Abstract
A particle induced γ-ray emission methodology employing the 18O(p,p′γ)18O (E γ = 1982 keV) nuclear reaction is described for the non-destructive determination of bulk oxygen in materials. The development of the methodology follows a comprehensive measurement of the thick target yields of the 1982 keV prompt γ-rays in the 3.0–4.2 MeV proton energy region and a systematic assessment of such analytical features as the limit of detection, probing depth, precision and accuracy. The methodology is validated by analyzing binary, ternary and multinary oxides. It is simple and rapid, and in combination with prompt γ-ray producing reactions involving the other constituents, enables the complete compositional analysis of oxygen bearing materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The determination of oxygen has long been a subject of interest due to the profound influence of the element on the properties of materials [1, 2]. The materials can be thin films or bulk compounds wherein oxygen prevails either as a major constituent or as an impurity element. Amongst the several different methods of oxygen determination, ion beam analysis (IBA) holds an important position by virtue of its non-destructive nature and versatility. An account of the capabilities of IBA for oxygen determination is exquisitely presented in a review article published by Cohen and Rose in early 90s [3]. The IBA methods, by taking cognizance of their probing depths, can be subsumed into two broad categories: those suitable for the determination of surface oxygen and those applicable for ‘bulk’ oxygen determination. Techniques such as nuclear reaction analysis (NRA), Rutherford backscattering spectrometry (RBS) and 3.05 MeV 16O(α,α)16O resonant scattering have a probing depth of a few microns and, therefore, have been extensively used for the determination of oxygen in films and in the surface regions of bulk materials [4,5,6]. In fact, 16O(α,α)16O resonant scattering with a detection sensitivity of ~ 1 at% and a depth resolution of ~ 30 nm, is the most popular method of depth profiling oxygen in materials [7]. Methods based on particle induced γ-ray emission (PIGE), on the other hand, have probing depths up to several tens of μm and therefore provide the determination of ‘bulk oxygen’ in materials. But the instances of the applications of these methods are far and few. In probably one of the most exemplary applications, Vickridge et al. used PIGE for the precise determination of oxygen in high temperature superconductors. In fact, the measurement was accomplished by the 16O(d,pγ)17O nuclear reaction (E γ = 871 keV) and therefore, the authors referred to the method as the deuteron induced gamma emission (DIGME) technique [8, 9]. The other PIGE methods of oxygen determination utilize the 16O(p,p′γ)16O (E γ = 6.1, 6.9 and 7.1 MeV) and 18O(p,αγ)15N (E γ = 5.2 MeV) nuclear reactions [10,11,12].
The objective of the present study is to standardize a simple and an easily adaptable PIGE methodology for the routine determination of bulk oxygen in materials which, as stated earlier, has not received much attention despite its immense importance. The 16O(d,pγ)17O nuclear reaction, though possessing excellent analytical attributes, has limited applicability in view of the fact that deuterons are prolific neutron producers and laboratories operating deuteron beams must have adequate shielding against neutrons. The 16O(p,p′γ)16O nuclear reaction, on the other hand, has several limitations. For example, (a) it occurs only above 6.8 MeV proton energy which precludes the use of low energy accelerators and (b) it suffers nuclear interference from 19F(p,αγ)16O, one of the most sensitive nuclear reactions for fluorine [10]. The likelihood of a significant neutron production at 6.8 MeV or higher proton energy from nuclear reactions involving the other constituents of the target is yet another drawback of the method. Therefore, the 16O(p,p′γ)16O nuclear reaction is not a favourable choice for routine applications.
The 18O(p,p′γ)18O nuclear reaction that emits 1982 keV γ-rays, offers an alternate method of bulk oxygen determination using low energy accelerators. Although the thick target yields of the γ-rays have been measured on a few occasions, the analytical capability of the reaction has not been comprehensively probed [10, 13]. Presently, continuing our endeavor to devise simple yet effective methodologies for oxygen determination, we have carried out a systematic investigation on the analytical potential of the 18O(p,p′γ)18O nuclear reaction which involved (a) the measurement of thick target yields of 1982 keV γ-rays in the 3.0–4.2 MeV proton energy range, (b) an assessment of analytical features such as limit of detection and probing depth and (c) the identification of sources of interferences. The applicability of the method was evaluated by analyzing several binary, ternary and multinary oxides. The compounds examined included lithium titanate and lithium iron phosphate which are important energy materials. It is shown that with the feasibility of oxygen determination, PIGE, by virtue of its simultaneous multi-element detection capability can enable the determination of the overall atomic composition of these compounds in a single measurement.
Experimental details
PIGE measurements
Target preparation
Calcium carbonate (CaCO3) and several oxides which included silicon dioxide (SiO2), titanium dioxide (TiO2), zinc oxide (ZnO) and gadolinium oxide (Gd2O3) of known stoichiometry were used as targets. The former four chemicals were of Merck origin whereas the last one was procured from Acros Organics. CaCO3 was used for the measurement of thick target γ-ray yields. CaCO3 also served as a standard for quantifying oxygen while the oxides were used as samples for the validation of the methodology. Subsequently, the method was applied for the analysis of other materials such as zirconium oxide, uranium oxide, tungsten (W) powder and tin sulphide of unknown oxygen content. Incidentally, zirconium oxide powders were synthesized from zircon while uranium oxide was in the form of disc, obtained on sintering a green disc of uranium oxide at 1700 °C in air. So far as W powder is concerned, it was obtained from a fusion research facility while the SnS powder was prepared wet-chemically and was stored in the laboratory ambient for a period of about 6 months before analysis.
In order to ascertain the applicability of the method to the matrices of diverse elemental composition, lithium titanate (Li2Ti2O4 and Li2TiO3), lithium cobalt oxide (LiCoO2) and lithium iron phosphate (LiFePO4) were also analysed. Li2TiO3 and LiCoO2 were of Sigma-Aldrich origin while the other two were from other laboratories: Li2Ti2O4 was synthesized by solid-state reaction route while LiFePO4 was prepared by a surfactant based sol–gel approach. Since the complete compositional analysis of the materials is one of the objectives of the study, lithium carbonate (Li2CO3) (Qualigens), cobalt carbonate (CoCO3), potassium dihydrogen phosphate (KH2PO4) (s d fine chemicals) were used as the standards of Li, Co and P respectively while single crystals of Si (Semiconductor Wafer Inc.) and high purity (> 99.5%) foils (thickness ≤ 0.5 mm) of Ti, Fe and Zr (Alfa Aesar) were used as the standards of the respective elements. The single crystal of Si was etched in HF medium while the foils were abraded under argon atmosphere before loading them in the scattering chamber in order to remove the layers of native oxide.
The compounds were in powder form. The purity of the materials used as standard or for validation was better than 99.0%. The compounds, prior to measurements, were mixed with 20 wt% high purity graphite powder and pressed into 10–20 mm circular discs (thickness ~ 1 mm) to serve as targets. The homogeneity of the mixtures was ascertained by measuring the yields of 1982 keV γ-rays at a particular proton beam energy at different locations on the targets.
Irradiation and γ-ray detection
The PIGE experiments were conducted with a 3.0–4.2 MeV proton beam (Φ ~ 3 mm) obtained from the 3 MV Tandetron (High Voltage Engineering Europa, The Netherlands) at NCCCM, Hyderabad. The specimens of the standard and the samples were fixed on an electrically insulated manipulator mounted on a scattering chamber. The manipulator was surrounded by a negatively biased secondary electron suppressor for current measurement and charge integration. The chamber was maintained at ~ 5 × 10−6 torr pressure during the experiments which involved the irradiation of the targets at normal incidence with protons of the requisite energy and the detection of the prompt γ-rays by a high purity germanium detector (HPGe) (Bruker Baltic, efficiency: 36%, energy resolution: 1.78 keV at the γ-ray energy of 1332 keV of 60Co) placed in the direction of the beam in air. The detector subtended a solid angle of 0.46 sr and was surrounded by a 2.0 cm thick cylindrical lead shield.
Depending on the nature of the matrix, the proton beam current during irradiation ranged from 3 to 50 nA; the lighter matrices were irradiated with lower beam currents while the heavier matrices, with higher beam currents. The duration of the measurements varied accordingly; it ranged from ~ 45 (heavier matrices) to ~ 90 min (light matrices). The data was acquired on a PC based 8 K multichannel analyzer.
Quantification
The content of oxygen or the atomic composition of a material was determined by the relative method. A binary oxide can be represented by a general formula A x O y where A is an element and x + y = 1. The atomic ratio i.e. x/y in the binary oxides was determined using the following formula that was derived from the standard thick-target yield equation [14]:
where
In Eq. (1) C A(std) and C O(std) represent the concentration of element A and oxygen in their respective standards, Y i is the charge-normalised yield of the characteristic γ-rays of the element i (A or oxygen) in the sample (samp) or standard (std); ε A(std) and ε O(std) are the stopping cross-sections of the proton in the standards of element A and oxygen respectively. It is worthwhile mentioning that Eq. (1) it is valid for cases wherein the standard of the element A and that of oxygen are two different chemical entities but it can be suitably modified if the same compound serves as the standard for both elements.
It is implicit that the Eq. (1) is applicable for compounds whose constituent A is also sensitive to PIGE (Table 1). However, if this is not the case or there is a lack of a suitable standard of A, it is more appropriate to represent the compound by the formula A 1−x O x and the concentration of oxygen in terms of atomic fraction (i.e. x) can be calculated using the formula
where ε A and ε O are the stopping cross-sections of element A and oxygen respectively while M and N represent the weight fractions of compound (A 1−xOx) and the graphite in the target respectively. Also, W A and W O are the atomic weights of element A and oxygen respectively.
The ternary (e.g. LiCoO2) or quaternary (LiFePO4) oxides can be represented by chemical formula AxByOz or AxByCzOp where the sum of the atomic fractions of all the elements in a compound is unity. The atomic composition of these compounds can be calculated using formula similar to Eq. (1) which is not mentioned here for the sake of brevity. Importantly, it is presumed that all the constituent elements of the compounds are sensitive to PIGE. The reactions involving these and several other elements relevant to the present study and their characteristic prompt γ-rays are listed in Table 1 for referencing [13]. The stopping cross-sections of the elements and compounds obtained from SRIM-2013 were used in calculations [15].
Proton elastic backscattering spectrometry (p-EBS)
In addition to PIGE, the specimens of lithium titanate and lithium iron phosphate were also analysed by p-EBS. The experiments were performed with 1.5 or 2.0 MeV protons in another scattering chamber maintained at ~ 5 × 10−6 torr pressure. The samples were in the form of 10 mm diameter discs prepared by pressing the compounds without any additive i.e. graphite. The beam diameter was about 1.5 mm while the beam current was about 5 nA. The beam was incident normally on the samples and the backscattered protons were collected by a Si surface barrier detector positioned at a backward angle of 165 (± 0.3)°. The atomic composition of the compounds was determined by simulating the experimental data using SIMNRA [16]. Since the scattering of 1.5–2.0 MeV protons from lithium, carbon or oxygen is non-Rutherford, the experimentally determined differential cross-sections of 6Li(p,p)6Li, 7Li(p,p)7Li, C(p,p)C and O(p,p)O scatterings were used in the simulations [17,18,19,20]. In fact, the cross-sections for the 6Li(p,p)6Li scattering reported in Ref. [17] are for 164° angle but were used for simulations due to the absence of the data for the scattering at 165° angle under the premise that the cross-sections at the two angles are not significantly different. Furthermore, any discrepancy in the overall atomic composition arising from the application of the data at 164° angle is expected to be marginal since the compounds contain Li in natural isotopic abundance. Incidentally, the Rutherford scattering cross-sections for P, Ti and Fe at the relevant energies were used in the simulations.
Results and discussion
Analytical features
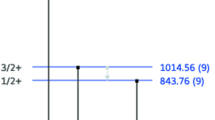
Figure 1 shows a typical prompt γ-ray spectrum recorded with the HPGe detector on irradiating the CaCO3 disc with 3.0 MeV proton beam. The 1982 keV γ-rays emitted from the 18O(p,p′γ)18O nuclear reaction are prominently present in the spectrum. The spectrum also contains 495 and 871 keV γ-rays, attributable to the 16O(p,γ)17F (Q = 0.596 MeV) and 17O(p,p′γ)17O nuclear reactions respectively. These reactions occur simultaneously with the 18O(p,p′γ)18O reaction during irradiation. It is important to note that the relative natural abundance of the 16O, 17O and 18O isotopes is 99.758, 0.037 and 0.204% respectively. A perusal of energy level schemes reported in references [21, 22] shows that the 495 keV γ-ray results from the transition from the first excited state with J = 1/2+ to the ground state with J = 5/2+ of the 17F nucleus; the 871 keV γ-ray, from the first excited state with J = 1/2+ to the ground state with J = 5/2+ of the 17O nucleus while the 1982 keV γ-ray emanates following the transition from the first excited state with J = 2+ to the ground state with J = 0+ of the 18O nucleus.
In order to ascertain the analytical proficiency of these reactions, the thick-target yields of the 495, 871 and 1982 keV γ-rays were measured at 0° as well as 90° angles (with respect to the direction of the beam) in the 3.0–4.2 MeV proton energy range at 200 keV energy intervals. The counts under a γ-ray peak obtained after the subtraction of a linear background were normalized to the total charge and the solid angle of the measurement which served as the yield of the corresponding γ-ray. The yields of the γ-rays measured as a function of beam energy at 0° and 90° angles are shown in Fig. 2 for illustration. The important inferences drawn from the yield measurements are: (a) the yield of the γ-rays, more pronouncedly that of the 1982 keV γ-rays, increases with proton beam energy, (b) amongst the three, the 1982 keV γ-ray has the highest yield above E p = 3.6 MeV and (c) the 495 as well as 871 keV γ-rays have, irrespective of incident beam energy, nearly identical yields at 0° and 90° while the 1982 γ-rays have a comparatively higher yields at the 0° detection angle. The near constancy of the yields of the 495 or 871 keV γ-rays at 0° and 90° angles is consistent with the angular isotropic distribution of γ-rays emitted as a result of the transition from a J = 1/2+ state.
Figure 2 also shows the limits of detection (LOD) of the nuclear reactions in the calcium carbonate (25 wt%) and graphite (75 wt%) mixture at different bombarding energies and detection angles. The LODs are calculated on the basis of three times the standard deviation of the background under the relevant γ-ray peaks. These follow the trend witnessed for thick target yields and accordingly, the best LOD of ~ 1.7 at% is obtained for the 18O(p,p′γ)18O nuclear reaction for measurements at 0° angle with the 4.2 MeV proton beam. The LODs of 16O(p,γ)17F and 17O(p,p′γ)17O nuclear reactions, on the other hand, measure about 18 and 14 at% respectively under similar experimental conditions. Therefore 18O(p,p′γ)18O is the most suitable reaction for oxygen determination. The analysis of different materials showed that the LOD of the reaction varies considerably with the nature of the matrix. In materials composed of high Z elements that are not prolific prompt γ-ray emitters (e.g. lanthanides, tungsten, uranium etc.), the 18O(p,p′γ)18O nuclear reaction has a LOD of about 2 at%. Conversely, in materials consisting of low Z elements which are sensitive to PIGE (e.g. B, Li, Na, Al etc.) the LOD of the reaction is 15–20 at% due to a higher background around 1982 keV in the γ-ray spectra. Notably, for such elements as Al, P and S, the peak of 1982 keV γ-rays is situated on the Compton edge of the 2211, 2230 or 2230 keV γ-rays emitted from the 27Al(p,p′γ)27Al, 31P(p,γ)32S and 32S(p,p′γ)32S nuclear reactions respectively. This is well illustrated by the γ-ray spectrum of SnS powder containing oxygen as an impurity in Fig. 3.
Apart from nuclear or spectral interferences, the prevalence of moisture in the compounds can also be a cause of concern, as it may result in an overestimation of their oxygen contents. Presently, though an independent measurement on the content of moisture in the samples was not performed, it, if existing, is presumably removed during the creation of the vacuum. This is manifested in the stability of the targets under proton beam irradiation which is indicated by the constancy of the charge normalized yields of the 1982 keV γ-rays for repeated measurements. So far as the probing depth is concerned, for the 18O(p,p′γ)18O reaction it is ~ 60 µm in SiO2, ~ 40 µm in TiO2 and ~ 30 µm in UO2 at E p = 4.2 MeV considering that the reaction commences at E p = 3.0 MeV. In view of the rather large probing depth, the method can be conveniently employed for the determination of bulk oxygen in materials.
Validation and analytical results
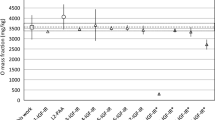
The analytical efficacy of the 18O(p,p′γ)18O nuclear reaction was ascertained by analysing several binary oxides with well-defined stoichiometry. Table 2 lists the measured contents of oxygen in some of these oxides along with their theoretical oxygen stoichiometry. The prompt γ-ray spectra of representative oxides of low, mid and high Z elements namely SiO2, TiO2 and Gd2O3 respectively used for the determination are shown in Fig. 4 for illustration. The spectrum of SiO2 consists of a strong γ-ray peak at 1779 keV and that of TiO2, at 981 keV attributable to the 28Si(p,p′γ)28Si and 48Ti(p,p′γ)48Ti nuclear reactions respectively. However, gadolinium emits low energy γ-rays and therefore these are not shown in the spectrum. The quantitative analyses of SiO2 and TiO2 specimens were carried out using Eq. (1) and, for the sake of comparison, also using Eq. (3) while the content of oxygen in Gd2O3 was determined using only Eq. (3) due to the unavailability of a proper standard of gadolinium. It can be observed from Table 2 that the measured contents of oxygen in the first three and also in the other materials are in good (3–7%) agreement with the respective theoretical concentrations. Insofar as the Eqs. (1) and (3) are concerned, the former is expected to yield more accurate results since it utilises standards for both the elements. However, as evidenced by the data in Table 2, both the equations provide nearly identical results. This inference underscores the adequacy of the Eq. (3) for the determination of oxygen in matrices composed of heavy metals, many of which do not produce prompt γ-rays (e.g. Sn, La) in the proton energy range used in the present study. In fact, the absence of strong prompt γ-ray producing reactions facilitates measurements with better precision (~ 1%) in heavier matrices as compared to the precision of ~ 3% observed for the lighter matrices.
The method was utilised to determine oxygen in a sintered disc of uranium oxide (UOx) and tungsten powders. Figure 5 shows the prompt γ-ray spectra of the two kinds of samples acquired with 4.2 MeV protons wherein the peaks due to the 1982 keV γ-rays are conspicuously present. Uranium does not emit any prompt γ-ray and the 1001 keV γ-ray in the spectrum of uranium oxide has its origin in 234mPa, a progeny of 238U. Tungsten, on the other hand, emits 101, 111 and 122 keV γ-rays by way of the 182W(p,p′γ)182W, 184W(p,p′γ)184W and 186W(p,p′γ)186W nuclear reactions respectively. The 1014 or 1779 keV γ-rays in the spectra are due to minor (~ 1 wt%) impurities of Al and or Si prevailing in the samples. The concentration of oxygen in the two specimens calculated using Eq. (3) is presented in Table 2. It is important to mention that the precision of the oxygen content in the UOx specimen, estimated on the basis of five measurements with each lasting for about 45 min at a beam current of about 50 nA, is about 1%. The method, therefore, is well suited for the determination of oxygen and, indirectly O/U ratio, in uranium oxide samples with non-destructive analysis, rapidity and insensitivity to uranium oxidation states being the major advantages. So far as the other specimen is concerned, it serves as yet another example to demonstrate the applicability of the method to high Z matrices. Moreover, W powders are extensively used in fusion research and the method can be applied to study oxygen pick up and or its retention during processing.
The method is equally applicable to ternary oxides, namely lithium titanate and lithium cobaltate as well and provides their analysis with reasonably good accuracy and precision. A typical PIGE spectrum of lithium titanate is shown in Fig. 6(a) while the measured atomic compositions of the titanate and cobalate ceramics are presented in Table 3. PIGE exhibits high sensitivity (~ 10 ppm) to lithium. As a result, the spectrum consists of a very strong peak of the 478 keV γ-rays emitted from the 7Li(p,p′γ)7Li reaction. The higher sensitivity also requires the acquisition of data at a proton beam current of 2-3 nA to keep the dead time within acceptable (< 5%) levels. Furthermore, the peak due to the 1982 keV γ-rays of oxygen is situated on an elevated background caused by the 7Li(p, γ)8Be nuclear reaction that emits 17.6 MeV γ-rays [23]. As a result, the data are collected for a comparatively longer period of time (~ 90 min) in order to ensure measurements with good statistics.
Lithium titanate and lithium cobaltate are important energy materials but are analytically intractable. It was, therefore, considered worthwhile to the analysis of the titanate ceramics by p-EBS in order to cross-validate the PIGE results. It is important to mention that p-EBS, by virtue of higher cross-sections of the 16O(p,p)16O scattering, has previously been utilised for analysing oxygen bearing materials including high temperature superconductors, for instance yttrium barium copper oxide (YBa2Cu3Ox) [24, 25]. The spectrum (of Li2Ti2O4) shown in Fig. 6(b) represents a typical proton-backscattered spectrum of titanate ceramics wherein the signal of O and also that of Ti/Li are distinctly observed. Notably, as is evident by the superimposed curve, it was simulated satisfactorily for quantification. The data presented in Table 3 show that the backscattering spectrometry technique provides good results too but these are not entirely consistent with the theoretical compositions or those measured by PIGE. Rutherford backscattering spectrometry (RBS) is an absolute method but the accuracy of the method, as described recently by Colaux et al. depends on a number of parameters [26]. p-EBS is analogous to RBS except that the scattering cross section is non-Rutherford in nature. As a result, the accuracy of p-EBS depends, in addition to the factors applicable to RBS, on the accuracy of the experimentally or theoretically determined scattering cross-sections as well. Therefore the accuracy of the differential cross-sections of 6Li(p,p)6Li, 7Li(p,p)7Li, and O(p,p)O scattering and the assumption that scattering cross-sections for Ti and Co are entirely Rutherford may be among the factors behind the discrepancy in the compositions determined by p-EBS. The present method, however, does not suffer from such limitation and, therefore, can be considered to be superior to p-EBS. It is, in fact, superior even to the PIGE method described in Ref. [11] (which is performed with 8 MeV protons and is susceptible to interference from fluorine) and thus is the preferred choice for the complete compositional analysis of titanate and cobaltate ceramics. Furthermore, it is instructive to mention that due to the sensitivity of PIGE to Cr and Mn, the methodology can be extended to the analysis of LiCrO4 and LiMn2O4 which are also important energy materials.
In order to further probe the potential of the method, it was applied to multinary compounds such as LiFePO4 and LiFePO4/C which, understandably, are analytically more complex and challenging than the titanates. LiFePO4 and its composite with carbon (i.e. LiFePO4/C) are promising cathode materials for Li-ion batteries. Figure 7 shows the PIGE spectrum of LiFePO4 acquired with 4.0 MeV protons wherein the peaks of the constituent elements are vividly present. However, two features of the spectrum need special mention. Firstly, the signal of oxygen is relatively weak since it is riding over the Compton edge of 2230 keV γ-rays emitted from the 31P(p, γ)32S reaction. Secondly, the γ-peak at 843 keV, attributable to Fe, is unusually broad and consists of a shoulder around about 837 keV. The origin of the 837 keV γ-rays lies in the 73Ge(n,γ)74Ge and 72Ge(n,n′γ)72Ge reactions taking place in the Ge crystal of the HPGe detector [27] while the neutrons involved in inducing these reactions are produced from the 7Li(p,n)7Be reaction (Q = − 1.664 MeV) occurring in the LiFePO4 target. Therefore, the true contribution of Fe was determined by fitting the envelope around 843 keV into two components. Meanwhile, the content of carbon in the LiFePO4/C composite specimen was determined by means of the 13C(p,p′γ)13C reaction that emits 3089 keV γ-rays [28]. The compositions of the compounds thus measured are listed in Table 4. The combined uncertainty of the analysis which has contributions from the peak area measurement, the stopping cross section and the charge integration of the sample as well as the standard is estimated to be about 5% for the binary and about 8% for the quaternary or multinary compounds. In fact, standardless PIGE measurements can provide results with much better uncertainty, however, it requires a precise knowledge of reaction cross-sections over a wide range of proton energy, the efficiency of the detector for γ-rays of different energies and other parameters appearing in the equation relating the yield of the γ-rays with the concentration of the elements [29].
For the sake of cross-validation, the multinary compounds were also analysed by proton backscattering spectrometry with a typical backscattered spectrum of LiFePO4 shown in Fig. 7(b). Though the signal (step) of Fe, P or O is prominent, that of Li is not as prominent as in the spectrum of Li2Ti2O4 in Fig. 6(b) due to its comparatively lower content in the compound and relatively higher background. An examination of the data presented in Table 4 shows that for LiFePO4 the PIGE and p-EBS results are in good agreement but for LiFePO4/C, a disagreement in values for Fe and, particularly for P prevails which, apart from the uncertainty associated with the differential scattering cross-sections of the different isotopes, may also arise due to the inhomogeneous distribution of the elements. It is to be noted that as compared to the probing depth of tens of microns of PIGE with 4.0 MeV protons, EBS performed with 1.5 MeV protons has a probing depth of only about 8 micron in LiFePO4. These considerations suggest that PIGE provides a more effective approach for the compositional analysis of this important class of materials.
Conclusions
The 18O(p,p′γ)18O nuclear reaction emitting 1982 keV γ-rays is employed in PIGE measurements for the determination of bulk oxygen in materials. The method is simple, rapid and non-destructive. It is endowed with a detection limit of ~ 2 at% in heavy matrices and ≥ 15 at% in light matrices, and has a probing depth of more than 30 μm at the 4.2 MeV proton energy. High precision measurement of oxygen in mid and high Z matrices is one of the discerning features of the method. It can, therefore, be applied to determine subtle changes in the oxygen stoichiometry in such materials. The method is largely free from nuclear or spectral interferences. However, due to the higher sensitivity of PIGE to low Z elements such as Li, B, Na, Al etc., the presence of these elements in high abundance can impair its detection limit and rapidity. Nevertheless, the simultaneous multielement detection capability of the technique can be exploited to determine the complete elemental composition of such compounds. It is amply demonstrated by analyzing complex oxides such as Li2TiO3 and LiFePO4. Finally, though not probed explicitly, the simultaneous occurrence of the 16O(p,γ)17F, 17O(p,p′γ)17O and 18O(p,p′γ)18O nuclear reactions emitting 495, 871 and 1982 keV γ-rays raises the possibility of isotopic analyses of oxygen in favourable materials.
References
Zheng J, Xiao J, Zhang Ji-Guang (2016) The roles of oxygen non-stoichiometry on the electrochemical properties of oxide-based cathode materials. Nano Today 11:678–694
Ganduglia-Pirovano MV, Hofmann A, Sauer J (2007) Oxygen vacancies in transition metal and rare earth oxides: current state of understanding and remaining challenges. Surf Sci Rep 62:219–270
Cohen DD, Rose EK (1992) Analysis of oxygen by charged particle bombardment. Nucl Instr Meth B 66:158–190
Amsel G, Samuel D (1967) Microanalysis of the stable isotopes of oxygen by means of nuclear reactions. Anal Chem 39(14):1689–1698
Csedreki L, Huszank R (2015) Application of PIGE, BS and NRA techniques to oxygen profiling in steel joints using deuteron beam. Nucl Instr Meth B 348:165–169
Luomajarvi M, Rauhala E, Hautala M (1992) Oxygen detection by non-Rutherford backscattering below 2.5 MeV. Nucl Instr Meth B 9:255–258
Leavitt JA, McIntyre LC Jr, Ashbaugh MD, Oder JG, Lin Z, Dezfouly-Arjomandy B (1990) Cross sections for 170.5° backscattering of 4He from oxygen for 4He energies between 1.8 and 5.0 MeV. Nucl Instr Meth B 44:260–265
Vickridge IC, Tallon J, Presland M (1994) High precision determination of 160 in high Tc superconductors by DIGME. Nucl Instr Meth B 85:95–99
Vickridge IC, Tallon J, Presland M (1995) 16O DIGME of high Tc materials. Nucl Instr Meth B 99:450–453
Raisanen J (1986) A rapid method for carbon and oxygen determination with external proton induced gamma-ray emission analysis. Nucl Instr Meth B 17:344–348
Chhillar S, Acharya R, Tripathi R, Sodaye S, Sudarshan K, Rout PC, Mukerjee SK, Pujari PK (2015) Compositional characterization of lithium titanate ceramic samples by determining Li, Ti and O concentrations simultaneously using PIGE at 8 MeV proton beam. J Radioanal Nucl Chem 305(2):463–467
Kumar S, Sunitha Y, Reddy GLN, Sukumar AA, Ramana JV, Sarkar A, Verma R (2016) Oxygen determination in materials by 18O(p,αγ)15 N nuclear reaction. Nucl Instr Meth B 378:38–44
Kiss AZ, Koltay E, Nyako B, Somorjai E, Anttila A, Raisanen J (1985) Measurements of relative thick target yields for PIGE analysis on light elements in the proton energy interval 2.4–4.2 MeV. J Radioanal Nucl Chem 89:123–141
Mateus R, Jesus AP, Ribeiro JP (2005) A code for quantitative analysis of light elements in thick samples by PIGE. Nucl Instr Meth B 229:302–308
Ziegler JF, Biersack JP, Ziegler D (2013) SRIM- The Stopping and Range of Ions in Matter. http://www.srim.org/
Mayer M (1997) SIMNRA user’s guide, Report IPP 9/113. Max Planck Institute for Plasmaphysik, Garching, Germany
Bashkin S, Richards HT (1951) Proton bombardment of the lithium isotopes. Phys Rev 84:1124–1129
Malmberg PR (1956) Elastic scattering of protons from Li7. Phys Rev 101:114–117
Gurbich AF (1998) Evaluation of non-Rutherford proton elastic scattering for cross section carbon. Nucl Instr Meth B 136–138:60–65
Gurbich AF (1997) Evaluation of non-Rutherford proton elastic scattering for cross section oxygen. Nucl Instr Meth B 129:311–316
Ajzenberg-Selove F (1986) Energy levels of light nuclei A = 16–17. Nucl Phys A 460(1):1–110
Ajzenberg-Selove F (1987) Energy levels of light nuclei A = 18–20. Nucl Phys A 475(1):1–198
Sunitha Y, Kumar Sanjiv (2017) Depth profiling Li in electrode materials of lithium ion battery by 7Li(p, γ)8Be and 7Li(p, α)4He nuclear reactions. Nucl Instr Meth B 400:22–30
Rauhala E, Keinonen J (1988) Ion beam analysis of oxygen distribution in superconducting YBa2Cu3Ox. Appl Phys Lett 52:1520–1522
Habrioux A, Surblé S, Berger P, Khodja H, D’Affroux A, Mailley S, Gutel T, Patoux S (2012) Nuclear microanalysis of lithium dispersion in LiFePO4 based cathode materials. Nucl Instr Meth B 290:13–18
Colaux JL, Jeynes C, Heasman KC, Gwilliam RM (2015) Certified ion implantation fluence by high accuracy RBS. Analyst 140:3251–3261
Fehrenbacher G, Meckbach R, Paretzke HG (1996) Fast neutron detection with germanium detectors: computation of response functions for the 692 keV inelastic scattering peak. Nucl Instr Meth A 372:239–245
Sunitha Y, Kumar Sanjiv (2017) 10B/11B isotopic ratio and atomic composition of boron carbide: determination by proton induced γ-ray emission and proton elastic backscattering spectrometry. Appl Radiat Isot 128:28–35
Mateus R, Jesus AP, Braizinha B, Cruz J, Pinto JV, Ribeiro JP (2002) Proton-induced γ-ray analysis of lithium in thick samples. Nucl Instr Meth B 190:117–121
Acknowledgements
The authors gratefully acknowledge the useful comments and suggestions of Dr. P. D. Naik, Associate Director, Chemistry Group, BARC and Dr. Sunil Jai Kumar, Head, NCCCM, Hyderabad. The authors thank Prof. C. Sudakar for providing LiFePO4 and LiFePO4/C samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunitha, Y., Kumar, S. 18O(p,p′γ)18O nuclear reaction in the determination of oxygen by proton induced γ-ray emission. J Radioanal Nucl Chem 314, 1803–1812 (2017). https://doi.org/10.1007/s10967-017-5575-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5575-5