Abstract
Nanoporous silicas of the type SBA-15 (Santa Barbara Amorphous) and MCM-48 (Mobile Composition of Material) were modified with dipyridylamine (dipy) and used as solid phases for the extraction of Pd(II) ions. The experimental conditions (pH, sample and eluent flow rates, type and quantity of eluent) were optimized. The recovery values were ~ 99.7 and ~ 93.4% for dipy-MCM-48 and dipy-SBA-15, respectively, the limits of detection were <0.08 and <0.11 ng L−1, the pre-concentration factors were 725 and 550, and the adsorption capacity was >78 mg g−1. The procedure was applied to the preconcentration of Pd(II) in real samples.

Nanoporous silicas of the type SBA-15 and MCM-48 were modified with dipyridylamine and used as solid-phase for the extraction of Pd(II) ions. The experimental conditions were optimized and the recovery values were determined. The procedure was applied to the pre-concentration of Pd(II) in real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Palladium is one of the most widely used of the platinum group metals (PGM) [1]. Palladium is especially valued for its catalytic functions, electrical conductivity, chemical resistance to corrosion, and extensive use in the petroleum, pharmaceutical, jewelry, and dentistry industries [2–4]. Palladium can also be used in biomedical devices, as a catalyst in fuel cells, and as anti-pollution devices for the automobile industry, amongst other. Consequently, this metal is released into the environment in considerable amounts as a new pollutant, and especially by the technical use of automobile catalyst convertors that contains the active palladium metal [5–8]. This metal may enter the environment and interact with complex materials such as humic substances [9]. Palladium has no biological role, and all of its compounds should be regarded as highly toxic and carcinogenic [10, 11]. Thus, due to the increasing price of palladium on the one hand, and the toxicity of its compounds to mammals, fish and plants, on the other, the separation and determination of palladium are of special interest to analytical sciences [12, 13]. Several methods have been available and used for the pre-concentration and separation of trace elements according to the nature of the sample, the analyte concentration, and the measurement technique [14] including co-precipitation [15], ion exchange [16], solvent extraction [17], and solid-phase extraction (SPE) [18–21]. Solid-phase extraction (SPE) is used most often because it has several advantages such as flexibility, low cost, low solvent consumption, simplicity, fast rate of analysis, ease of automation, and less environmental pollution [22]. Different solid-phase extractors such as Amberlite XAD resins [23], polyurethane foam [24], activated carbon [25], silica gel [26], and mesoporous silicas [27] with chelating groups have been widely used as sorbents. Among the many types of adsorbents used in SPE, functionalized nanoporous silicas such as MCM-41, MCM-48, and SBA-15 have received a considerable attention for their good mechanical and thermal stability, and also for their lower susceptibility to swelling and shrinking [28]. Among the variety of adsorption applications, the preparation of highly effective adsorbents for trapping heavy metal ions, grafted or ligand-incorporated nanoporous silicas is clearly one of the most promising for environmental clean-up. Thus, in this study we utilized novel modified Si-SBA-15 and Si-MCM-48 for selective sorbents for the separation, pre-concentration, and determination of palladium ions by inductively coupled plasma-atomic emission spectrometry. In this work, for the first time, SBA-15 and MCM-48 nanoporous silicas were functionalized with dipyridylamine (dipy-Si-SBA-15, dipy-Si-MCM-48) and their application as adsorbents for the separation of ultra-traces amounts of palladium ions was studied. The effects of type and concentration of the eluent, solution pH, flow rate, breakthrough volume, and adsorption capacity were investigated. The present method was conducted on a few natural samples, and its accuracy and precision was measured.
Experimental
Reagents and solutions
All analytical grade reagents were purchased from Merck (http://www.merck.de) (Darmstadt, Germany) or Fluka (http://www.fluka.org) (Buchs SG, Switzerland) and used without further purification. The following buffer solutions were used for the various pH levels; pH 1–2, KCl/HCl; pH 4–6, CH3COOH/CH3COONH4; pH 6–8, Na2HPO4/ NaH2PO4, and pH 8–10, NH3/NH4Cl. A standard 1,000 mg L−1 Pd(II) solution was purchased from Merck (Darmstadt, Germany), and all solutions were prepared using deionized water. Hexadecyl trimethyl ammonium bromide, TEOS, HCl, and NaOH for the synthesis of Si-MCM-48 and Si-SBA-15 were purchased from Merck, and Pluronic P123 and 2,2′-dipyridylamine were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com) (Missouri, United States). The mine stones were obtained from a mineral for which a certified concentration of palladium was reported by the Geological Survey of Iran (http://www.gsi.ir).
Preparation of nanoporous silicas and modification by dipyridylamine
The nanoporous silicas, Si-MCM-48 [29] and Si-SBA-15, [30] were prepared according to the reported procedures and functionalized by 3-choloropropyl triethoxysilane, similar to the general procedure used for the functionalization of silica by amine [31, 32]. In a typical reaction, 1 g of nanoporous silica was suspended in 50 mL toluene and the mixture was stirred for 1 h followed by the addition of 2.0 g of 3-choloropropyl triethoxysilane and refluxed for 2 h under a nitrogen atmosphere. The white solid was removed from the solvent by filtration and extracted with toluene using a Soxhlet extractor and then dried under reduced pressure at 70 °C. This solid was reacted with 1 g of 2,2′-dipyridylamine in 50 mL of toluene in the presence of diethylamine. After 12 h stirring at room temperature the white solid was removed from the solvent by filtration and extracted with toluene and then with ethanol using a Soxhlet extractor. A schematic diagram of the modified Si-SBA-15 and Si-MCM-48 with dipyridylamine is shown in Fig. 1. These materials were designated dipy-Si-SBA-15 and dipy-Si-MCM-48. Functionalization of Si-SBA-15 and Si-MCM-48 with 2,2′-dipyridylamine were confirmed by small-angle X-ray diffraction, IR spectroscopy and elemental analysis. The small-angle X-ray diffraction patterns of mesoporous silicas, as shown for dipy-Si-SBA-15 in Fig. 2, revealed that the mesoporous structures of the materials were maintained after functionalization with 2,2′-dipyridylamine: IR (KBr, cm-1): 3,450 (NH), 2,913 (CH, aliphatic), 1,650 (C = N), 1,600 (C = C), 1,080 (Si-O-Si). Elemental analysis of dipy-Si-SBA-15 (C, 26.52%; N, 7.41% and H, 2.38%), and dipy-Si-MCM-48 (C, 28.88%; N, 8.06% and H, 2.59%) samples yielded dipyridylamine concentrations of 1.70 and 1.85 mmol g−1, respectively.
Instrumentation
Determination of palladium was performed with a Shimadzu (Kyoto, Japan) Model 7,000 inductively coupled plasma-atomic emission spectrometer (ICP-AES) equipped with a mini-torch-type and conventional pneumatic concentric nebulizer. The operation conditions are provided in the Supplementary Material (Table S1). A vacuum pump was purchased from Leybold (Germany) and used during the experiment. A vacuum gauge controller from Analytichem International (Harber City, CA) was used to control the flow rate during the extraction. The pH measurements were determined using a digital WTW Metrohm 827 Ion analyzer (Switzerland) equipped with a combined glass-calomel electrode. All pH measurements were made at 25 ±1 °C. The CHN analyses were performed on a Thermo Finnigan Flash-1112EA microanalyzer (Italy). The X-ray diffraction patterns were obtained on a STOE diffractometer with Cu Kα radiation. The IR spectra were recorded using a BOMEM/MB series Spectrometer.
Column procedure
A glass column, 120 mm in length and 20 mm in diameter, was blocked by polypropylene filters at each ends, filled with 200 mg of dipy-functionalized nanoporous silica and used for the experiments. Before extraction, the column was treated with 20 mL hydrochloric acid (2 mol L−1), 20 mL nitric acid (2 mol L−1), 20 mL toluene, 20 mL ethanol, and 50 mL distilled water to remove any organic and inorganic contaminants.
Pre-concentration procedure
A solution containing 1 ng mL−1 of palladium ions at pH 7 was prepared. The pH was adjusted with a Na2HPO4/ NaH2PO4 buffer solution and then 50 mL of a solution containing palladium ions was passed through the column at a flow rate of 10 and 12 mL min−1 for dipy-Si-SBA-15 and dipy-Si-MCM-48, respectively. The column was eluted with 4 mL of 0.1 mol L−1 thiourea solution and the palladium ions in the eluent were analyzed by ICP. Each measurement was replicated for five times and the results were averaged.
Real sample pre-treatment
The real samples were obtained from tap water, seawater (Caspian Sea and the Persian Gulf), river water (Chalus River) and jewelry wastewater. The samples were stored in cleaned polyethylene bottles and were filtered through nylon filters (Millipore) before use. The pH adjustment was performed with Na2HPO4/ NaH2PO4 buffer solutions. Then, the solutions were passed through the present column under the optimum conditions. In addition, two mine stone samples with a certified palladium content, which was reported by the Geological Survey of Iran, were obtained. These samples were digested in an 8 mL mixture of 5% aqua regia with the assistance of a microwave digestion system. Digestion was carried out for 2 min at 250 W, 2 min at 0 W, 6 min at 250 W, 5 min at 400 W, and 8 min at 550 W, and the mixture was then vented for 8 min. The residue from this digestion, as well as a control digestion was then diluted with deionized water [26]. Finally, the abovementioned method was applied to separate and pre-concentrated palladium ions from the aforementioned samples.
Result and discussion
Palladium ions were adsorbed onto dipy-Si-SBA-15 and dipy-Si-MCM-48 in the column and the possible reaction that resulted in the formation of a square planner complex is shown in Fig. 1. Various parameters such as the pH of the sample solutions, the type, concentration and volume of the eluent, and the sample and eluent flow rates were optimized. The recovery percentages of the palladium ions on the solid-phases were calculated from the values obtained from the measurements of palladium ions in the effluent and eluent solutions.
Influence of pH
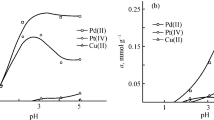
In order to study the effect of pH on extraction, the pH of 10 mL of the different sample solutions containing 1 ng mL−1 palladium ions was adjusted within the range of pH 2 to 9 (in pH 10 the nanoporous silica structures will collapse). The samples were passed through the column and eluted with 4 mL of 0.1 mol L−1 thiourea in 0.1 mol L−1 H2SO4 solutions. The eluents were analyzed by ICP and the results are shown in Fig. 3. It was observed that the highest recovery for palladium extraction was obtained at neutral pH. Separation in neutral pH may be considered as an advantage of these novel solid phases. This observation can be attributed to the presence of the free lone pair of electrons on nitrogen of the dipyridylamine groups, which are suitable donors for coordination to the palladium ions.
Effect of type, concentration and volume of eluent solution
Different eluent solutions were used for desorption of the palladium ions from the functionalized nanoporous silicas. These eluents contained H2SO4, HCl, HNO3, thiourea, and methanol in different concentrations. It was found that 0.1 mol L−1 thiourea in 0.1 mol L−1 H2SO4 solution was the best desorbing eluent and recoveries of more than 93% for dipy-Si-SBA-15 and 99.7% for dipy-Si-MCM-48 were obtained with this eluent. The effect of eluent volume on desorption was also studied. It was found that 4 mL was the optimum eluent volume for desorption of palladium ions from these functionalized nanoporous silicas.
Sample and eluent flow rates
In order to study the effect of sample and eluent flow rates on the extraction recovery, 10 mL of 1 ng mL−1 palladium solution was used. The pH of the solution was adjusted to pH 7 and it was passed through the column at different flow rates within the range of 1–14 mL min−1 using a peristaltic pump. Finally, the column was washed with 4 mL of a selected eluent at different flow rates. As shown in the Supplementary Material (Figs. S1 and S2), the optimum flow rate for adsorption and desorption was obtained within the range of 1 to11 and 1 to 5 mL min−1 for dipy-Si-MCM-48 and 1 to 8 and 1 to 7 for dipy-Si-SBA-15, respectively.
Column reuse
The long-term stability of the solid-phase of the column, dipy-Si-MCM-48 and dipy-Si-SBA-15, was investigated by successive sorption and elution cycles by passing 10 mL of 1 ng mL−1 palladium ions through the column under the optimum conditions. The sorbed palladium ions were then eluted from dipy-Si-MCM-48 and dipy-Si-SBA-15 with 4 mL of 0.1 mol L−1 thiourea solution. The stability of the column was assessed by monitoring the change in the recoveries of the extracted palladium ions. The results showed that there was no change up to ten cycles, and after ten sorption-desorption cycles the recovery decreased.
Influence of interference ions
In order to investigate the selectivity of the method, the effect of the presence of different cations in the extraction of palladium was studied. Cations of Na+, K+, Cs+, Mg2+, Ca2+, Cd2+, Fe2+, Cu+2, Ni+2, Mn+2, Cr+3, Au+3 and Pt+2 as chloride salts of various concentrations were added to 10 mL of a single solution containing 1 ng of palladium ions and the extraction procedure was followed. The selectivity for palladium ions by the sorbents is shown in Table 1.
Maximum adsorption capacity
In order to determine how much sorbent was required to quantitatively remove a specific amount of a metal ion from the solution, the capacity of the sorbent was calculated. To evaluate this factor, 1,000 mL of a solution containing 50 mg palladium ions underwent the extraction procedure and the maximum capacity was calculated. A maximum adsorption capacity of 97.7 and 78.4 mg g−1 (0.9 and 0.7 mmol g−1) was obtained for dipy-Si-MCM-48 and dipy-Si-SBA-15, respectively.
Analytical performance
In order to study the breakthrough volume, 1 μg of palladium ions was dissolved in 100, 200, 500, 750, 1,000, 1,500, and 2,000 mL distilled water and the aforementioned procedure was followed. The palladium ions were quantitatively retained with a recovery of more than 2,000 mL for dipy-Si-MCM-48 and almost 750 mL for dipy-Si-SBA-15. Thus, the breakthrough volume for the aforementioned SPE method for 1 μg palladium ions should be greater than 2,000 mL for dipy-Si-MCM-48 and greater than 750 mL for dipy-Si-SBA-15. Therefore, it can be concluded that dipy-Si-MCM-48 has high a trapping efficiency of the palladium ions.
The enrichment factor was calculated by the recommended column procedure using volumes of 10 μg L−1 palladium ion solution. The maximum sample volume was found to be 2,900 or 2,200 mL with recoveries greater than 99.5% or 93% for dipy-Si-MCM-48 and dipy-Si-SBA-15, respectively. The loaded palladium ions were desorbed from the solid phases with their respective eluent volumes. As a result, enrichment factors of 725 and 550 for dipy-Si-MCM-48 and dipy-Si-SBA-15, respectively, were obtained. We believed that dipyridylamine functionalized Si-MCM-48 and Si-SBA-15 had low limits of detection compared to the other solid phases for the extraction of palladium ions. In order to determine the limits of detection (LOD) for this method, ten 50 mL blank solutions were passed through the column under the optimal conditions. The LOD values of 0.08 (dipy-MCM-48) and 0.11 (dipy-SBA-15) ng mL−1 were obtained from CLOD = KbSb/m for a numerical factor of kb = 3. The analytical performance of the present method under the optimized conditions was obtained from the results of the ICP measurements. The recovery of the palladium ions extraction procedure was found to be 99.7% or 93.4% with relative standard deviations of 0.1 and 0.8 for ten replicated analyses for dipy-Si-MCM-48 and dipy-Si-SBA-15, respectively (Table 2). This technique was validated via a comparison to the mine stone samples containing certified palladium contents (see Supplementary Material Table S2). In addition, the present method was performed on real samples to study its accuracy.
Based on presented results it seems that dipy-SBA-15 and dipy-MCM-48 have lower detection limits and higher capacities compared with other procedures for the extraction of Pd(II) ions (Table 3). Apparently, the high surface area of the nanopours materials and the high loading of the modifier ligand on their surface, make these materials a special sorbent for palladium ion.
Conclusion
The solid-phase extraction of palladium as a “precious or noble” metal was investigated. Using dipyridylamine-functionalized Si-SBA-15 and Si-MCM-48 nanoporous silicas as solid-phase materials for the extraction of palladium is fast and selective. The dipyridylamine-functionalized Si-SBA-15 and Si-MCM-48 nanoporous silicas showed high performance characteristics, such as a high capacity factor, a low detection limit and a high enrichment factor, which makes them special sorbents for palladium ions.
References
Sari A, Mendil D, Tuzen M, Soylak M (2009) Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J Hazard Mater 162:874
Yoshida H, Nakajima T, Yazawa Y, Hattori T (2007) Support effect on methane combustion over palladium catalysts. Appl Catal B 71:70
Tripodia P, Gioacchinob D, Darja Vinko J (2009) AC electrical resistance measurements of Pd samples versus composition. J Alloy Compd 486:55
Doucet H, Hierso J (2007) Palladium coupling catalysts for pharmaceutical applications. Curr Opin Endocrinol Diab 10:672
Edmiston Ch, Goheen MP, Seabrook GR, Johnson Ch, Lewis B, Brown KR, Towne JB (2006) Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am J Surg 192:344
Zhu Y, Khan Z, Masel RI (2005) The behavior of palladium catalysts in direct formic acid fuel cells (205). J Power Sources 139:15
Hooda PS, Miller A, Edwards AC (2007) The distribution of automobile catalysts cast platinum, palladium and rhodium in soils adjacent to roads and their uptake by grass. Sci Total Environ 374:384
Sures B, Zimmermann S (2007) Impact of humic substances on the aqueous solubility, uptake and bioaccumulation of platinum, palladium and rhodium in exposure studies with Dreissena polymorpha. Environ Pollut 146:444
Shemirani F, Rahnama Kozani R, Jamali MR, Assadi Y, Milani Hosseini MR (2006) Cloud-point extraction, preconcentration, and spectrophotometric determination of palladium in water samples. Int J Environ Anal Chem 86:1105
Wataha JC, Hanks CT (1996) Biological effects of palladium and risk of using palladium in dental casting alloys. J Oral Rehabil 23:309
Tokalioğlul S, Yılmaz V, Kartal S, Delibaş A, Soykan C (2009) Solid phase extraction of Pd(II) on a newly synthesized chelating resin prior to determination by flame atomic absorption spectrometry. Microchim Acta 165:347
Jamali MR, Assadi Y, Shemirani F, Salavati-Niasari M (2007) Application of thiophene-2-carbaldehyde-modified mesoporous silica as a new sorbent for separation and preconcentration of palladium prior to inductively coupled plasma atomic emission spectrometric determination. Talanta 71:1524
Mohamadi M, Mostafavi A (2010) A novel solidified floating organic drop microextraction based on ultrasound-dispersion for separation and preconcentration of palladium in aqueous samples. Talanta 81:309
Soylak M, Nilgun DE (2006) Copper(II)–rubeanic acid coprecipitation system for separation–preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J Hazard Mater 137:1035
Oguria K, Shimodab G, Tatsumi Y (1999) Quantitative determination of gold and the platinum-group elements in geological samples using improved NiS fire-assay and tellurium coprecipitation with inductively coupled plasma-mass spectrometry (ICP-MS). Chem Geol 157:189
Pearson DG, Woodland SJ (2000) Solvent extraction/anion exchange separation and determination of PGEs (Os, Ir, Pt, Pd, Ru) and Re–Os isotopes in geological samples by isotope dilution ICP-MS. Chem Geol 165:87
Igarashi Sh, Ide N, Takagai Y (2000) High-performance liquid chromatographic–spectrophotometric determination of copper(II) and palladium(II) with 5,10,15,20-tetrakis(4 N-pyridyl)porphine following homogeneous liquid–liquid extraction in the water–acetic acid–chloroform ternary solvent system. Anal Chim Acta 424:263
Philippeit G, Angerer J (2001) Determination of palladium in human urine by high-performance liquid chromatography and ultraviolet detection after ultraviolet photolysis and selective solid-phase extraction. J Chromatogr B 760:237
Fang J, Jiang Y, Yan XP (2005) Selective quantification of trace palladium in road dusts and roadside soils by displacement solid-phase extraction online coupled with electrothermal atomic absorption spectrometry. Environ Sci Technol 39:288
Pyrzynska K (1998) Recent advances in solid-phase extraction of platinum and palladium. Talanta 47:841
Anitha Mary T, Murty DSR (2008) Solid phase extraction and preconcentration of gold, palladium, and silver on chitin for their determination in silicate rocks by Flame Atomic Absorption Spectrometry. At Spectrosc 29:69
Armenta S, Garrigues S, dela Guardia M (2008) Green analytical chemistry. TrAC Trends Anal Chem 27:497
Venkatesh G, Singh AK (2007) Enrichment and flame atomic absorption spectrometric determination of palladium using chelating matrices designed by functionalizing Amberlite XAD-2/16 and silica gel. Microchim Acta 159:149
Moawed EA (2006) Preparation of novel ion exchange polyurethane foam and its application for separation and determination of palladium in environmental samples. Anal Chim Acta 580:263
Tavallali H, Yazdandoust S, Yazdandoust M (2010) Cloud point extraction for the preconcentration of silver and palladium in real samples and determination by Atomic Absorption Spectrometry. Clean Soil Air Water 38:242
Zheng H, Zhang D, Wang WY, Fan YQ, Li J, Han HP (2007) Highly selective determination of palladium(II) after preconcentration using Pd(II)-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Microchim Acta 157:7
Yousefi R, Shemirani F, Jamali MR, Salavati-Niasari M (2010) Extraction and preconcentration of ultra trace amounts of beryllium from aqueous samples by nanometer mesoporous silica functionalized by 2,4-dihydroxybenzaldehyde prior to ICP OES determination. Microchim Acta 169:241
Guliants VV, Carreon MA, Lin YS (2004) Ordered mesoporous and macroporous inorganic films and membranes. J Membr Sci 235:53
Hartmann M, Bischof Ch (1999) Mechanical Stability of Mesoporous Molecular Sieve MCM-48 Studied by Adsorption of Benzene, n-Heptane, and Cyclohexane. J Phys Chem B 103:6230
Zhao D, Huo Q, Feng J, Chmelka B, Stucky G (1998) Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J Am Chem Soc 120:6024
Johnson JS, Stein A (2001) Surface Modification of Mesoporous, Macroporous, and Amorphous Silica with Catalytically Active Polyoxometalate Clusters. Inorg Chem 40:801
Pirouzmand M, Amini MM, Safari N (2008) Immobilization of iron tetrasulfophthalocyanine on functionalized MCM-48 and MCM-41 mesoporous silicas: catalysts for oxidation of styrene. J Colloid Interface Sci 319:199
Daniel S, Rao PP, Rao TP (2005) Investigation of different polymerization methods on the analytical performance of palladium(II) ion imprinted polymer materials. Anal Chim Acta 536:197
Ebrahimzadeh H, Tavassoli M, Amini MM, Fazaeli Y, Abedi H (2010) Determination of very low levels of gold and palladium in wastewater and soil samples by atomic absorption after preconcentration on modified MCM-48 and MCM-41 silica. Talanta 81:1183
Ghaedi M, Shokrollahi A, Niknam Kh, Niknam E, Najibi A, Soylak M (2009) Cloud point extraction and flame atomic absorption spectrometric determination of cadmium(II), lead(II), palladium(II) and silver(I) in environmental samples. J Hazard Mater 168:1022
Tavakoli L, Yamini Y, Ebrahimzadeh H, Nezhadali A, Nourmohammadian F (2008) Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater 152:737
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 84 kb)
Rights and permissions
About this article
Cite this article
Mehrani, K., Mehrani, A., Amini, M.M. et al. Dipyridylamine-modified nanoporous silicas as new sorbents for the separation and pre-concentration of palladium. Microchim Acta 173, 521–527 (2011). https://doi.org/10.1007/s00604-011-0590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0590-7