Abstract
An isotope dilution multicollector inductive coupled plasma mass spectrometry (ID-MC-ICP-MS) method for determining age of trace Pu through measuring 241Pu/241Am, 240Pu/236U ratio was established. At the same time, other two methods-α-spectrometry combined with MC-ICP-MS and liquid scintillator combined with α-spectrometry through measuring 241Pu/241Am ratio to determine the age of trace Pu were also studied. The techniques were explored for the age determination of nanogram grade Pu sample on the basis of Pu/Am, Pu/U separation. The ages of two Pu samples—one with known and the other with unknown age—were determined by the three methods. The determined ages by the three methods were all in agreement with the reference value. The established methods for determining the age of trace Pu could be adopted in the verification activities of nuclear safeguards and nuclear arms control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the verification technologies for arms control, age attribution of plutonium is of far reaching importance.
At present, γ-spectrometry and mass spectrometry were primarily used to determine the age of Pu. γ-spectrometry method was based on the determination of gamma rays of 241Pu(237U) and 241Am, a relatively large amount of plutonium was needed [1, 2]. More parent/daughter nuclides were used in Mass spectrometry method, such as 238Pu/234U, 239Pu/235U, 240Pu/236U and 241Pu/241Am ratios [3–5]. And only a relatively small quantity of sample was enough to get corrected results.
In our lab, three techniques for the age determination of nanogram grade Pu sample were studied on the basis of radiochemical separation of Pu/Am, Pu/U and isotopic dilution mass spectrometry.
Methodology
“Age” refers to the date of the last separation of parent nuclide from its daughters. The determination of the age of plutonium was based on the parent/daughter ratio of 240Pu/236U or 241Pu/241Am. 236U and 241Am are the first generation daughters of 240Pu and 241Pu, so the age of plutonium can be calculated by Eq. 1.
In this equation, \( t \) is the age of plutonium, \( \lambda_{1} \) and \( \lambda_{2} \) are the decay constants of Pu and the respective daughter nuclide and \( N_{1} \) and \( N_{2} \) are the amounts of the mother and daughter nuclide at the time of analysis.
Other parent/daughter radios (238Pu/234U, 239Pu/235U) were not adopted for potential contamination from the background of natural uranium.
Experimental
Instrumentation
GV Micromass Isoprobe MC inductively coupled plasma mass spectrometer, α-spectrometry (ORTEC Octête plus) and liquid scintillator (Tri-Carb3170) were used for studies.
Samples and reagents
Certified reference material (233U solution, NPL A051018 and 243Am solution, AEA Technology UK ATP10040) and standard reference material (242Pu solution, IRMM-044) were prepared as spike solutions for quantification of the analysis. Uranium isotopes standard solution (EC-NCM 199) and a mixture of standard reference solution (233U, NPL A051018 and 236U, NPL E5552) were used to correct mass discrimination and ion multiplier efficiency variance.
BV–III grade nitric acid and hydrochloric acid (Institute of Chemical Reagent of Beijing) were used for sample preparation. High-purity water (18 MΩ cm−1) was prepared with a Millipore Milli-Q-Element water purification system (Millipore, USA). TEVA and TRU (all 50–100 μm, Eichrom, USA) were used for the separation of U, Pu and Am.
Radiochemical separation
Separation of Pu and Am
About 0.04 g plutonium sample (contained 107 ng Pu g−1) was weighed. Approximately 0.4 g 243Am spike solution (47.93 pg g−1), 0.01 g 242Pu spike solution (1.0409 ng g−1) and 600 μL 4 × 10−3 mol L−1 NaNO2 were added to the sample. The samples were vibrating for 10 min, heated to vaporize to nearly dry and re-dissolved with 2 mL 2 mol L−1 HNO3 for the following solid phase extraction procedure.
TEVA (1 mL) was added to 2 mL glass column and conditioned with 5 mL 2 mol L−1 HNO3. The sample was then loaded on the column and rinsed with 1.5 mL 2 mol L−1 HNO3 to remove americium. The TEVA column was then rinsed with 5 mL 2 mol L−1 HNO3 to eliminate the residual americium completely. Plutonium was then eluted using 6 mL 0.15 mol L−1 HNO3–0.025 mol L−1 H2C2O4.
TRU (1 mL) was added to 2 mL glass column and conditioned with 5 mL 5 mol L−1 HCl. The americium fraction from TEVA column was loaded on the column and rinsed with 3 mL 5 mol L−1 HCl to remove sodium ions. Americium was then eluted using 11 mL 5 mol L−1 HCl.
Separation of Pu and U
About 0.04 g plutonium sample (contained 107 ng Pu g−1) was weighed. Approximately 0.1 g 233U spike solution (7.285 pg g−1), 0.2 g 242Pu spike solution (1.0409 ng g−1) and 1 mL 6 mol L−1 HNO3 were added to the sample. The samples were vibrating for 10 min, heated to vaporize to nearly dry and re-dissolved with 0.5 mL 6 mol L−1 HNO3 for the following solid phase extraction procedure.
TEVA (1 mL) was added to 2 mL glass column and conditioned with 5 mL 3 mol L−1 HNO3. The samples were then loaded on the column and rinsed with 7 mL 3 mol L−1 HNO3 to collect uranium. Plutonium was then eluted using 6 mL 0.15 mol L−1 HNO3–0.025 mol L−1 H2C2O4.
Measurement
Multi-collector inductively plasma mass spectrometry
Uranium, plutonium and americium isotope ratios were measured using MC-ICP-MS. The operating condition of ICP-MS is listed in Table 1. 233U/236U ratio of the mixture of standard reference solution was quantitative analyzed using EC-NRM 199 as standard to correct the mass discrimination. 241Am/243Am and 241Pu/242Pu ratios of sample after the separation of Pu and Am and 233U/236U and 240Pu/242Pu ratios of sample after the separation of Pu and U were measured using the mixture of standard reference solution (233U, NPL A051018 and 236U, NPL E5552) as reference.
α-Spectrometry
The separated Am fraction was vaporized to nearly dry and re-dissolved with 8 mL 0.15 mol L−1 HNO3–0.025 mol L−1 H2C2O4. The solution was transferred to electrodeposit cell, adjusted to pH 1–2 and electrodeposited for 2 h. The 241Am/243Am ratio was measured using α-spectrometer. For all measurements the counting time was optimized to achieve an uncertainty of the concerned isotopes due to counting statistics inferior to 1%.
Liquid scintillator
A series of 241Pu standard samples of the different quenching degree were used to scale the efficiency of liquid scintillator. About 0.04 g plutonium age standard solution (contained 107 ng Pu g−1) was weighed and measured using liquid scintillator.
Results and discussion
ID-MC-ICP-MS method
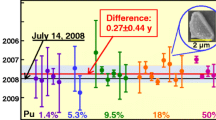
241Pu/241Am and 240Pu/236U ratios were determined by MC-ICP-MS. Determinations were performed on a plutonium age standard solution (PU 1) and a plutonium solution with unknown age (PU 2), and four sub-samples for each were measured. The obtained ages were shown in Table 2. As can be seen from Table 2, the ages determined by 241Pu/241Am ratio corresponded well with the 240Pu/236U ratio. The discrepancy between the obtained age and reference age of PU 1, which maybe come from unknown system error, was about 1 year. The results of sub-samples were comparatively consistent, so the method had good precision.
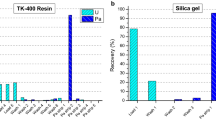
241Pu and 241Am would interfere each other during the ICP-MS measuring, so the completely separation of Pu and Am was the key technique. TEVA and TRU resins were used to separate Pu and Am in this work. The decontamination factor was >250 for Pu in Am fraction and >1000 for Am in Pu fraction. These separation factors were adequate for the preparation of sample determined by ICP-MS. The chemical yields were about 80% for Am, 96% for Pu and 70% for U, respectively.
The primary interference during the measurement of 240Pu/236U ratio may come from the residual 236U in the initial Pu sample. According to the portion of 236U in the irradiated low enriched reacted uranium was about 0.49%. The amount of the residual uranium in the separated Pu was assumed to be 100 μg g−1 and 240Pu content was 5%, the initial 240Pu/236U ratio would be about 105. 240Pu/236U ratio was <103 for the plutonium with the age of about 20 years. So the deviation from the residual 236U should be <1%.
ID-α-spectrometry combined with MC-ICP-MS method
The other way to obtain the age of plutonium was that α-spectrometry combined with MC-ICP-MS to determine 241Pu/241Am ratio. The amount of 241Pu was measured by using MC-ICP-MS as 4.1, and 241Am was electrodeposited on stainless steel plate and determined by α-spectrometer. The result was shown in Table 3. In this method, the interference of 241Pu on 241Am and the correction of Am by uranium standard during the MC-ICP-MS measurement were avoided. In the α-spectra, the peaks of 238Pu and 241Am were overlapped. But the radioactivity of 238Pu could be estimated less than one-ten thousandth of 241Am. So the interference of 238Pu on 241Am could be ignored. The result of the method was consistent with the foregoing method. The obtained age of PU 1 was in agreement with the reference one.
ID-α-spectrometry combined with liquid scintillator method
A method using liquid scintillator to determine the amount of 241Pu instead of ICP-MS and α-spectrometry to determine the amount of 241Am to obtain the age of plutonium was also developed. The result of the method was shown in Table 4. The ages determined by α-spectrometry combined with liquid scintillator were lower than the ones obtained by the two foregoing methods. The deviation was about 10% and considered to be mainly from the measurement of liquid scintillator. The method could be used in the normal radiochemistry labs without ICP-MS.
Conclusions
Three methods for determining the age of trace plutonium on the basis of separation of U, Pu and Am were developed in this work. A plutonium age standard solution and a plutonium solution of unknown age were analyzed by the three methods, respectively. The determined ages were quite consistent and agreed well with the reference age. The three methods could be adopted in the verification activities of nuclear safeguards and nuclear arms control.
References
Keegan, R.P., Gehrke, R.J.: A method to determine the time since last purification of weapons grade plutonium. Appl. Radiat. Isot. 59, 137–143 (2003)
West, D., Sherwood, A.C.: γ-Rays from an unirradiated plutonium-uranium oxide fuel pin and a method of measuring the age of plutonium since chemical processing. Ann. Nucl. Energy 8, 441–453 (1981)
Wallentus, M., Tamborinl, G., Koch, L.: The “age” of plutonium particles. Radiochim. Acta 89, 55–58 (2001)
Wallenius, M., Mayer, K.: Age determination of plutonium material in nuclear forensics by thermal ionisation mass spectrometry. Fresenius J. Anal. Chem. 366, 234–238 (2000)
Nygren, U., Rameback, H., Nilsson, C.: Age determination of plutonium using inductively coupled plasma mass spectrometry. J. Radioanal. Nucl. Chem. 1, 45–51 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Chang, Zy., Zhao, Yg. et al. Studies on the age determination of trace plutonium. J Radioanal Nucl Chem 281, 675–678 (2009). https://doi.org/10.1007/s10967-009-0056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0056-0