Abstract
Barium borosilicate glass (BaBSG) is proposed as a potential candidate for vitrification of nuclear waste generated from thoria based nuclear reactors. Along with fission products, activation products and many inactive chemicals, like fluorine in the form of HF are expected to be present in the dissolver solution with nuclear waste. As vitrification occurs at high temperature, it is important to quantify fluorine in BaBSG. Due to its complex matrix, most of the wet chemical and nuclear analytical methods encounter problems in the estimation of fluorine. Particle induced γ-ray emission (PIGE) method has been standardized for non-destructive determination of fluorine contents in BaBSG samples utilizing measurement of prompt gamma-rays from 19F (p, p’γ) 19F reaction. Experiments have been carried out with thick pellet targets prepared in cellulose matrix using 4 MeV proton beam from the folded tandem ion accelerator at BARC, Mumbai. For obtaining current normalized count rate of interest, beam current variation was monitored by the Rutherford backscattering spectrometry (RBS) method as well as by the in situ approach using an externally added element sensitive to PIGE. In this paper standardization of PIGE methods for F determination, validation of methods using synthetic samples, and application to BaBSG samples are reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High level radioactive liquid waste (HLW) generated from reprocessing of spent nuclear fuel mainly contains fission products, corrosion products, minor actinides and inactive chemicals added at different stages of reprocessing. Thoria containing spent fuels offer resistance with respect to dissolution because of the refractory nature of thoria. Developmental work carried out by several researchers [1] envisages use of mixture of HNO3 and HF for dissolution. In view of the corrosive nature of F− ions, Al(NO3) is also added to complex excess amount of F− ions. Thus, F− ions are present in significant amount in the HLW generated during reprocessing stage of spent fuel. HLW needs to be immobilized in a suitable inert matrix before their long term disposal in geological repositories.
BaBSG is being used in India for vitrification of HLW [2]. It is reported that BaBSG can accommodate up to 16% by weight ThO2 without any phase separation [3, 4]. Also, it has been reported that BaBSG can contain F− ions up to 4 wt% and F− exists as F–Na(x)Ba(y) structural units. Only negligible amount of F− ions form Si–F linkages in the glass [5]. Typical composition of barium borosilicate based vitrified glass is SiO2 (34%), B2O3 (20%), Na2O (17%), BaO (8%), TiO2 (3%) and waste oxides (Th, Al, fission products etc., 18%). In general, losses of ingredients during preparation of glass because of carryover/volatilization are of the order of 1–2% for borosilicate glasses without fluoride. Suitable modifications have been made to incorporate Th and F in borosilicate glass. Presence of F− in glass is likely to affect physico-chemical properties of glass namely melt temperature, glass transition temperature, viscosity, coefficient of thermal expansion and chemical durability. Therefore, it is very important to understand the behaviour of fluoride during vitrification of HLW. It is important to add here that vitrification process is carried out at high temperature (~1,000 °C) and steps like evaporation, calcinations, fusion, soaking are main events during vitrification. Retention of fluoride in the glass therefore becomes important to ascertain extent of loss, if any, on account of volatilization or carryover. Hence it is very important to estimate fluorine content in the complex matrix of BaBSG with a suitable analytical method so that its retention in glass matrix, prepared for vitrification of waste, can be studied. F determination in the BaBSG samples is difficult by conventional analytical methods like AAS, ICP-MS, and ICP-AES as well as non-destructive methods like neutron activation analysis (NAA), particle induced X-ray emission (PIXE) and XRF. Wet chemical methods demand that the analyte should be in solution form and dissolution of solid glass itself is difficult. During dissolution of glass containing F at high temperature, probability of F to volatilise in the form of SiF4 or BF3 or alkali metal fluoride is very high. X-ray based techniques (e.g., PIXE, and XRF) are not used for low Z elements due to self attenuation of X-rays by the sample itself. In NAA, neutron activation products of 27Al (28Al; t 1/2 = 2.24 min; 1,779 keV) and 23Na (24Na; t 1/2 = 15 h; 1368.5 keV; 2,754 keV) give high dead time and Compton background during gamma ray spectrometry. 23Na also undergoes (n, αγ) threshold reaction with fast/high energy neutrons in reactor and produces 20F (t 1/2 ~11.6 s), which is an interference reaction for 19F present in the BaBSG matrix. Presence of B reduces the neutron flux as B is a neutron poison. Therefore, NAA is not suitable to estimate 19F in BaBSG matrix. The only suitable method for non-destructive determination of F in this matrix appears to be PIGE [6, 7] using proton beam.
Particle induced γ-ray emission, an ion beam analysis (IBA) technique, is exclusively used for determination of low Z elements like Li, Be, B, F, Na, Mg, Al and Si in diverse matrices [8, 9] such as geological [10], biological [11, 12], environmental [10, 13], alloys [14], and archaeological [15] samples. PIGE has advantages over PIXE, since it uses measurement of γ-rays, which experience less attenuation in matrix. Thus, PIXE and PIGE can be used simultaneously for complete compositional analysis of the sample [8, 9]. In the PIGE technique, a charged particle beam falls on the sample surface and induces the emission of characteristic gamma-rays, following in-elastic, (p, p′γ) excitation and nuclear reactions like, (p, γ) and (p, αγ). These emitted gamma-rays are the signature of a particular isotope of an element whose intensities are proportional to its concentration in the sample. In the present work, we have used 19F (p, p′γ) 19F reaction over 19F (p, αγ) 16O reaction for estimation of F in BaBSG samples, due to high detection efficiency of HPGe for low energy gamma-rays (110 and 197 keV) emitted in former reaction.
Since PIGE is an on-line technique, sample and standards are measured separately. It is therefore important to monitor the beam current fluctuations for the purpose of normalizing count rate of interest element. When samples are thick and non-conducting, the beam gets fully stopped inside the sample pellet and current measurements cannot be performed from the target. Instead, current normalization is carried out by using Rutherford backscattering spectrometry (RBS), in which backscattered ions from thin foils of high Z metals like Au, Ag and W placed just before the sample, can be measured using a Si based surface barrier detector kept at a fixed backward angle with respect to the ion beam. In the RBS method, gamma-ray counts from the isotope of the element of interest are normalized by the RBS counts of protons. Alternatively, current fluctuations can also be measured by an in situ method, where an element, that is not present in the sample and has good sensitivity by PIGE technique, is added externally to the samples. In this approach the gamma-ray counts from the isotope of element of interest are normalized by sensitivity of internal standard. The samples were diluted with cellulose matrix to keep the effective ‘Z’ of the pellet constant for sample and standards, so that the LET of proton is same in standard and samples. The present work reports on the use of both RBS and in situ current normalization techniques for accurate calculation of count rate of γ-ray peak of interest used for concentration determination of F in the said glass samples.
Experimental
Method 1 (RBS method)
Standard pellets were prepared by mixing varying amount of NaF, as it is part of BaBSG matrix, (corresponding to F amount 1,000–40,000 mg kg−1) in cellulose (Aldrich-Sigma) matrix. For method validation five synthetic samples were used; one synthetic sample pellet was prepared by mixing LiF (~4 mg) in cellulose matrix and four synthetic sample pellets were prepared by mixing cellulose and base glass (~100–150 mg), containing known fluorine amount in the range of 0.3–1.8 wt%. Sample pellets were prepared by mixing about 100–150 mg of BaBSG sample and 650–600 mg of cellulose. All the pellets (20 mm diameter and 2 mm thick) had a net mass of ~750 mg.
The pellets were irradiated, under high vacuum (10−6 torr) conditions, by 4 MeV proton beam (beam current ~5 nA) obtained from the folded tandem ion accelerator (FOTIA), BARC, Mumbai. The duration of irradiation time was varied between 0.5 and 2 h depending on the F amount. The proton beam passed through a thin Au foil (thickness ~3.5 mg cm−2) before falling on the target. The backscattered protons from the thin gold foil were counted using a Si solid surface barrier detector placed at an angle of 150° with respect to the beam, and used for current normalization. The 197 keV prompt gamma-rays of F from the 19F (p, p′γ) 19F reaction were measured using a 30% HPGe detector placed perpendicularly to the beam axis at a distance of 5 cm from the sample ladder. The energy calibration of the HPGe was performed using a standard source of 152Eu (gamma energies of 152Eu used for calibration are 121.8; 244.7; 344.3; 444; 778; 964; 1,112; 1,408 keV).
Method 2 (in situ current normalization approach)
For this method Li was chosen as an in situ current normalization standard as it was not a part of the sample matrix. Standard pellets were prepared by mixing varying amount of NaF (~3.5–68 mg) with constant amount of Li2SO4·H2O (34 mg) and cellulose powder. For validation of this approach synthetic sample pellets were prepared by mixing base glass sample (~200 mg, having different amount of F ~0.1–1.0 wt%) with fixed amount of Li2SO4·H2O (~25 mg) and cellulose powder. The sample pellets were prepared by mixing glass sample (~200 mg) with fixed amount of lithium sulphate (~25 mg) and cellulose. All pellets were of same dimensions (20 mm diameter and 2 mm thick) and weight (~750 mg).
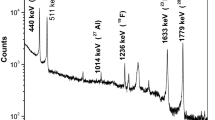
Sample irradiations protocols were followed similar way to that of RBS method. Typical γ-ray spectra of BaBSG samples containing F without and with current normalizing standard Li are shown in Figs. 1 and 2, respectively. The γ-ray spectra were analyzed using peak fitting software (PHAST) [16] to determine the peak areas under the γ-ray peaks of 197 keV from the 19F (p, p′γ) 19F and 478 keV from the 7Li (p, p′γ) 7Li reactions respectively.
Calculations
In the RBS method, the count rate (counts per second, CPS) corresponding to the 197 keV peak of fluorine was normalized for beam current using the backscattered protons from Au. The normalized count rates of 197 keV (CPS N ) gamma-rays relative to RBS count rates are taken as:
Calibration plot of current normalized 197 keV peak count rate (CPS N ) from standard samples (Fig. 3) were used to calculate the sensitivity (S N ). The concentration of fluorine in the BaBSG samples was calculated using the following formula:
As the method is on-line, the principal back ground at the gamma-ray of interest was due to Compton events from high energy gamma-rays from the sample.
In the in situ method the gamma-ray of 478 keV from 7Li (p, p′γ) 7Li reaction was used for current normalization. The CPS of the 197 keV gamma-ray of F was normalized using sensitivity of Li [CPS/(mg kg−1)] from same spectrum as:
Here peak (197 keV) count rate was used for fluorine concentration calculation in the synthetic samples and in BaBSG samples. Following formula was used for calculation of fluorine:
Here CPSF,Li is counts per second and SF,Li is the relative sensitivity of 19F with respect to Li for the chosen pair of γ-ray, obtained from calibration plot (Fig. 4). The 3σ detection limits (LD) for both the methods using following formula:
where Cb is background counts under 197 keV peak of F in sample spectrum, LT is the live time of counting and SF is the sensitivity of F (CPS/(mg kg−1)). This calculation is valid for the same proton beam current condition for sample and standard.
Results and discussion
Method 1 (RBS approach)
The calibration plot was obtained by plotting current normalized (using proton counts in RBS) count rate of 197 keV gamma-ray of 19F. Fluorine amount was determined in four synthetic borosilicate glass samples and also in one lithium fluoride (LiF) standard sample using sensitivity of F. The concentration values so determined were in the range of 623–3,980 mg kg−1 and the results are shown in Fig. 5. It can be clearly seen that the amount of F in the synthetic samples are in good agreement (within ±5%) with the added amounts of F. The present method was applied to determine the concentration of fluorine in five borosilicate glass samples. The measured concentrations of fluorine in the borosilicate glass samples were found to be in the range of 700–5,000 mg kg−1 in the pellet (cellulose + sample), which correspond to 0.36–3.76 wt% of F in the original samples (Table 1). Uncertainties in the determined concentrations of F in BaBSG samples are due to the counting statistics on the peak areas and peak fitting error, which are in the range of 1.0–4.0%. Other parameters contributing to the uncertainties during the experiment are, the mass of the sample and standards (0.05–1%), concentration of standards (0.3%), and count rate of RBS (0.1%). The geometries of samples and standards were identical for irradiation and counting, and thus the uncertainties due to the said parameters are negligible. Though BaBSG contains many other low Z elements (B, Al, Na and Si) along with C,H and O from cellulose used for pelletization, there were no gamma-ray interference for 197 keV peak of F.
Method 2 (in situ approach)
The calibration plot was obtained by plotting the Li sensitivity normalized CPS of 197 keV of F with sensitivity of Li. The plot shows linearity for F concentration in the range 1,000–40,000 mg kg−1. Since, F count rates are normalised by Li sensitivity in the samples, to take care of current variations, the slope of the calibration plot gives relative sensitivity of F with respect to Li (SF, Li). This relative sensitivity of F was used for calculation of F in five synthetic borosilicate glass samples for validation of the approach. Control glass samples did not show any Li peak which ensures that Li can be used as internal standard for beam current normalization in this case. The results of F concentrations in synthetic samples are shown in Fig. 6. The percentage deviations of F determined in synthetic samples are in the range of ±0.5–5% with respect to the expected values. The method has been applied to several samples of barium borosilicate glass; results for the two samples (BSG 6 and 7) are shown in Table 1.
The uncertainty in the results (Table 1) of seven samples of BaBSG was in the range of ±1.0–4.0%. The 3σ detection limits were calculated for both the methods using Eq. 5. The detection limits achieved by the PIGE methods used are 16–19 mg kg−1, which are due to varying background counts of samples.
Conclusions
Two methods of PIGE using 4 MeV protons were optimized for determination of F in BaBSG samples. In situ approach of current normalization of count rate of interest is simpler as it does not require separate measurement set-up like in the case of RBS and is independent of sample to detector geometry. The only requirement for in situ approach is that the chosen low Z element should not be present in the sample. The F concentrations in pellets were found in the range of 700–5000 mg kg−1 that correspond to 0.36–3.76 wt% in the BaBSG samples.
References
Moore RL, Goodall C, Hepworth JL, Watts RA (1957) Ind Eng Chem 49:885–887
Raj K, Prasad KK, Bansal NK (2006) Radioactive waste management practices in India. Nucl Eng Des 236:914–930
Mishra RK, Sudarsan V, Kaushik CP, Raj K, Kulshreshta SK, Tyagi AK (2006) J Nucl Mater 359:132–138
Mishra RK, Sudarsan V, Tyagi AK, Kaushik CP, Raj K, Kulshreshta SK (2006) J Non Cryst Solids 352:2952–2957
Mishra RK, Sudarsan V, Kaushik CP, Raj K, Vatsa RK, Body M, Tyagi AK (2009) J Non Cryst Solids 355:414–419
Borbely-Kiss I, Jozsa M, Kiss AZ, Koltay E, Nyako B, Somorjai E, Szabo Gy, Seif El-Nasr S (1985) J Radioanal Nucl Chem 92:391–398
Mosbah M, Metrich N, Massiot P (1991) Nucl Instrum Method B58:227–231
Volfinger M, Robert JL (1994) J Radioanal Nucl Chem 185:273–291
Boni C, Caruso E, Cereda E, Marcazzan GMB, Redaelli P (1989) Nucl Instrum Method B40/41:620–623
Valkovic O, Jaksic M, Fazinic S, Valkovic V, Moschini G, Menapace E (1995) Nucl Instrum Method B99:372–375
Yosnda K, Hai VH, Nomachi M, Sugaya Y, Yamamato H (2007) Nucl Instrum Method B260:207–212
Salah H, Arab N (2007) J Radiochem Nucl Sci 8:31–34
Edward SM, Radcliffe CD, Lewis WC, Carole RS (1978) Anal Chem 50:1120–1124
Ene A, Popescu IV, Badica T, Besliu C (2006) Rom J Phys 51:595–602
Weber G, Martinot L, Strivay D, Garnir HP, George P (2003) X-ray Spectrom 34:297–300
Mukhopadhyay PK (2001) Proceedings of the symposium on intelligent nuclear instrumentation (INIT-2001), Bhabha Atomic Research Centre, Mumbai, p 313
Acknowledgments
One of the authors (SC) thanks HBNI, DAE for the fellowship. Authors thank Dr. V. K. Manchanda, Ex-Head, RCD and Dr. A. Goswami, Head, RCD, BARC for their keen interest and support. Authors thank Dr. P. Singh, Mr. S. K. Gupta, Mr. A. Aggarwal and other personnel of LEHIPA project, Physics Group for their support during experiment at FOTIA, BARC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chhillar, S., Acharya, R., Sodaye, S. et al. Application of particle induced gamma-ray emission for non-destructive determination of fluorine in barium borosilicate glass samples. J Radioanal Nucl Chem 294, 115–119 (2012). https://doi.org/10.1007/s10967-011-1525-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1525-9