ground, and homogenized. In addition, the samples were heated to 105°C for a day to remove moisture, and 323 g of the dried powder was collected and shaped similarly to the reference material that was used for the calibration process. This powder should be tightly sealed in a cylindrical plastic container for 28 days to accumulate the daughter radon 222Rn, which is formed in the gaseous state [11].
Gamma spectrometer setup. Nondestructive technique was used in this study. It relies on hyperpure germanium (HP-Ge) detector with the relative detection efficiency of 50% and a resolution of 1.95 keV. The applied bias is 3.3 kV. The detector is connected to a Canberra DSA-1000 multichannel analyzer (MCA). The HP-Ge crystal was fully immersed in liquid nitrogen to maintain the temperature of the crystal at –200°C to reduce the noise and the leakage current. The HP-Ge detector is shielded by a 10 cm thick lead material to eliminate the effect of the background radiation on the counting process. This experimental setup was used for identifying the radionuclides through their gamma-ray energies and for determining their activities by analysis of the obtained spectrum using the Genie-2000 software [7].
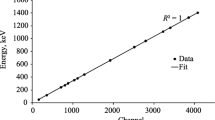
Gamma spectrometer energy calibration. The energy calibration was performed using standard IAEA sources (point sources), model RSS8 and MGS-1, which contain certain radioisotopes (133Ba, 137Cs, 60Co, 22Na, and 152Eu). These radioactive sources have known activities and half-lives (Table 1) [12]. The relation between the energy and the channel number was deduced according to the energy calibration and is presented in Eq. (1):
Gamma spectrometer efficiency calibration. The efficiency calibration is an essential factor to attain accurate and valid measurements. The standard sources that are used for the efficiency calibration should have the same geometry and density as those of the unknown sample. In addition, the calibration process should take place under the same conditions of the experimental setup as those used for identifying these unknown samples [13]. The efficiency calibration is performed using a standard IAEA-RGU uranium ore reference material, which is incorporated in the evaluation of the environmental samples. This standard source was measured for 1 day to accumulate reasonable counts sufficient to enhance the calibration process [14]. The absolute efficiency can be calculated using the following equation:
where εabs is the absolute efficiency, C is the net counts of photopeak for a certain isotope in the reference material, AS is the known specific activity for each isotope in the reference material, Iγ is the branching ratio, and T is the counting time.
Spectrum analysis and activity calculation. The emitted gamma rays from each uranium isotope have certain energies. They will interact and deposit their energy in the detector, which converts this energy into detected peaks. Each of them in the energy spectrum indicates the gamma energy line corresponding to a certain radioisotope. The activity of this radioisotope will be determined from the counts under the peak of high intensity, which is defined as a photopeak. The identification of uranium isotope (238U) relies on the emitted gamma rays of different energies from its daughters, 234Th (63.29 keV) and 234Pa (1001.03 and 766.36 keV). These daughters are considered to have the same activity as that of 238U. Uranium-235 can be identified by its own energy lines (143.7, 163.3, 185.7, and 205.3 keV). In addition, 234U can be distinguished according to 53 and 120.9 keV gamma energy lines. The activity (A) of the radioactive isotope will be calculated using the following equation:
In this study, the comparator method was applied to overcome the issues related to the difficulty of the efficiency calibration, to reduce the systematic and random errors, and to enhance the accuracy of the identification process. In this case, the most important factor (efficiency) that can affect the measurement accuracy will be excluded. Therefore, the relation between the activity of the experimental sample and that of the standard source will be determined using Eqs. (4) and (5), where the calculation will not depend on the efficiency calibration for simplifying the radiation measurements and improving their accuracy:
where AS and AU are the specific activities of the standard and the unknown material, respectively; CS and CU are the net counts of the standard and unknown material, respectively; εS and εU are the efficiencies of the standard and unknown material respectively, which are the same for each energy line. This method will be used under some constraints. Namely, the unknown material should have the same energy lines, weight, and density as the standard material.
RESULTS AND DISCUSSION
Specific activity and activity ratio results. It has been found that the average value of the specific activity of 235U isotope is 1269 ± 98 Bq/kg. This value is based on the specific activities (1167.4, 1353.3, 1201.2, and 1352.8 Bq/kg) corresponding to the energy lines of 235U (143.7, 163.36, 185.72, and 205.23 keV, respectively). In addition, for 238U, the average specific activity is 27186 ± 376 Bq/kg, which is related to the specific activities of 238U daughters (234Th and 234mPa) with the energy lines of 63.29 and 1001 keV, respectively. Finally, the average specific activity of 234U is 22067 ± 82 Bq/kg, based on its two energy lines (53.24 and 120.9 keV), for which the specific activity is 22067.8 and 22125.8 Bq/kg, respectively (Table 2).
The 234U/238U activity ratio is 0.8, as calculated from the results presented in Table 3. This ratio is less than unity (<1), which means that there is disequilibrium between the parent and daughter nuclei in the decay series. The 234U/238U activity ratio should be equal to unity if there is radiological equilibrium between these two isotopes, which is attained if the uranium is in a closed system. However, the system may be exposed to a disturbance, which is caused by the groundwater circulation or by the effect of weather changing, in addition to the alpha recoil effect. This system disturbance will cause the fractionation between 234U and 238U, which leads to a change in the activity ratio to become higher or lower than unity [15]. Our results are consistent with the results of another study at the same location (El-Sella), according to which the 234U/238U activity ratio was from 0.4 to 0.92 [16].
The enrichment calculations and results. The enrichment (%) of the uranium sample can be estimated using the atom ratio equation:
where N235U, N234U, and N238U are the numbers of radionuclides of 235U, 234U, and 238U, respectively, which can be estimated from the decay constant and the activity of these radioisotopes using the decay equation
Thus, the enrichment equation can be written as follows:
where λ234U, λ235U, and λ238U are the decay constants and (τ1/2)234U, (τ1/2)235U, and (τ1/2)238U, are the half-lives of 234U, 235U, and 238U, respectively. The enrichment percentage was obtained using Eq. (8), which shows that the collected sample is naturally enriched (0.729%) as presented in Table 3. This result shows that the predicted equation can be used for determining the enrichment of any uranium sample based on the average specific activity of uranium isotopes in this sample.
Uranium isotopic mass and total uranium ore concentration (UOC). The uranium mass in a certain sample can be calculated by knowing the activity and the decay constant for each uranium radioisotope, which will be applied to the following equations:
where m238U, m235U, and m234U are the masses of 238U, 235U, and 234U, respectively; M238U, M235U, and M234U are the atomic masses of 238U, 235U, and 234U, respectively; NA is Avogadro’s number. The total uranium ore concentration (UOC) in the sample was determined using the ratio of the total masses of uranium isotopes and the total sample mass [Eq. (10)].
The masses of 234U, 235U, and 238U were calculated using Eq. (9) from the activity of each isotope (Bq), decay constant (s–1), molar mass (g mol–1), and Avogadro’s number. The masses of these uranium isotopes are summarized in Table 3. As we found, the total uranium amount in the sample according to Eq. (10) is 710.2 mg, and the sample weight is 323 g. Thus, the total uranium ore concentration is 2198.8 ppm. These results are consistent with the results of another study, according to which the total uranium ore concentration at (El-Sella) site ranges from 203 to 6147 ppm [16].
Dating method and calculations. Naturally occurring radioisotopes belong to the decay chains of 235U, 238U, and 232Th, where the activities of the parent and daughter isotopes in each chain will reach a state of secular equilibrium in the naturally occurring undisturbed materials within several million years. The radioactive decay series is in secular equilibrium if the activities of all radionuclides of the series (daughters) are equal to that of the parent [17]. Therefore, if one of these daughters has been lost from the geological system due to any process other than radioactive decay, the equilibrium will be disrupted. This disturbance state is the key of the U-series disequilibrium dating methods [15]. This U-series disequilibrium can be produced by two different mechanisms.
The first mechanism is related to the α-recoil effect, which is based on the ejection of α-particle, where the daughter recoils in the opposite direction. Both the α-particle and the recoiled daughter will have the kinetic energy according to the conservation of momentum law. Hence, the atoms that are close to the surface of the mineral will be able to leave the surface of the mineral after gaining a great amount of this kinetic energy [18, 19]. The second mechanism is due to the physical or chemical changes of the parent and daughter in the environment by different processes such as precipitation, adsorption, and dissolution. The causes of the disorder of the secular equilibrium are usually related to the geochemical processes, which result in the mobilization of radionuclides during a period comparable to the half-life of the daughter [20].
The uranium age equation is deduced from the Bateman equation based on the relation between the parent and daughter atom ratio. The Bateman equation is a mathematical model describing the abundances and activities in the decay chain as a function of time, decay rates, and the initial abundances as described in the following equations considering the simplest case of a parent feeding a single daughter:
Solving this first-order differential equation for N2 and assuming zero concentration for all daughters at time t = 0, we obtain
where λ = (ln 2)/t1/2, the 234U half-life is 2.455 × 105 years, the 230Th half-life is 7.538 × 104 years, and the activity is A = λN. For 234U as a parent and 234Th as a daughter, Eq. (15) can be written as follows.
Thus, the age of any uranium sample can be determined directly by knowing only the activity of 230Th and 234U in this sample.
It has been found that the age of the investigated sample is 163271.9 years (Table 3). The age of this sample can be expressed as the time that passed since the nuclear material has been chemically separated from its daughter nuclides or since any event or process that caused any elemental or isotopic fractionation. The age of 163271.9 years may be a questionable value as it is short compared to the common life time of environmental samples. However, this is due to the disequilibrium and the disturbance of the state of equilibrium for the system, which is related to the geochemical processes that cause the mobilization of the radionuclides during a period comparable to the half-life of the daughter. Therefore, this age is considered as the time after that passed since an event or process causing elemental or isotopic fractionation. In this case, the disequilibrium was caused by the uranium migration and secondary mineral formation during which the Eastern Desert was flooded by surface water. In addition, this age is coincident with the pluvial periods in Egypt.
CONCLUSIONS
Nondestructive analysis has been performed using a gamma-ray spectrometer with an HP-Ge detector calibrated for the efficiency and energy by a modified method to improve the measurement accuracy. This detector was used to determine the radioactivity of granite samples collected from the South Eastern Desert, El-Sella site in Egypt. These samples were prepared by a set of steps such as crushing, sieving, drying, and sealing. The specific activity of different uranium isotopes (235U, 238U, and 234U) was determined: 1269 ± 98, 27186 ± 376, and 22068 ± 82 Bq/kg, respectively. The 235U/238U activity ratio has a natural value of 0.046. This 234U/238U ratio is lower than unity, which suggests the occurrence of uranium disequilibrium in the samples. In addition, an equation for determining the sample enrichment percentage was derived. The enrichment of the investigated sample is 0.72%, which agrees with the natural value. The masses of uranium isotopes (234U, 235U, and 238U) were calculated to determine the total uranium ore concentration (UOC), which has a high value of 2198.8 ppm. Finally, the age of the sample, counting from an event or process that caused elemental or isotopic fractionation, was determined using the parent-to-daughter dating method based on the 234U/230Th ratio. The sample age was found to be 163271.9 years. This result is coincident with the pluvial periods in Egypt.
REFERENCES
IAEA Nucl. Secur. Ser., 2015, no. 2-G, pp. 1–80
Wallenius, M., Morgenstern, A., Apostolidis, C., and Mayer, K., Anal. Bioanal. Chem., 2002, vol. 374, pp. 379–384. https://doi.org/10.1007/s00216-002-1555-9
Zhao, J. and Collins, L.B., Encyclopedia of Modern Coral Reefs Hopley, D., Ed., Dordrecht: Springer, 2011. https://doi.org/10.1007/978-90-481-2639-2_161
Ibrahim, E.M., Hassan, S.F., and El Feky, M.G., Int. J. Recent Sci. Res., 2016, vol. 7, no. 2, pp. 8849–8858.
Keegan, E., Kristo, M.J., Colella, M., Robel, M., Williams, R., Lindvall, R., Eppich, G., Roberts, S., Borg, L., Gaffney, A., Plaue, J., Wong, H., Davis, J., Loi, E., Reinhard, M., and Hutcheon, I., Forensic Sci. Int., 2014, vol. 240, pp. 111–121. https://doi.org/10.1016/j.forsciint.2014.04.004
Tohamy, M., Abd El-Ghany, S., El-Minyawi, S.M., Fayez-Hassan, M., El-Hakim, E.H., El-Mongy, S.A., and Comsan, M.N.H., Ann. Nucl. Energy, 2016, vol. 87, no. P2, pp. 186–191. https://doi.org/10.1016/j.anucene.2015.09.001
Abdel-Rahman, M.A.E., Shady, A., and El-Mongy, S.A., Z. Anorg. Allg. Chem., 2018, vol. 644, pp. 477–482. https://doi.org/10.1002/zaac.201800125
Belew, W.L., Abstract of Papers, Conf.: Department of Energy’s (DOE) Int. Safeguard Meet., Vienna (Austria), March 22–23, 1993, pp. 2–4.
Nuclear Fuel Cycle Information System, Vienna: IAEA, April 2009, pp. 1–82.
Salman, A.B., Shalaby, M., Noseir, L., El Kholy, D., Roz, M., Abu Zeid, M., Mostafa, M., Amin, N., Ayoub, R., and Khamis, H., Abstract of Papers, Second Arab Conf. on the Peaceful Uses of Atomic Energy, Cairo, Nov. 5–9, 1994.
Abdel-Rahman, M.A.E., Sabry, M., Khattab, M.R., El-Taher, A and El-Mongy, S.A., Z. Anorg. Allg. Chem., 2020, vol. 646, pp. 1–9. https://doi.org/10.1002/zaac.202000176
Erickson, M.D., The Procedures Manual of the Environmental Measurements Laboratory, New York: US Department of Energy, 1997, vol. 1.
Shabaka, A.N., Omar, A., El-Mongy, S.A., and Tawfic, A.F., Int. J. Environ. Anal. Chem., 2020, vol. 100, pp. 1–14. https://doi.org/10.1080/03067319.2020.1724985
Abdel-Rahman, M.A.E. and El-Mongy, S.A., Nucl. Eng. Technol., 2017, vol. 49, pp. 1752–1757. https://doi.org/10.1016/j.net.2017.07.020
Ibrahim, E.M., Int. J. Recent Sci. Res., 2017, vol. 8, pp. 15126–15141.
Gawad, A.E.A., J. Radioanal. Nucl. Chem., 2016, vol. 308, no. 1, pp. 129–142. https://doi.org/10.1007/s10967-015-4374-0
Ivanovich, B.M., Radiochim. Acta, 1994, vol. 94, pp. 81–94. https://doi.org/10.1524/ract.1994.64.2.81
Fantle, M.S., Maher, K., and Depaolo, D.J., Rev. Geophys., 2010, vol. 48, pp. 1–38. https://doi.org/10.1029/2009RG000306
Eggeling, L., Genter, A., Kölbel, T. and Münch, W., Geothermics, 2013, vol. 47, pp. 80–88. https://doi.org/10.1016/j.geothermics.2013.03.002
Papadopoulos, A., Christofides, G., Koroneos, A., Stoulos, S., and Papastefanou, C., Appl. Radiat. Isot., 2013, vol. 75, pp. 95–104. https://doi.org/10.1016/j.apradiso.2013.02.006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabry, M., El-Mongy, S.A., Abdel-Rahman, M.A.E. et al. Nondestructive Analysis of Uranium Isotopic Activity, Enrichment, Concentration, and Age with a Sensitive γ-Ray Spectrometer for El-Sella Site Samples. Radiochemistry 63, 620–626 (2021). https://doi.org/10.1134/S1066362221050106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362221050106