Abstract
Adsorption and separation performances of a microporous silica-based (DAMIA-EH + TOA)/SiO2-P adsorbent towards Pd(II) were systematically investigated under the effect of contact time, temperature, concentration of nitric acid, chromatography etc. in a simulated high-level liquid waste containing 14 kinds of representative co-existing metal ions. Successful recovery of around 97.95% Pd(II) was achieved under the elution with 0.01 M Tu (pH = 2) solution in column experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high-level liquid waste (HLLW) coming from the reprocessing of spent nuclear fuel consists of several waste liquids, the main, in terms of radioactivity and volume, being the raffinate of the solvent extraction process [1]. As a result, large amounts of nonvolatile fission products (FPs), actinides, and trace amounts of uranium and plutonium, together with corrosion products are contained in HLLW [2]. Due to its high corrosiveness, high concentration of nitric acid, generation of decay heat, high rates of radioactivity and related generation of hydrogen, storage and disposal of HLLW requires strict control and complicated management from the perspective of environmental protection. Palladium, as one of main FPs, is not extracted with U and Pu in the solvent extraction process of PUREX and finally remains in HLLW [3]. High concentration of Pd(II) in the HLLW raises problems during the raffinate solution has to be concentrated and solidified prior to its interim storage and final disposal. When Pd(II) is accumulated, it will form separate phases in the borosilicate glass matrix, not chemically incorporated in the final glass. Sedimentation of these dispersed phases in the absence of convective mixing in the melt leads to the formation of a layer with increased viscosity (up to a factor of 4) and electric conductivity (up to a factor of 100), possibly causing a short circuit and temperature gradients, and finally resulting deterioration in the stability of the glasses, discontinuous operation of the vitrification process and heterogeneous molten glass formation (lowering the quality of the final product) in waste vitrification process [4]. In order to decrease the volume of vitrificated glass units to be disposed and to achieve better stability of the vitrification process and higher quality of the final product, effective recovery and separation of Pd(II) from HLLW is very important.
Many methods have been adopted in the treatment and disposal of HLLW. Extraction chromatography as one of the most effective one, can decrease the formation of third phase; operate with smaller elution volumes, adjustable flow rates; minimize the accumulation of secondary waste, etc. [5]. In extraction chromatography, a certain extractant is impregnated either alone or in combination with a suitable diluent into an inert support, to prepare a solid sorbent capable of selectively removing metal ions from HLLW. Based on the Hard–Soft Acid–Base (HSAB) theory by Pearson, palladium(II) is classified as soft acid, showing affinity to soft bases with atoms S and N [6]. Thiodiglycolamide (TDGA) and its derivatives act as classic S donor chelating agent, have been widely studied in the separation of Pd(II) in acid solution [7]. Xu et al. [8] reported the adsorption behaviors of Pd(II) onto a silica-based (Crea + 1-dodecane)/SiO2-P extraction resin from simulated HLLW, their results found (Crea + 1-dodecane)/SiO2-P extraction resin exhibited good adsorption selectivity for Pd(II) over the other fission products in HNO3 solution, the distribution coefficient (Kd) of Pd(II) was more than 104 even the concentration of HNO3 reached 5 M. Even though S donor ligands showed superior separation performances towards Pd(II) in acid solution, the unstable and unfriendly (not fulfill CHON criteria) S group is still a problem for the effective recovery of Pd(II) in HLLW. Earlier it has been reported that S donor ligands namely DHS etc. undergo oxidation in strong nitric acid medium [9]. Ruhela et al. [10] studied the extraction of Pd(II) from simulated HLLW in T(2EH)TDGA/n-dodecane solvent system. It was found that Kd of Pd(II) decreased from 1000 to less than 30 when the adsorbed does reached 0.5 MGy. And the degradation phenomenon of T(2EH)TDGA was caused by the cleavage of amide bond, thioether bond and carboxyl linkage of amide. On the other hand, utilizing N donor ligand has also been considered as one of the possible methods to separate Pd(II) in HLLW, though water solubility caused by protonation in highly acidic condition is frustrating. Amberlite XAD-16 adsorbent functionalized with 2-acteyl pyridine by coupling it with 2-chloro pyridine has been synthesized in Ruhela et al. [11] group. Their results showed with an increase of acidity, N atom got gradually protonated and consequently there was a huge decrease in Kd of Pd(II). Mehrani et al. [12] prepared a chemical dipyridylamine-modified nano-porous silicas as a novel adsorbents for the pre-concentration and recovery of Pd(II). It was observed that the highest recovery for Pd(II) was obtained at neutral pH area. When the pH was lowered to 2, the extraction percentage of Pd(II) decreased to only 20%. As we known, soft N donor can maintain the selectivity towards Pd(II), hard O donor such as amide oxygen donor can lower the basicity of the N donor and then could be utilized in more acidic condition [13]. Therefore, to develop a ligand merging both soft N donor and hard O donor without S donor may become a feasible ligand to be utilized in the practical separation of Pd(II) from HLLW.
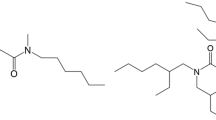
In this paper, to seek the possibility of Pd(II) removal from HLLW, a hybrid N and O donor ligand, 2,2′-[(2-ethylhexyl)imino]bis[N,N-bis(2-ethylhexyl)acetamide] (DAMIA-EH) was selected as the extractant [14, 15]. Tri-n-octylamine (TOA) was added as synergist. DAMIA-EH and TOA were impregnated and immobilized into the pores of a microporous silica (SiO2-P, “P” refers to the styrene–divinylbenzene copolymer) particles with a mean diameter of 50 µm (mean pore size of 50 nm) by wet vacuum immersion method to give the (DAMIA-EH + TOA)/SiO2-P adsorbent [16,17,18,19,20]. Its adsorption properties concerning effect of adsorption speed, effect of HNO3 concentration, effect of temperature, extraction chromatography etc. towards Pd(II) will be evaluated in simulated HLLW.
Experimental
Materials
All chemical reagents used in this research, such as nitric acid (HNO3), diethylene triamine pentaacetic acid (DTPA), thiourea (Tu), palladium (II) nitrate (Pd(NO3)2) solution with 10 wt% of Pd(II) in 10 wt% nitric acid (99.99% trace metals basis), ruthenium(III) nitrosyl nitrate (Ru(NO)(NO3)x(OH)y, x + y = 3) with 1.4 wt% of Ru(III) in dilute nitric acid, rhodium(III) nitrate solution (Rh(NO3)3) with ~ 10 wt% of Rh(III) in > 5 wt% etc. were all chemical pure from Wako Pure Chemical Industries, Inc. and Aladdin Industrial, Inc. The extractant DAMIA-EH (chemical structure is shown in Scheme 1) was purchased from Chemicrea Inc and used directly without further purification. All aqueous solutions were prepared by deionized water with a specific resistance of 18.3 MΩ cm or greater.
Preparation of (DAMIA-EH + TOA)/SiO2–P adsorbent
The relevant synthesis procedures were described briefly as follows: SiO2–P particles were firstly washed three time (each time 1 h) by methanol to remove the industrial residuals in their pores and then dried in vacuum for 24 h [21]. Equal quality of 10 g DAMIA-EH and TOA were placed in a beaker and dissolved using 300 mL dichloromethane (CH2Cl2) as diluent. 20 g of weighed SiO2-P particles were slowly added into the solution. The mixture was transferred to a round-bottomed flask and rotated mechanically for 1 h at room temperature. With increasing the temperature of water bath to 318 K, the CH2Cl2 solution was gradually evaporated under reduced pressure to immobilize DAMIA-EH and TOA into the pores of SiO2–P. After drying for further 24 h in vacuum at 323 K, the light-yellow silica-based (DAMIA-EH + TOA)/SiO2–P adsorbent was obtained [22]. The surface morphology of synthesized (DAMIA-EH + TOA)/SiO2–P was characterized by scanning electron microscopy (SEM, Hitachi S-3100H) as shown in Fig. 1 to prove the successfully impregnate DAMIA-EH and TOA into the pores of SiO2–P.
Batch adsorption experiments
Adsorption behaviors, such as effect of adsorption speed, effect of concentration of HNO3, effect of temperature etc. towards Pd(II) by (DAMIA-EH + TOA)/SiO2–P adsorbent was firstly studied in simulated HLLW. The solutions used in batch experiments were all prepared in 2 M HNO3, containing 14 types of different kinds of co-existing metal ions, such as Cs(I), Sr(II), Ba(II), Ru(III), Rh(III) etc. and the concentration of each metal ion was prepared as 5 mM. Firstly, a weighed amount of (DAMIA-EH + TOA)/SiO2–P adsorbent 0.2 g as a solid phase was combined with 4 mL of solution in a 13.5 mL glass vial with a plastic cap. Then, the mixture was stirred vigorously at 160 rpm in a water batch, followed by a solid and aqueous phase separation by using syringe and water membrane filter with the average pore size of 0.2 µm. The concentration of Cs(I) remained in solution was analyzed by atomic absorption photometer (AAS, Shimazu AA-6200). The concentration of other metal ions was detected by inductively coupled plasma atomic emission spectrometer (ICP-AES, Shimazu ICPS-7510), and the experiments were triplicate and the average values were recorded. The adsorption percentage (E, %), distribution coefficient (Kd, cm3/g), separation factor (SF) and adsorbed amount (q, mmol/g) were decided by the following equations [23]:
where C0, Ct, Ce are the concentration of each metal ion in the aqueous phase (mM) at the initial, certain time and equilibrium state, respectively. m and V indicate the mass of dry (DAMIA-EH + TOA)/SiO2–P adsorbent (g) and the volume of the aqueous phase (mL), respectively. A and B mean different metal ions.
Dynamic chromatography adsorption
Prior to the separation experiments, 5 g of dry (DAMIA-EH + TOA)/SiO2–P adsorbent was slowly added in a glass column with 10 mm diameter and 170 mm height by wet packing method. A constant temperature outside the column was set to 298 K in the loading and elution process by circulating the thermo-stated water through an EYELA model water jacket [24]. The flow rate of feed solution was controlled as 0.5 mL/min. after 5 mL, 2 M HNO3 solution containing 5 mM of 14 types of different kinds of co-existing metal ions, such as Cs(I), Sr(II), Ba(II), Ru(III), Rh(III) etc. passed through the column, the certain volumes of 50 mL, 2 M HNO3 solution, 30 mL, 0.01 M Tu (pH = 2) solution, 30 mL, 0.01 M DTPA solution as eluents were subsequently pumped into the column. The effluent flowed out from column was collected with fraction of 5 cm3 aliquot by a DC-1500 model auto fractional collector (EYELA). The concentration of Cs(I) was measured by AAS, others were measured by ICP-AES.
Results and discussion
Effect of contact time
To investigate the adsorption kinetics of (DAMIA-EH + TOA)/SiO2–P towards Pd(II), the effect of contact time on adsorption performance was carried out in the presence of 2 M HNO3 solution through batch method. The results for adsorption speed were illustrated as shown in Fig. 2. It was found that the adsorption kinetics of Pd(II) and Re(VII) were fairly fast, the adsorption equilibrium can be attained within only 10 min. With increasing the contact time, the uptake amount of Pd(II) and Re(VII) onto (DAMIA-EH + TOA)/SiO2–P was increased to 90.93% and 58.09%, respectively, comparatively higher than other alkali metals (Cs(I)), alkaline earth metals (Sr(II), Ba(II)) and rare earth metals (La(III), Ce(III) etc.) [25]. It revealed that the co-existing metal ions contained in simulated HLLW would not affect the uptake selectivity towards Pd(II) onto (DAMIA-EH + TOA)/SiO2–P adsorbent. Due to (DAMIA-EH + TOA)/SiO2–P containing both soft N and hard O donors, the strong uptake performance of Pd(II) and Re(VII) resulted from the efficient complexing ability with N donor in DAMIA-EH. The weak uptake performance towards other co-existing La(III), Ce(III) etc. derived from the amide group (hard O donors) [26]. Moreover, the adsorption kinetics of Ru(III) was previously reported to be slow in many cases. In contrast, in the present study, the adsorption equilibrium of Ru(III) was attained in around 5 h. This can be attributed to the high surface reactivity and the large surface area of the porous silica [27].
To analyze the adsorption kinetics of representative metal ions by (DAMIA-EH + TOA)/SiO2–P, the linear Pseudo-second order kinetic model was applied to the experimental data. And it was descried as shown in Eq. 5 [28]:
where qe and qt (in mg/g) are adsorption capacity of the metal ions at equilibrium and certain time t, respectively. k2 represents the rate constant of the second order at the equilibrium state (g/(mg h)).
The kinetic data of t/qt versus t for Pd(II), Re(VII), Ru(III) adsorption were plotted as depicted in Fig. 3. The kinetic parameters including the calculated adsorption amount (qe, calc), rate constant (k2), correlation coefficient (R2) were calculated from the plots and summarized as listed in Table 1. The results showed the R2 of Pd(II), Ru(III), Re(VII) were more than 0.99, indicating the use of linear Pseudo-second model was feasible for the predicating the adsorption process. And it can be predicted the adsorption behavior of Pd(II), Ru(III), Re(VII) onto (DAMIA-EH + TOA)/SiO2–P by chemisorption and the complexation reaction between metal ions with ligands was the rate controlling step of the adsorption process [29].
Effect of HNO3 concentration
In the adsorption process, the complexation reactions of N donors in DAMIA-EH with metal ions and HNO3 were considered as two competition reactions. As a result, the association of DAMIA-EH and HNO3 may decrease the complexation of tested metal ions with DAMIA-EH, causing the decreasing of adsorption ability of metal ions onto (DAMIA-EH + TOA)/SiO2–P adsorbent [30]. However, DAMIA-EH was classified as a hybrid N and O donor ligand, through introduction of O donors into DAMIA-EH, the adsorbent was expected to be used in more acidic condition.
Therefore, the influence of HNO3 concentration on the sorption of 15 types of metal ions has been studied under an initial metal concentration of 5 mM in a wide HNO3 concentration varying from 0.1 to 5 M at 298 K. Figure 4 presented with an increase in the HNO3 concentration, the Kd of Pd(II) decreased gradually from 2159.04 cm3/g in 0.1 M HNO3 to 12.09 cm3/g in 5 M HNO3. The Kd of Re(VII) decreased gradually from 1355.56 cm3/g in 0.1 M HNO3 to 1.86 cm3/g in 5 M HNO3. The decrease of Kd values of Pd(II) can be explained as the competition reactions between protonation of soft N donors and complexation of metal ions. While in the case of Re(VII), it was attributed to the increasing amounts of NO3− with increasing the [HNO3] [31]. The higher Kd of Pd(II) indicated that (DAMIA-EH + TOA)/SiO2–P adsorbent showed a better preference to Pd(II) than Re(VII). For Ru(III), the Kd slightly increased to 6.82 when the HNO3 concentration reached 2 M, and then decreased gradually to 1.67 in 5 M HNO3, which meant the 2 M HNO3 was an optimum acidity for the adsorption of Ru(III). Meanwhile, the adsorption performance towards other metal ions showed relatively weak adsorption. These results indicated that (DAMIA-EH + TOA)/SiO2–P adsorbent had an excellent adsorption and high selectively for Pd(II) over other metal ions through introduction of O donors, the acidity resistance of (DAMIA-EH + TOA)/SiO2–P adsorbent was enhanced and was expected to be feasible in the separation of Pd(II) from HLLW [32].
Adsorption capacity and isotherm
The adsorption capacity and isotherm of representative metal ions such as Pd(II), Re(VII), Ru(III) onto (DAMIA-EH + TOA)/SiO2–P adsorbent at 298 K were studied by plotting the amount of metal ions adsorbed versus the equilibrium concentration as show in Fig. 5. Adsorption amount of Pd(II) increased gradually with increasing the initial concentration of Pd(II) in the aqueous phase, and then gradually reached to a stable state as it further increased, which meant the adsorption of Pd(II) onto (DAMIA-EH + TOA)/SiO2–P reached saturated. While the adsorption of Re(VII) and Ru(III) still have not approached a stable state. In order to further study the adsorption isotherm of metal ions onto (DAMIA-EH + TOA)/SiO2–P, Langmuir and Freundlich isotherm models described as follows were selected to analyze the obtained experimental data. Langmuir isotherm model applies to the adsorption reaction is a monolayer adsorption with constant adsorption energy. Freundlich isotherm model assumes to multilayer adsorption. The expressions of these models were descried as follows [33]:
where Qeq (mmol/g) is the amount of metal ions adsorbed at equilibrium state, Ceq (mmol/L) is the equilibrium concentration, Qmax (mmol/g) is the theoretical maximum of metal ions adsorbed, and KL (L/mmol) and KF (mmol/g) are the Langmuir and Freundlich constant, respectively. 1/n is the Freundlich isotherm exponent constant.
Non-linear theoretical fitting details of Pd(II), Re(VII), Ru(III) were shown in Fig. 5 and summarized Table 2. The R2 of Pd(II), Re(VII), Ru(III) obtained suggested that the Langmuir isotherm model was well fitted with the experimental data, indicating the adsorption process was identified as a monolayer adsorption [34]. The Qmax values of Pd(II), Ru(III) and Re(VII) were estimated to be 0.573, 0.714, and 0.757 mmol/g, respectively.
Effect of temperature
The effect of temperature on the adsorption performance of tested 15 types metal ions onto (DAMIA-EH + TOA)/SiO2–P adsorbent in 2 M HNO3 was studied. Based on experimental results shown in Fig. 6a, it was found that except Pd(II), Re(VII), Ru(III), the adsorption abilities of other metal ions still remained in a relatively low level (Kd < 1). Furthermore, the plots of Ln Kd versus 1/T only gave the fitting lines of Pd(II), Re(VII), Ru(III) as shown in Fig. 6b. With increasing the T, the adsorption ability towards Pd(II) and Re(VII) onto (DAMIA-EH + TOA)/SiO2–Pd(II) decreased gradually, while the adsorption towards Ru(III) slightly increased, which meant the high temperature was not benefit for the effective adsorption towards Pd(II). In order to further clarify the effect of T in more details, van’t Hoff equation (as shown below) was used to calculate the thermodynamic parameters [35]. ΔH° and ΔS° were provided as the slope and intercept of the fitting lines in Fig. 6, and the theoretical fitting results were summarized as shown in Table 3.
where R is the universal gas constant (8.314 J/(K mol)), Kd is distribution coefficient. ∆G°, ∆H°, ∆S° are the standard changes in Gibbs free energy (J/mol), enthalpy (J/mol), entropy (J/(K mol)), respectively.
a Effect of temperature on the adsorption percentage of 15 types of tested metal ions onto (DAMIA-EH + TOA)/SiO2–P; b Relationship between Ln Kd versus 1/T for the adsorption of Pd(II), Ru(III), Re(VII). [Metal] = 5 mM; [HNO3] = 2 M; Phase ratio = 0.2 g/4 mL; Shaking speed: 160 rpm; Shaking time: 5 h; Temperature: 288–323 K
Based on the results, it was found that all the ∆G° values were negative, indicating the adsorption process was spontaneous and thermodynamically favorable. The corresponding thermodynamic parameters ∆H° of Pd(II), Re(VII) adsorption onto (DAMIA-EH + TOA)/SiO2–P were calculated as − 16.625 and − 7.796 kJ/mol, respectively. The negative value of ∆H° indicated that Pd(II), Re(VII) adsorption was exothermic, while the positive values of ∆H° for Ru(III) revealed its adsorption process was endothermic. The negative ∆S° of Pd(II) can be explained as a result of stable arrangement onto (DAMIA-EH + TOA)/SiO2–P [36].
Chromatography separation experiment
The chromatographic separation results were illustrated in Fig. 7, and the recovery yield of representative metal ions were summarized in Table 4. As can be seen, with the feed solution was supplemented, the alkali metals (Cs(I)), alkaline earth metals (Sr(II), Ba(II)) and rare earth metal (La(III), Ce(III), Nd(III) etc.), Mo(VI), Zr(IV) etc. showed almost no adsorption ability and was quickly flowed out with 2 M HNO3, which was consistent with the results from batch adsorption experiments. While Pd(II) and Re(VII) were still strongly adsorbed inside the column. Next, 0.01 M Tu (pH = 2) solution was employed to separate the adsorbed Pd(II) from Re(VII) in (DAMIA-EH + TOA)/SiO2–P adsorbent. The concentration of Pd(II) in effluent was found to be quickly increased with the flowing in of 0.01 M Tu (pH = 2) solution. The phenomenon shown in Fig. 7 can be seen as the sharp elution peak of Pd(II). It can reveal the disassociation speed was also very rapid, which will be benefit in the practical utilization. After measuring by ICP-AES, it was calculated that about 97.95% of Pd(II) was recovered due to the stronger affinity of S donor in Tu (S = C functional group) than N donor in DAMIA-EH. From previous studies, four Tu molecules formed a 1:4 complex with one Pd(II) ion, the mechanism of complexation behavior between Tu and Pd(II) can be described as the following equation in details [37]:
Conclusions
The adsorption behaviors of a hybrid soft N and hard O donor (DAMIA-EH + TOA)/SiO2–P adsorbent towards Pd(II) in simulated HLLW solution containing 14 kinds of co-existing metal ions was investigated. The results found that the adsorption speed was fairly fast and the equilibrium state can be reached at only 10 min. With increasing the concentration of HNO3, the adsorption ability towards Pd(II) decreased gradually. Kd of Pd(II) decreased gradually from 2159.04 cm3/g in 0.1 M HNO3 to 12.09 cm3/g in 5 M HNO3. Such a decrease was explained as the competition reactions between protonation and complexation behaviors of N donors. The results also revealed that (DAMIA-EH + TOA)/SiO2–P had a good selectivity of Pd(II) in a wide HNO3 range from 0.1 to 5 M compared with other co-existing metal ions. The experimental data fitted well with Langmuir isotherm model, indicating the adsorption process was identified as a monolayer adsorption. Furthermore, with an increase in the temperature of solution, the adsorption performance of Pd(II) slightly decreased, which meant the adsorption process was high temperature unfavorable. The values of calculated thermodynamic parameters revealed that the adsorption process of Pd(II) was spontaneous and exothermic. The chromatography separation experiment of Pd(II) was successfully completed under elution with 0.01 M Tu (pH = 2) solution, and about 97.95% of Pd(II) was selectively recovered. The results introduced above revealed that the utilization of a hybrid donor (DAMIA-EH + TOA)/SiO2–P adsorbent to directly separate Pd(II) from simulated HLLW was feasible.
References
Natarajan R (2017) Reprocessing of spent nuclear fuel in India: present challenges and future programme. Prog Nucl Energy 101:118–132
Jayakumar M, Venkatesan KA, Srinivasan TG, Rao PRV (2009) Studies on the feasibility of electrochemical recovery of palladium from high-level liquid waste. Electrochim Acta 54:1083–1088
Ruhela R, Singh AK, Tomar BS, Hublia RC (2014) Separation of palladium from high level liquid waste–a review. RSC Adv 4:24344–24350
Uruga K, Sawada K, Enokida Y, Yamamoto I (2008) Vitrification of high-level radioactive waste considering the behavior of platinum group metals. Prog Nucl Energy 50:514–517
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J Nucl Sci Technol 49:320–327
Turanov AN, Karandashev VK, Proshin AN (2008) Extraction of Palladium(II) from nitric acid solutions with 1-Benzoyl-3-[6-(3-benzoyl-thioureido)-hexyl]-thiourea. Solvent Extr Ion Exch 26:360–374
Ruhela R, Sharma JN, Tomar BS, Panja S, Tripathi SC, Hubli RC, Suri AK (2010) N, N, N′, N′-tetra(2-ethylhexyl) thiodiglycolamide T(2EH)TDGA: a novel ligand for the extraction of palladium from high level liquid waste (HLLW). Radiochim Acta 98:209–214
Xu YL, Kim SY, Ito T, Tokuda H, Hitomi K, Ishii K (2015) Adsorption behavior of platinum group metals onto a silica-based (Crea + Dodec)/SiO2-P extraction resin from simulated high level liquid waste. Sep Sci Technol 50:260–266
Narita H, Tanaka M, Morisaku K (2008) Palladium extraction with N, N, N′, N′-tetra-n-octyl-thiodiglycolamide. Miner Eng 21:483–488
Ruhela R, Sharma JN, Tomar BS, Singh KK, Kumar M, Bajaj PN, Hubli RC, Suri AK (2012) Studies on hydrolytic and radiolytic stability of N, N, N′, N′-tetra-(2-ethylhexyl) thiodiglycolamide T(2EH)TDGA. Radiochim Acta 100:37–44
Ruhela R, Singh KK, Tomar BS, Sharma JN, Kumar M, Hubli RC, Suri AK (2012) Amberlite XAD-16 functionalized with 2-acetyl pyridine group for the solid phase extraction and recovery of palladium from high level waste solution. Sep Purif Technol 99:36–43
Mehrani K, Mehrani A, Amini MM, Sadeghi O, Tavassoli N (2011) Dipyridylamine-modified nanoporous silicas as new sorbents for the separation and pre-concentration of palladium. Microchim Acta 173:521–527
Xiao CL, Wang CZ, Yuan LY, Li B, He H, Wang SA, Zhao YL, Chai ZF, Shi WQ (2014) Excellent selectivity for actinides with a tetradentate 2,9-diamide-1,10-phenanthroline ligand in highly acidic solution: a hard—soft donor combined strategy. Inorg Chem 53:1712–1720
Sasaki Y, Tsubata Y, Kitatsuji Y, Sugo Y, Shirasu N, Morita Y, Kimura T (2013) Extraction behavior of metal ions by TODGA, DOODA, MIDOA, and NTaamide extractants from HNO3 to n-dodecane. Solvent Extr Ion Exch 31:401–415
Sasaki Y, Morita K, Shimazaki S, Tsubata Y, Ozawa M (2016) Masking effects for Mo, Re, Pd and Ru by S and N-donor reagents through MIDOA and Ntaamide extraction. Solvent Extr Res Dev Jpn 23:161–174
Ning SY, Zhou J, Zhang SC, Zhang W, Wei YZ (2019) Synthesis of soft N-donor isoPentyl-BTBP and study on its removal of actinides from high level liquid waste. Radiochim Acta 108:5619
Ning SY, Zhang SC, Zhang W, Zhou J, Wang SY, Wang XP, Wei YZ (2020) Separation and recovery of Rh, Ru and Pd from nitrate solution with a silica-based IsoBu-BTP/SiO2-P adsorbent. Hydrometallurgy 191:105207
Zhang SC, Ning SY, Zhou J, Wang SY, Wang XP, Wei YZ (2020) New insight into the adsorption of ruthenium, rhodium, and palladium from nitric acid solution by a silica-polymer adsorbent. Nucl Sci Tech 31:201
Wu Y, Lee CP, Mimura H, Zhang XX, Wei YZ (2018) Stable solidification of silica-based ammonium molybdophosphate byallophane: application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J Hazard Mater 341:46–54
Wang QL, Sang HJ, Chen LF, Wu Y, Wei YZ (2020) Selective separation of Pd(II) through ion exchange and oxidation-reduction with hexacyanoferrates from high-level liquid waste. Sep Purif Technol 231:115932
Yu Q, Ning SY, Zhang W, Wang XP, Wei YZ (2018) Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent. Hydrometallurgy 181:74–81
Khayambashi A, Wang XL, Wei YZ (2016) Solid phase extraction of uranium (VI) from phosphoric acid medium using macroporous silica-based D2EHPA-TOPO impregnated polymeric adsorbent. Hydrometallurgy 164:90–96
Shu QD, Khayambashi A, Wang XP, Wei YZ (2018) Studies on adsorption of rare earth elements from nitric acid solution with macroporous silica-based bis(2-ethylhexyl)phosphoric acid impregnated polymeric adsorbent. Adsorpt Sci Technol 36:1049–1065
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Study on selective separation of cesium from high level liquid waste using a macroporous silica-based supramolecular recognition absorbent. J Radioanal Nucl Chem 293:13–20
Takeda Y, Ishida K (2001) Thin-layer chromatographic behavior of rare earths on silica gel with aqueous alkaline earth metal nitrate solutions as mobile phases. Fresenius J Anal Chem 370:371–376
Sasaki Y, Ozawa M, Kimura T, Ohashi K (2009) 2,2′-(Methylimino)bis(N, N-dioctylacetamide) (MIDOA), A new tridentate extractant for Technetium(VII), Rhenium(VII), Palladium(II), and Plutonium(IV). Solvent Extr Ion Exch 27:378–394
Petalaa E, Dimos K, Douvalis A, Bakas T, Tucek J, Zboril R, Karakassides MA (2013) Nanoscale zero-valent iron supported on mesoporous silica: characterization and reactivity for Cr(VI) removal from aqueous solution. J Hazard Mater 261:295–306
Liu RQ, Wang XP, Ning SY, Wei YZ, Yang JL, Zhao YP, Ding YQ (2015) Adsorption behavior of 241Am(III) and Eu(III) by silica/polymer-based isoHex-BTP adsorbent from nitric acid solution. Nucl Sci Tech 26:61–68
Ito T, Kim SY, Xu YL, Hitomi K, Ishii K, Nagaishi R, Kimura T (2013) Adsorption behaviors of platinum group metals in simulated high level liquid waste ssing macroporous (MOTDGA-TOA)/SiO2-P silica-based absorbent. Sep Sci Technol 48:2616–2625
Ning SY, Zhang SC, Zhou J, Zhang W, Wei YZ (2019) Salt-free separation of 241Am(III) from lanthanides by highly stable macroporous silica-polymer based Me2-CA-BTP/SiO2-P adsorbent. J Radioanal Nucl Chem 322:1023–1030
Favre-Réguillon A, Draye M, Cote G, Czerwinsky KR (2019) Insights in uranium extraction from spent nuclear fuels using dicyclohexano-18-crown-6-fate of rhenium as technetium homolog. Sep Purif Technol 209:338–342
Yuan LY, Zhu L, Xiao CL, Wu QY, Zhang N, Yu JP, Chai ZF, Shi WQ (2017) Large-Pore 3D cubic mesoporous (KIT-6) hybrid bearing a hard—soft donor combined ligand for enhancing U(VI) capture: an experimental and theoretical investigation. ACS Appl Mater Interfaces 9:3774–3784
Wu Y, Zhang XX, Kim SY, Wei YZ (2016) Simultaneous separation and recovery of Cs(I) and Sr(II) using a hybrid macrocyclic compounds loaded adsorbent. Kinetic, equilibrium and dynamic adsorption studies. J Nucl Sci Technol 53:1968–1977
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Peng XJ, Cui Y, Ma JF, Li YL, Sun GX (2017) Extraction of lanthanide ions with N, N, N′, N′-tetrabutyl-3-oxadiglycolamide from nitric acid media. Nucl Sci Tech 87:9077
Ghosal PS, Gupta AK (2017) Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J Mol Liq 225:137–146
Zou Q, Wu Y, Shu QD, Ning SY, Wang XP, Wei YZ, Tang FD (2018) Separation of palladium along with minor actinides by isoBu-BTP/SiO2-P Adsorbent from high-level liquid waste. J Chem Eng Data 63:2931–2939
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Kubota, M., Osawa, N. et al. Adsorption and separation behavior of palladium(II) on a silica-based hybrid donor adsorbent from simulated high-level liquid waste. J Radioanal Nucl Chem 326, 1323–1331 (2020). https://doi.org/10.1007/s10967-020-07414-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07414-z