Abstract
The article gives the summary of our R&D contributions of in situ current normalized particle induced gamma-ray emission (PIGE) method utilizing proton beams from accelerators to quantify low Z elements from Li to Ti in various samples. Measurement of prompt gamma-rays from proton induced reactions was performed by HPGe detector system coupled to MCA. PIGE methods were utilized for non-destructive quantification of low Z elements in glass, ceramics like lithium titanate, Li-ion batteries and archaeological artifacts, environmental and food samples, and simultaneous quantification of total B and its isotopic composition in boron-based neutron absorbers, alloys and refractory materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical characterization of fabricated or developed materials is the most important step under chemical quality control (CQC) to ensure the material suitability as per the specified composition. CQC involves quantification of major, minor and trace elemental concentrations by a suitable analytical method with adequate accuracy and precision. The materials of interest are solid samples having matrices like metals and alloys, glass and ceramics, various oxides, carbides and refractory materials as well as other matrix samples of geological, biological and environmental origin. The routinely used analytical methods are mostly wet-chemical and spectroscopic techniques like atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectroscopy (ICP-OES) and ICP mass spectrometry (ICP-MS) and chromatographic techniques. Though these techniques serve the purpose even at low concentration levels, they are destructive in nature involving cumbersome chemical dissolution using stronger acids and separation and/or pre-concentration and, also, they are not free from reagent blank. The method of choice for analysis of solid samples mainly are X-ray fluorescence (XRF) [1], nuclear analytical techniques (NATs) namely neutron activation analysis (NAA), prompt gamma-ray NAA (PGNAA) [2,3,4,5], photon activation analysis (PAA) [6] and charge particle activation analysis (CPAA), laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) [7], laser induced breakdown spectrometry (LIBS) and ion beam analysis (IBA) techniques like particle induced X-ray/gamma-ray emission (PIXE/PIGE) [8,9,10,11,12,13,14,15]. NATs like PGNAA, INAA, CPAA, PAA and PIGE are isotope specific techniques and have several advantages like simultaneous multielement determination capability, non-destructive in nature (as direct solid samples are used), negligible matrix effect, high sensitivity and selectivity for many elements, and inherent precision and accuracy in the analytical results.
In samples like ceramics, glass, soil, sediment and geological origin, low Z elements like Si, Al, Na, K, Mg, and Ca are the major matrix elements whereas in samples like lithium based ceramics and boron carbides, again low Z elements are the main constituents. Low Z elements are also minor and trace constituents in various samples. Thus, quantification of low Z elements in these solid samples without sample destruction is a challenge to the analyst. INAA, XRF and PIXE techniques are mainly used for determination of medium and high Z elements in solid samples, and thus not suitable for low Z elements. INAA is a very good non-destructive technique used for determination of the elements starting from Na. Non-destructive approaches of PIXE and XRF techniques are not suitable for low Z elements due to lower X-ray yield and higher Compton background at low energy region. Practically, XRF and PIXE techniques are used for the elements starting from K, though in some cases reports are there from Si onwards. Other two activation techniques namely PAA (using Bremsstrahlung radiation from electron beam accelerators) and CPAA (using energetic charge particles like p and α from particle accelerators) can determine low Z elements, however many of the products of low Z elements are neutron deficient decaying by 511 keV annihilation gamma-ray (non-characteristic), which makes it difficult to determine simultaneously as well as non-destructively. PGNAA is a suitable technique for a few low Z elements including H, neutron poisons like B, Cd and Gd and rare earth elements (REEs). On the other hand, PIGE is the most suitable nuclear analytical method for simultaneous determination of low Z elements from Li to S using low energy proton beam (2–5 MeV) [9,10,11,12,13,14] and for C, N, O as well as medium to higher Z elements using medium energy proton beam (7–9 MeV) [15]. PIGE involves measurement of prompt gamma-rays when energetic charged particles (p, d and α) are bombarded on the target. The proton induced reactions involve measurement of prompt gamma rays from inelastic-scattering (p,p′γ) or from nuclear reactions like (p,αγ), (p,nγ) and (p,γ). For quantitative information on elemental concentrations using peak areas under characteristic gamma-ray spectra, it is important to have the knowledge of parameters like gamma-ray yields or cross-sections, stopping power of material and beam current. Table 1 gives some relevant reactions of low Z elements and corresponding thick target gamma-ray yields at two selected proton energies (4 and 7 MeV) [14, 15]. The thick target gamma-ray yields are facility independent and efficiency normalized values and, thus, they give idea about relative sensitivities of low Z elements for same and different proton energies and also for different gamma-rays of same elements like in the cases of F, Li, B, Na, Al, Si, Mg and Ti (Table 1). The most prominent reaction is (p,p′γ) compared to (p,γ), (p,nγ) and (p,αγ) reactions. Though many elements like Li, F, B and Al have good sensitivity at 4 MeV proton beams, elements like C, N, O, Al, P, S, Cl, Mg, K and Ti have better sensitivities [except for (p,γ) reaction] in higher energy (7 MeV) proton beam due to higher effective cross sections or higher thick target gamma-ray yields. Thus PIGE, a complementary technique to PIXE, XRF and INAA, has several advantages like simultaneous determination of low and medium Z elements, often solid sample for analysis, thus non-destructive in nature, less matrix effect for thick and diluted pellet samples and no or very less spectral interference.
In addition to above PIGE literature references, various samples of geological, archaeological, ceramic, steel, dust, aerosol and biomedical origin have been analyzed by conventional PIGE since 1960. Sippel and Glover [16] for the first time showed that gamma-rays emitted by using energetic protons of the order of MeV could be used for determining low Z elements like Li, Be, C, N, O, F, Na, Mg Al and P in geological samples. PIGE using deuteron beam was used for carbon and 4 MeV proton beam was used for Si in different kind of steel samples [17]. Fluorine was quantified by PIGE in SiFx etch residues on silicon using 197 keV of 19F [18]. The PIGE technique was utilized for determination of C, N, O, Si and S in coal samples using 9.5 MeV proton beam [11]. Coote, in 1992 reviewed specifically the nuclear reactions for PIGE analysis of F and other low Z elements in different materials including biological (like teeth, bone and fish scales), archaeological and atmospheric samples [19]. PIGE was employed to determine Li, Be, B and F in the individual grains of micas using alpha particle beam of energy 1–3 MeV [12]. Clay samples were analyzed by Savidou et al. using 4 MeV proton beam for low Z elements namely Li, B, F, Na, Mg, Al, Si and P [14]. Nsouli et al. analyzed F concentration in a drug as a part of CQC exercise [20]. A number of glass samples of archaeological importance have been studied using PIGE-PIXE combination, wherein PIGE was used to determine Na, Mg, Al and Si [21]. Different samples of geological importance and environmental reference materials have been analyzed by Valkovic et al. using PIGE methods [9]. In addition to in-beam PIGE, external PIGE (beam in air) keeps promise for analysis of many solid and non-standard geometry samples of importance including archaeological samples/ceramics. Using external PIGE, Saarela et al. determined low Z elements like Na, Mg, Al, P and Mn in plant samples using 3 MeV proton beam [22]. Sunitha and Kumar et al., have determined O in materials and 10B/11B atom ratio in B4C by conventional PIGE methods [23, 24].
In the above mentioned literature surveys, mainly conventional PIGE methods using RBS or charge (µC) normalized approaches have been used to the best of our knowledge. Present article gives a summary of our work on development and application of in situ current normalized as well as conventional PIGE methods for quantification of low Z elements using proton beams (4–8 MeV) from accelerators and their applications to various samples including glass, ceramics, carbides and alloys [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Experimental
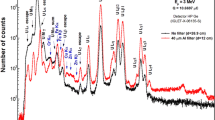
In the present work, both conventional and in situ current normalized PIGE methods were standardized using 4 and 5 MeV proton beams from tandem particle accelerators in India namely FOlded Tandem Ion Accelerator (FOTIA), BARC, Mumbai and 3 MV Tandetron of Ion Beam Laboratory (IBL), Institute of Physics (IOP), Bhubaneswar and 8 MeV proton beam from BARC-Tata Institute for Fundamental Research (TIFR), Mumbai. Samples analyzed were glass, lithium based ceramics, clay ceramics, and boron based neutron absorbers as well as geological, environmental and biological samples. Samples were in pellet forms either in cellulose or graphite matrix, keeping in mind of similar or negligible stopping power. Samples were irradiated in vacuum (~ 10−6 torr) with proton beam current in the range of 10–50 nA. In the present work, we have followed an in situ current normalization method in which either F or Li (not present in the sample) was mixed in the sample and standard, the details of the method is described below. Otherwise conventional RBS approach using thin gold foil and conducting target using graphite matrix were employed for beam current monitoring/normalization. The prompt gamma-rays of low Z elements were measured by HPGe detector based conventional high resolution gamma-ray spectrometry in the range of 110 keV (19F) to 6129 keV (16O). The typical gamma-ray spectra of samples in PIGE using proton beams (4, 5 and 8 MeV) are given in the Figs. 1, 2, 3 and 4: Fig. 1 for soda-lime glass using 4 MeV proton beam showing Si, Al, Na peaks along with 1236 keV of 19F (as in situ current normalizer), Fig. 2 for lithium titanate sample using 8 MeV proton beam showing Li, Ti and O peak, Fig. 3 for ZrB2 sample using 5 MeV proton beam showing peaks of 10B and 11B along with 197 keV of 19F (current normalizer) and Fig. 4 for rock phosphate reference material (BCR No. 32) along with Li as in situ current normalizer, showing gamma-rays of low Z elements like Si, Al, Na, P and F. Gamma-ray spectra were analyzed using peak-fit software called pulse height analysis software (PHAST) for the peak area determination. For obtaining lower counting statistical errors, higher counts (peak area) were acquired for the analyte of interest in the range of 20,000–1,00,000 as well for the in situ current normalizers (like Li, F or Al) in the range of 50,000–2,00,000.
The in situ current normalized PIGE method for concentration calculation
The count rate R (counts per second, cps) of gamma-rays of interest emitted in bombardment of a thick target by a proton beam of energy (Emax) is given by [35],
where NA is the Avogadro number, M is the atomic mass, ρ is elemental density (in g cm−3), εr is the absolute detection efficiency, θ is isotopic abundance of analyte, Io is the beam current, σ(E) is the energy dependent gamma-ray production cross-section for a particular nuclear process, (dE/dx)E is the stopping power of target (S) at the beam energy E and C is the concentration (wt% or mg kg−1) of analyte in the pellet. The count rate ratio of a gamma-ray corresponding to an element/isotope in the sample (Sam) and the standard (Std) is given below.
When exact sample composition is unknown the stopping power correction is difficult. In the case of powder samples, they are mixed with cellulose or graphite as the major matrix and care is taken to achieve same stopping powers of sample and standard pellets for the proton beam and thus the ratio becomes unity. Then the above equation becomes simple as given below.
Now it is necessary to know the value of beam current ratio [(Io)Sam/(Io)Std], which is an important aspect of accelerator based experiments. The beam current variation in thick sample is monitored or normalized by measuring the current directly from the conducting sample [27] or by RBS method using thin foils like Au and Ag in which backscattered particles are measured using a Si based surface barrier detector kept at a fixed backward angle with respect to the ion beam [25, 35]. In the present work, we have optimized an in situ current normalization approach wherein an element namely F or Li not present in the sample and having higher sensitivity in PIGE, is mixed homogenously in the sample and standard in constant amount. If it is difficult to mix with the samples like paraffin wax or any metal and alloy, the in situ current normalizer (like Al) can be kept or wrapped over the sample and standard [35]. The variation of beam current, if any, is obtained by measuring simultaneously the count rate of element of interest and the in situ current normalizer. This method does not demand sample to be conducting and also does not require a separate arrangement (like RBS set-up) for current measurement. The count rate (R in counts per second, CPS) of the gamma-ray of interest is normalized with the sensitivity (S = CPS per unit mass or concentration) of the added element to account for the current variations, if any, during the experiment. This normalization procedure makes the analysis independent of any fluctuation in beam current during irradiation as the count rate of the current normalizing standard as well as the element of interest changes proportionally with the beam current. For concentration calculation of analyte in sample, the in situ current normalized count rate of sample is simply compared with beam current normalized sensitivity of standard using the following relation,
where CSam,x, (Rx)Sam, (SIS)Sam and (Sstd,x)N are the concentration of analyte ‘x’ (mg kg−1) in the standard pellet, count rate of gamma-ray of interest of analyte in sample, sensitivity of in situ current normalizer (CN) for sample pellet and current normalized sensitivity of elemental standard (normalized CPS per mg kg−1 of standard element), respectively. Further details of calculations are found in our publications [25, 30, 35].
Results and discussion
Barium borosilicate glass (BaBSG) is a promising matrix for nuclear waste vitrification and such glass samples with varying composition of Si, B, Al and Na with F were prepared to examine the retention or loss of F during vitrification at a higher temperature. Both conventional and in situ current normalized PIGE method were utilized for determination of total F as well as other low Z elements (Si, Al, Na, B and/or Li) as a part of chemical quality control (CQC) exercise [25, 26]. The concentrations ranges of different elements determined were 0.1–3.8 wt% (F), 0.7–1.0 wt% (Li), 4.5–10.0 wt% (B), 8.0–13.0 wt% of Na, 16.0–18.0 wt% (Si) and 1.8–3.0 wt% (Al). The total propagated uncertainties in the results were less than ± 3.0%, which are due to uncertainties on peak areas of analyte of interest of sample, standard and in situ current normalizer and their corresponding masses. Application of PIGE was very important to determine F and other low Z elements without any sample destruction of the complex glass matrix.
The PIGE methods were extended to other glass samples (soda-lime, Fig. 1) and archeological clay ceramics (potteries and bricks) and (certified) reference materials from NIST and IAEA for quantification of low Z elements (Si, Al, Na and/or B) [27, 28]. Typical results of low Z elements in glass samples, clay ceramics and NIST SRM 1645 (sediment) are given in Table 2. Glass objects are widely studied by IBA techniques (PIXE/PIGE) for forensic applications [29].
The in situ current normalized PIGE method (using F as current normalizer) was extended for the determination of lithium and other low Z elements in sol–gel synthesized (1) Li doped neodymium dititanate and (2) lithium titanate (Li2TiO3) and lithium aluminate (LiAlO2), which are difficult to be analyzed using wet chemical methods. Concentration of Li was estimated in both heat-treated (at 800 °C) as well as precursor samples of Li doped neodymium dititanate for studying the ferroelectric properties. The concentrations of Li were found to be lower by 5–35% with respect to precursor samples [30]. PIGE method using 4 MeV proton beam was further used for the determination of Li and Ti in Li2TiO3 and Li and Al in LiAlO2, which are important proposed tritium breeder blanket materials in proposed D–T based fusion reactor under ITER programme [31, 32]. Li concentrations in the range of 11.0–12.7 wt% and Ti concentration in the range 42.7–44.7 wt% (by PIGE and INAA) with less than ± 3% uncertainties were determined. As O could not be determined using 4 MeV proton beam, PIGE method using 8 MeV proton beam was developed for simultaneous determination of Li, Ti and O (Fig. 2) [33]. Experiments were carried out using samples in graphite matrix and RBS method using thin Au foil for the current measurement. The concentrations of Li, Ti and O were in the range of 11.8–12.7, 43.3–43.8 and 43.7–44.3 wt% with respect to their stoichiometric concentrations of 12.67, 43.51 and 43.85 wt%, respectively. The corresponding propagated uncertainty values within ± 3%, ± 3% and ± 8%.
The in situ current normalized PIGE method applied to Lithium iron phosphate (LiFePO4) based Li-ion rechargeable batteries. While synthesizing these samples lithium has a tendency to sublime, hence a more amount of lithium is used. In order to know exact amount of Li in synthesized Li-ion batteries with respect to added Li, PIGE method using F as in situ current normalizer was applied [34]. The results of Li concentration are given in Table 3, clearly indicating loss of Li during preparation. It helped us to take slightly higher Li during preparation, so that intended stoichiometric compound can be prepared for its desired application.
Boron and its compounds, composites and alloys find extensive applications in various fields including nuclear technology due to its high thermal neutron absorption cross section. Various solid boron based materials (like boric acid, boron carbide, rare-earth and Ti and Ti–Cr based refractory borides) are extensively used in nuclear industry as neutron sensors, shielding against neutrons, control/shutoff rods and in nuclear material storage. Isotope specific nature of PIGE was advantageously utilized for simultaneous quantification of total boron as well as its isotopic composition (IC) i.e., 10B/11B atom ratio in natural and enriched boron based samples. In situ current normalization was carried out using F (by mixing in the target pellet) or thin foil of Al (using as a single wrapper) for total boron concentration determination. In addition to the knowledge of total boron, isotopic composition (10B/11B atom ratio) and 10B atom% (which gives 10B enrichments) with respect to their natural abundances of 19.8 atom% (10B) and 80.2 atom% (11B). The total boron concentrations obtained in various boron based compounds and materials were in the range of 5–78 wt% and 10B atom% was in the range of 19.8–67% [35,36,37] as shown in Table 4. The PIGE method was simple for determining the IC values as current normalization is not a requirement. Results of IC and total boron concentrations in natural stoichiometric compounds were used or evaluating the accuracy of the method. For method validation, total boron concentrations were also determined by conventional ICP-OES and titrimetry (Table 4). It has been observed that for complex matrix samples including carbide and refractory matrices, PIGE is a simple and fast method for determination of IC as well as total B as compared to TIMS and ICP-MS.
Additionally, PIGE method has been used for determination of F in environmental and food samples like soil, sediment, coal, coal fly ash, and food (rice, wheat and tea) samples. Soil and food samples from Fluoride affected and unaffected areas were collected and analyzed by in situ current normalized (taking Li as current normalizer) PIGE method using 4 MeV proton beam [38, 39]. F concentrations in the range of 40–1500 mg kg−1 in soil (still higher in some locations) and 25–200 mg kg−1 in rice and wheat samples were determined. Samples containing F concentration > 500 mg kg−1 in soil and > 50 mg kg−1 in food, were observed in fluoride affected regions. Tea samples were found to have higher concentration of F i.e., in the range of 250–600 mg kg−1. PIGE method is being applied for quantification of F and other low Z elements in various reference materials as well as environmental samples. The Fig. 4 shows a typical gamma-ray spectrum of a Rock Phosphate Reference Material (BCR No. 32) irradiated using 4 MeV proton beam at FOTIA, BARC.
Quality assurance/quality control (QA/QC) of PIGE method
The in situ current normalized PIGE methods were validated (as a part of QC) by analyzing stoichiometric chemical compounds and/or reference materials from NIST and IAEA. Since in many cases, it was difficult to obtain suitable reference materials, the methods were validated by analyzing synthetic samples in cellulose or graphite matrix. As a part of QA/QC, in addition to validation of methods, total propagated uncertainty in the measurements and detection limits all elements of interest were evaluated. The total propagated uncertainties were arrived at from the (1) counting statistics of samples, standard, in situ current normalizer, (2) uncertainties on their corresponding masses, and (3) uncertainty on the concentration of current normalizer. The energy uncertainty of the proton beam is about 0.2% for 4 MeV proton beam which was arrived from ± 2 kV uncertainties at 2 MV of terminal voltage of accelerator. The propagated uncertainties for elements of interest like F, Li, B, Si, Na, Al and Ti were in the range of ± 1–5% except for O in which propagated uncertainty was about ± 8%. The 3σ detection limits determined using 4 MeV proton beam were in the range of 5–50, 4–40 and 5–25 mg kg−1for F, Li and B, respectively, depending on the experimental conditions and energy of gamma-rays (197 keV for F, 478 keV for Li and 429 and 2125 keV for B). The detection limits estimated for Li, Ti and O were 4, 8 and 136 mg kg−1, respectively, in lithium titanate sample using 8 MeV proton beam. In summary, PIGE methods developed were simple, sensitive and non-destructive in nature and applied for quantification low Z elements (in addition to isotopic composition of B) in various materials including that are relevant to nuclear technology. The results helped in obtaining desired elemental concentration non-destructively, process/preparation method optimization as well as CQC of finished products.
Conclusions
PIGE using proton beams is a simple, sensitive and faster technique for non-destructive quantification of most of the low Z elements in various matrices including complex matrix samples like glass, ceramics, carbides, alloys and refractory materials. This method in conjunction with PIXE is capable of complete compositional characterization of materials in several cases. In addition to conventional PIGE method, efficacy of the in situ current normalized PIGE method has been successfully demonstrated utilizing 4–8 MeV proton beams for quantification of low Z elements in nuclear technology materials as well as in Li ion batteries and other samples. Most important is that PIGE method is capable of giving quantitative information on isotopic composition of B (10B/11B) and total B simultaneously in boron based materials. Quantification of Li in Lithium based ceramics as well as simultaneous determination of Li, Ti and O is important contribution for method optimization purposes. Compositional characterization of glass and ceramic samples will be of help for quality control purposes as well as forensic applications. All the results obtained by conventional and in situ current normalized PIGE methods are useful under CQC of prepared/synthesized materials, which are otherwise difficult to be analyzed by other radioanalytical and wet-chemical methods. Utilization of PIGE method using medium energy proton beam (7 or 8 MeV) will enhance the elemental capability (up to Z = 30 or higher) with improved sensitivities and detection limits. PIGE keeps promise for its application to various samples of environmental, biological, biomedical and pharmaceutical importance as well as to advanced and energy related materials including reactor materials, namely zircaloys, Zr–Nb alloys, stainless steels and other ceramics/oxides.
References
Civici N, Vata E (2013) Analysis of automotive glass of various brands using EDXRF spectrometry. Rom Rep Phys 65:1265–1274
De Soete D, Gijbels R, Hoste J (1972) Neutron activation analysis. Wiley, London
Acharya RN, Nair AGC, Reddy AVR, Manohar SB (2002) Validation of a neutron activation analysis method using k0-standardization. Appl Radiat Isot 57:391–398
Segebade C, Berger A (2008) Photon activation analysis. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, London
Molnar GL (ed) (2004) Handbook of prompt gamma activation analysis with neutron beams. Kluwer Academic Publishers, Dordrecht
Acharya R (2009) Prompt gamma-ray neutron activation analysis methodology for determination of boron from trace to major contents. J Radioanal Nucl Chem 281:291–294
Smith K, Trejos T, Watling RJ, Almirall J (2006) A guide for the quantitative elemental analysis of glass using laser ablation inductively coupled plasma mass spectrometry. At Spectrosc 27:69–75
Willy M (1988) Applications of ion beam analysis in biology and medicine, a review. Nucl Instrum Methods Phys Res B 35:388–403
Valković O, Jakšić M, Fazinić S, Valković V, Moschini G, Menapace E (1995) Quality control of PIXE and PIGE nuclear analytical techniques in geological and environmental applications. Nucl Instrum Methods Phys Res B 99:372–375
Smita Z, Milavecc T, Fajfarb H, Rehrend Th, Lanktone JW, Gratuze B (2013) Analysis of glass from the post-Roman settlement Tonovcov grad (Slovenia) by PIXE–PIGE and LA-ICP-MS. Nucl Instrum Methods Phys Res B 311:53–59
Macias ES, Radcliffe CD, Lewis CW, Sawicki CR (1978) Proton induced gamma-ray analysis of atmospheric aerosols for carbon, nitrogen, and sulfur composition. Anal Chem 50:1120–1124
Volfinger M, Robert JL (1994) Particle-induced-gamma-ray-emission spectrometry applied to the determination of light elements in individual grains of granite minerals. J Radioanal Nucl Chem 185:273–291
Kiss AZ, Koltay E, Nyako B, Somorjal E, Anttila A, Raisanen J (1985) Measurement of thick-target gamma-ray production yields of the 7Li(p,p′)7Li and 7Li(p,γ)8Be reactions in the near-threshold energy region for the 7Li(p,n)7Be reaction. J Radioanal Nucl Chem 89:123–141
Savidou A, Aslanoglou X, Paradellis T, Pilakouta M (1999) Proton induced thick target γ-ray yields of light nuclei at the energy region E p = 1.0–4.1 MeV. Nucl Instrum Methods Phys Res B 152:12–18
Raisanen J, Witting T, Keinonen J (1987) Absolute thick-target γ-ray yields for elemental analysis by 7 and 9 MeV protons. Nucl Instrum Methods Phys Res B 28:199–204
Sippel RF, Glover ED (1960) Sedimentary rock analysis by charged particle bombardment. Nucl Instrum Methods 9:37–48
Pierce TB, Peck PF, Henry WM (1965) The rapid determination of carbon in steels by measurement of the prompt radiation emitted during deuteron bombardment. Analyst 90:339–345
Van Ijzendoorn LJ, Haverlag M, Verbeek A, Politiek J, de Voigt MJA (1996) Quantification of fluorine in SiFx etch residues on silicon with proton induced γ-emission. Nucl Instrum Methods B113:411–414
Coote GE (1992) Ion beam analysis of fluorine: its principles and applications. Nucl Instrum Methods B66:191–204
Bejjani Nsouli A, Negra SD, Gardon A, Thomas JP (2010) Ion beam analysis and PD-MS as new analytical tools for quality control of pharmaceuticals: comparative study from fluphenazine in solid dosage forms. Anal Chem 82:7309–7318
Mosbah M, Metrich N, Massiot P (1991) PIGME fluorine determination using a nuclear microprobe with application to glass inclusions. Nucl Instrum Methods B58:227–231
Saarela KE, Harju L, Lill JO, Rajander J, Lindroos A, Heselius SJ (2000) Thick-target PIGE analysis of plant materials preconcentrated by dry ashing. Talanta 51:717–725
Sunitha Y, Kumar Sanjiv (2017) 18O(p,p′γ)18O nuclear reaction in the determination of oxygen by proton induced γ-ray emission. J Radioanal Nucl Chem 314:1803–1812
Sunitha Y, Kumar Sanjiv (2017) 10B/11B isotopic ratio and atomic composition of boron carbide: determination by proton induced γ-ray emission and proton elastic backscattering spectrometry. Appl Radiat Isot 128:28–35
Chhillar S, Acharya R, Sodaye S, Sudarshan K, Santra S, Mishra RK, Kaushik CP, Choudhury RK, Pujari PK (2012) Application of particle induced gamma-ray emission for non-destructive determination of fluorine in barium borosilicate glass samples. J Radioanal Nucl Chem 294:115–119
Chhillar S, Acharya R, Mishra RK, Kaushik CP, Pujari PK (2017) Simultaneous determination of low Z elements in barium borosilicate glass samples by in situ current normalized particle induced gamma-ray emission methods. J Radioanal Nucl Chem 312:567–576
Dasari KB, Chhillar S, Acharya R, Ray DK, Behera A, Das NL, Pujari PK (2014) Simultaneous determination of Si, Al and Na concentrations by particle induced gamma-ray emission and applications to reference materials and ceramic archaeological artifacts. Nucl Instrum Methods Phys Res B 39:37–41
Tamilarasu S, Velraj G, Ray DK, Acharya R (2016) Chemical analysis of archaeological clay potteries by PIGE and PIXE methods using proton beams from tandem accelerator for provenance study. J Radioanal Nucl Chem 310:363–370
Acharya R, Pujari PK (2018) Potential of conventional and internal monostandard NAA and PGNAA and PIGE in forensic sciences: an overview. Forensic Chem. https://doi.org/10.1016/j.forc.2018.01.002
Chhillar S, Acharya R, Pai RV, Sodaye S, Mukerjee SK, Pujari PK (2012) A simple and sensitive particle induced gamma-ray emission method for non-destructive quantification of lithium in lithium doped Nd2Ti2O7 ceramic sample. J Radioanal Nucl Chem 293:437–441
Chhillar S, Acharya R, Rao TVV, Bamankar YR, Mukerjee SK, Pujari PK, Aggarwal SK (2013) Non-destructive compositional analysis of sol–gel synthesized lithium titanate (Li2TiO3) by particle induced gamma-ray emission and instrumental neutron activation analysis. J Radioanal Nucl Chem 298:1597–1603
Modi KB, Acharya R, Munot S, Parida SC, Pujari PK (2017) Chemical characterization of lithium titanate and lithium aluminate as tritium breeders of fusion reactor by PIGE and INAA methods. J Radioanal Nucl Chem 314:1113–1120
Chhillar S, Acharya R, Triptahi R, Sodaye S, Sudarshan K, Rout PC, Mukerjee SK, Pujari PK (2015) Compositional characterization of lithium titanate ceramic samples by determining Li, Ti and O concentrations simultaneously using PIGE at 8 MeV proton beam. J Radioanal Nucl Chem 305:463–467
Halankar Kruti K, Mandal BP, Jangid MK, Mukhopadhyay A, Meena SS, Acharya R, Tyagi AK (2018) Optimization of lithium content in LiFePO4 for superior electrochemical performance: the role of impurities. RSC Adv 8:1140–1147
Chhillar S, Acharya R, Sodaye S, Pujari PK (2014) Development of particle induced gamma-Ray emission methods for nondestructive determination of isotopic composition of boron and its total concentration in natural and enriched samples. Anal Chem 8:11167–11173
Venkatesh K, Chhillar S, Kamble GS, PandeY SP, Venkatesh M, Kumar SA, Kumar S, Acharya R, Pujari PK, Reddy AVR (2014) Determination of boron concentration in borosilicate glass, boron carbide and graphite samples by conventional wet-chemical and nuclear analytical methods. J Radioanal Nucl Chem 302:1425–1428
Acharya R, Raja SW, Chhillar S, Gupta J, Sonber JK, Murthy TSRC, Bhushan KS, Rao RM, Majumdar S, Pujari PK (2018) Non-destructive quantification of total boron and its isotopic composition in boron based refractory materials by PIGE and inter-comparison study using TIMS and titrimetry. J Anal At Spectrom 33:784–791
Srivastava A, Chhillar S, Kaur N, Singh D, Acharya R, Pujari PK (2014) Determination of fluoride contents in soil samples from Punjab state using particle induced gamma-ray emission. J Radioanal Nucl Chem 302:1461–1464
Dhorge PS, Acharya R, Rajurkar NS, Chahar V, Tuli V, Srivastava A, Pujari PK (2017) Quantification of trace fluorine concentrations in soil and food samples from fluoride affected region by in situ current normalized particle induced gamma-ray emission method. J Radioanal Nucl Chem 311:1803–1809
Acknowledgements
Authors are thankful to all co-workers and operation crew members at FOTIA, BARC, Mumbai, IBL, IOP, Bhubaneswar and BARC-TIFR, Mumbai. Authors thank Head, IADD and Mr. A. Agarwal, OIC, FOTIA, IADD, BARC for their help and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acharya, R., Pujari, P.K. Development and applications of in situ current normalized PIGE method using proton beams for quantification of low Z elements. J Radioanal Nucl Chem 318, 1727–1735 (2018). https://doi.org/10.1007/s10967-018-6319-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6319-x