Abstract
Particle induced gamma-ray emission method was used to quantify total fluorine (F) concentrations in soil and food samples obtained from fluoride affected as well as non-affected areas of Punjab state, India. Samples, standards and reference materials in pellet form with lithium as in situ current normalizer were irradiated using 4 MeV proton beam from the tandem particle accelerator. Characteristic prompt gamma rays at 197 keV from 19F and 478 keV from 7Li were measured using a HPGe detector system. Fluorine concentrations in soil and food samples were 279–3138 and 51–211 mg kg−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
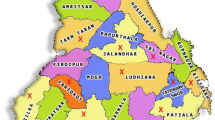
Fluorine (F) is an essential as well as toxic trace element and it is present ubiquitously in the environment. The toxicity of fluorine in the form of fluoride is associated with its high chemical and biological activity in human body [1]. Fluoride (F−) gets absorbed in the gastrointestinal tract and rapidly migrates across the biological membranes. It negatively influences many metabolic, structural, and cellular functions. The toxic effect of fluoride includes induction of inflammatory reactions, cell contractile responses, inhibition of protein synthesis, cell cycle progression, oxidative stress and DNA damage [2]. Many of these cellular events ultimately lead to cell death or apoptosis. F is found in the form of fluoride in soil and water. Small amount of fluoride is beneficial in preventing dental carries (1–1.5 mg L−1) which strengthens the enamel; whereas its long exposure is found to be harmful to bones and teeth. Fluoride is considered to be beneficial in drinking water at a level of about 0.7 mg L−1 but it is harmful once it exceeds the safe limit of 1.5 mg L−1 set by World Health Organization [3]. It has been reported that fluoride concentration in the range of 1.5–4 mg L−1 causes dental fluorosis [4], whereas prolonged exposure at higher concentrations i.e., 4–10 mg L−1 leads to skeletal fluorosis [5]. It has been reported by Central Ground Water Board (CGWB, Ministry of Water Resources, River Development and Ganga Rejuvenation) of the Government of India that the states of Andhra Pradesh, Bihar, Delhi, Gujarat, Haryana, Jammu and Kashmir, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Odisha, Punjab, Rajasthan, Tamil Nadu, and Uttar Pradesh are affected by fluoride related toxicity [6]. In the state of Punjab [7], the present study area, there are 11 districts namely Amritsar, Bhatinda, Faridkot, Fatehgrah Sahib, Firozepur, Gurdaspur, Mansa, Moga, Muktasar, Patiala, and Sangrur, which have shown fluoride concentration in ground water higher than the permissible limit of 1.5 mg L−1. It is known that main sources of fluorine in human body are water and food and they provide about 40% of the intake of total F [8]. The main sources of fluoride in soil are minerals like fluorite, fluorapatite, apatite, biotite, tourmaline, muscovite, phlogopite and cryolites [3], which are present in the earth’s crust. Fluorine is also known to be present in significant amount in products of coal combustion [9] besides pesticides [10] and fertilizers [11].
Various factors that govern the release of fluoride into water from fluoride-bearing minerals are (i) the chemical constituents present in the water, (ii) the presence and accessibility of fluoride minerals to water, and (iii) the contact time between the source mineral and water [12]. Overall water quality (e.g., pH, hardness, and ionic strength) also plays a key role by influencing mineral solubility, complexation and sorption/exchange reactions [13]. The irrigation of soil using fluoride rich water may further lead to increase in the fluoride content of the soil in addition to fluorine derived from use of agrochemicals. Therefore, it becomes essential to determine concentration of total F in soil, used for cultivation. Wet-chemical techniques like potentiometry [14], colorimetry, fluoride ion selective electrode [15, 16] and ion chromatography [17] are routinely used to determine trace concentration of F in water. However, samples like soil and food are not favorable matrices for these techniques. These methods are based on chemical dissolution/separation followed by detection. Thus, for solid samples the above mentioned chemical technique are destructive in nature, time consuming and requires tedious chemical treatments which may lead to contamination or evaporation of F. In view of this, a technique which can determine F non-destructively i.e., using directly solid samples without any chemical dissolution is preferred. In this respect, particle induced gamma-ray emission (PIGE) [18, 19], an ion beam analysis (IBA) technique, is suitable for determination of low concentration of fluorine using smaller mass of the sample. PIGE is an accelerator based isotope specific online technique for low Z elements which involves measurement of prompt gamma-rays from nuclear reactions like (p,p′γ), (p, αγ), (p,γ) and (p,nγ) [18]. Using low energy proton beam (2–5 MeV), It is capable of determining low Z elements (3 ≤ Z < 17, except for C, N and O) [18–22]. The determination of C, N, O by PIGE is possible though with poor (higher) detection limits due to use of less abundant isotopes like 13C and 18O [18]. However, at higher proton energy (>7 MeV) C, N and O can easily be determined at low (trace) concentration levels [18, 22]. Main advantage of the PIGE method is direct use of solid samples [23–25]. PIGE has been extensively applied for the determination of total fluorine in various fields like pharmaceuticals [26] glass [27–30], aerosols [31], geological [32, 33] and biological samples [24, 34–36], geological and environmental reference materials [37] along with thin films and functionalized carbon nanotubes [38]. For determination of F contents, PIGE is preferred over techniques like particle induced X-ray emission (PIXE), X-ray fluorescence (XRF) and neutron activation analysis (NAA), which are not routinely used due to low energy X-ray of F (for X-ray based techniques) or short half-life (20F, 11.4 s) (for NAA). Other techniques which can determine fluorine in samples like soil are colorimetry, spectrophotometry and F-ion-sensitive electrode [39], whereas direct solid sample analysis can be carried out by high-resolution graphite furnace molecular absorption spectrometry [40]. In the present study, the concentrations of F in soil and food samples, obtained from fluoride affected as well as non-affected areas districts of Punjab state, were determined using in situ current normalized PIGE method. Experimental methodology, results of F concentrations, total uncertainties and 3σ detection limits are presented.
Experimental
Sample collection and preparation
Soil samples
Soil samples were collected from different locations of Punjab at a depth ranging from 5 to 30 cm by using normal Auger method. The samples were dried below 60 °C for 12 h and ground into fine powder by using an agate mortar. Powder samples were homogeneously mixed with cellulose and constant amount of Li2CO3. The samples thus obtained were pressed using a hydraulic press to get pellet of uniform thickness.
Food samples
To estimate the F contents and also possible uptake in food samples grown in the very same soil, food samples (rice) from the same locations were also collected for analysis. Samples were dried at 60 °C and powdered. Powder samples were homogeneously mixed with cellulose and constant amount of Li2CO3. The samples thus obtained were pressed using a hydraulic press to get pellet of uniform thickness.
Standards and reference materials
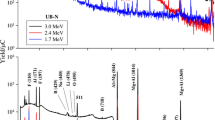
Standards for F were prepared by mixing homogeneously varying amount of NaF (100–3000 mg kg−1) and constant amount of lithium carbonate (Li2CO3) (about 10 mg of Li) in cellulose matrix. Reference materials were prepared in similar way to that of soil samples. Accelerator based experiments might be associated with variation in the beam current during the irradiation. Therefore, beam current measurement and normalization is a key aspect in PIGE. The normalization can be done either by measuring the beam current directly from conducting target or by making use of Rutherford back scattering (RBS) [41] or from the target (powdered sample) mixed with graphite, if target is non-conducting. In present work, current was normalized by externally adding the Li in the form of Li2CO3 to monitor current fluctuations in it. The in situ current normalizer works in the principle as given here: the relative count rate per mg kg−1 (ppm) of Li (current normalizer) of sample to sample or sample to standard is directly proportional to beam current fluctuation, if any, provided Li is homogeneously distributed in the sample. The condition for selecting an element as the current normalizer is that it is sensitive towards PIGE and it should not be present in the sample of interest. PIGE experiments were carried out at FOTIA, BARC, Mumbai. Some of the experiments were carried out at 3 MV Tandetron at IOP, Bhubaneswar. The target pellets in cellulose matrix with added Li were irradiated, under high vacuum (10−6 torr) conditions, and the characteristic gamma rays at 197 and 1236 keV from 19F (p, p′γ) 19F and at 478 keV from 7Li (p, p′γ) 7Li were measured using 30% relative efficiency high purity germanium (HPGe) detector coupled with PC based 8 k multi-channel analyzer (MCA). The detector was placed perpendicular to the direction of beam axis at a distance of 7 cm from the target ladder. The time of irradiation was kept 30–60 min depending on the F concentration and also for getting higher counts under the peaks of interest. The PIGE spectra for soil and food samples are given in Figs. 1 and 2, respectively. The gamma-ray spectra obtained from different samples were analyzed using peak-fit PHAST software [42]. The counts under the peaks of 197 and 478 keV were in the range of 10,000–50,000 so as to get higher counts (peak area) of interest and to get minimum or low counting statistical error.
Calculations
Relative method was used for the determination of absolute concentration of F. Concentration of F was determined using Eq. (1).
where C x is the concentration of analyte of interest (in this case F), CPS x is counts per second at 197 keV from 19F, S CN is sensitivity of current normalizer using 478 keV from 7Li and S x,N is current normalized sensitivity of F using count rate of 197 keV of F standard and sensitivity of current normalizer Li.
Current normalized sensitivity of F (S x,N ) was determined using Eq. (2),
where (CPS) x is the count rate of 197 keV gamma-ray of 19F, C x is the concentration of F in mg kg−1 in the pellet and S CN is the sensitivity of current normalizer (Li) obtained using following Eq. (3),
where (CPS)Li is the count rate of 478 keV gamma-rays of Li, and C Li is the concentration of Li in mg kg−1 in the pellet. Further details of calculations can be found in our earlier publications [19–21]. The concentration of F in soil samples was determined using the sensitivity of F obtained from the Eq. 1. For normalizing the effect of beam current fluctuations, the in situ current normalizing element (Li) was added in constant amount to each target of sample and standard.
The 3σ detection limit of F in the present method was calculated by standard calculation equation using sample background counts and elemental sensitivity at 197 keV peak of F obtained using 4 MeV proton beam as reported earlier in our previous paper [19, 20].
Results and discussion
The results of F concentrations of three RMs namely AGV-1, G-2 and 2695 (H) obtained by in situ current normalized PIGE method are given in Table 1. The concentrations are in the range on 426–1257 mg kg−1 and the percent deviations are in the range of ±0.2–2.5%, with respect to the certified values. The propagated uncertainties on the results are in the range of ±2–5%, which are due to counting statistics of peak areas of 197 keV (19F) of sample and standard as well as 478 keV of in situ current normalizer (7Li) and uncertainties on their corresponding masses.
The standardized method was applied to a total of 27 soil and 26 food (rice) samples for determining total F concentration. Concentrations of F determined in soil and food samples are in the range of 279–3138 mg kg−1 (Table 2) and 51–211 mg kg−1 (Table 3), respectively. The associated propagated (as described earlier) uncertainties are in the range of 1.1–2.4% for soil samples and 2–5% for food samples due to their varying concentrations. To the best of our knowledge literature data about safe limit of F (in mg kg−1) in soil and food are not available like permissible limit for drinking water. The F concentrations in soil samples reported are in the range of about 20–1000 mg kg−1, and based on this data it can be stated that soil samples analyzed have higher concentration of F than the control/uncontaminated soil. These results indicate that the soil samples are contaminated with fluorine. From literature data on food and diet samples [8, 16, 43], it was difficult to find F concentration data in rice samples. However, the reported data on F in diet/food samples (except for rice) are in the range of 10–30 mg kg−1 [8, 16, 43]. Based on these results, it can be stated that the higher concentrations of F (51–211 mg kg−1) in the rice samples might be due to fluoride contaminated soil and water in the study areas. We have attempted to find correlation of F concentration between soil and food from same location and it was observed that F concentration in food to soil are in the range of 3–21% indicating no direct correlation from our observed results. The detection limits of F by the present method (4 MeV proton beam, about 20 nA current, 30% HPGe detector at about 7 cm distance) are in the range of 10–50 and 9–24 mg kg−1 for soil and food samples, respectively. The detection limit values of F in food samples are given in Table 3.
The in situ current normalized PIGE method is advantageous as it does not need another set up/method for current measurement. PIGE method used here is promising for determination of low to high concentrations of F in food (biological/organic matrix) and soil samples as it does not need sample dissolution like other conventional wet-chemical methods. The advantages of the method are: small sample size, non-destructive in nature, on-line measurement thus less turn-around time and high sensitive particularly for F. It is also free from spectral interference as no peak at 197 keV was observed from cellulose matrix. From our work it can be concluded that PIGE is the best as well as simple method for precise and accurate determination of low concentration of F in solid powder samples (Table 4).
Conclusions
PIGE method using 4 MeV proton beam from FOTIA has been optimized for determination of trace to minor concentration of fluorine in food and soil samples obtained from fluoride affected and non affected areas. The method applied to three reference materials as a part of quality control exercise and the results are in good agreement with that of certified values. Our results on F concentrations will serve as data base for our future work as well as for other researchers.
References
Tebbutt THY (1983) Relationship between natural water quality and health. UNESCO, Paris
Agalakova NI, Gusev GP (2012) Molecular mechanisms of cytotoxicity and apoptosis induced by inorganic fluoride. ISRN Cell Biol 2012:1–6. doi:10.5402/2012/403835
Fawell J, Bailey K, Chilton J et al (2006) Fluoride in drinking-water. WHO, Cornwall
Salah H, Arab N (2007) Application of PIGE to determine fluorine concentration in human teeth: contribution to fluorosis study. J Nucl Radiochem Sci 8:31–34
Reddy DR (2009) Neurology of endemic skeletal fluorosis. Neurol India 57:7–12. doi:10.4103/0028-3886.48793
Susheela AK (1999) Fluorosis management programme in India. India Curr Sci 77:1250–1256
State Profile, Ground Water Scenario of Punjab, Central Ground Water Board, Punjab, http://cgwb.gov.in/gw_profiles/st_Punjab.htm. Accessed 15 July 2016
Farooqi AS, Arshed W, Akanle OA et al (1992) Fluorine determination in diet samples using cyclic INAA and PIGE analysis. J Radioanal Nucl Chem 161:71–78. doi:10.1007/BF02034881
Ando M, Tadano M, Yamamoto S et al (2001) Health effects of fluoride pollution caused by coal burning. Sci Total Environ 271:107–116. doi:10.1016/S0048-9697(00)00836-6
Singh K, Lataye DH, Wasewar KL (2013) Removal of fluoride from aqueous solution: status and techniques. Desalination Water Treat 51:3233–3247. doi:10.1080/19443994.2012.749036
Eyde B (1985) Determination of fluoride in fertilizers by means of the ion-selective electrode. Anal Chem 320:41–44
Thesai S, Krishnasamy J, Sundaram R et al (2014) Incidence and effects of fluoride in Indian natural ecosystem: a review. Adv Appl Sci Res 5:173–185
Vithanage M, Bhattacharya P (2015) Fluoride in drinking water: health effects and remediation, vol 5. Environmental chemistry for a sustainable world. Springer, New York, pp 105–151. doi:10.1007/978-3-319-11906-9_4
Light TS, Mannion RF (1969) Microdetermination of fluorine in organic compounds by potentiometric titration using a fluoride electrode. Anal Chem 41:107–111
Eyde B (1983) Determination of acid soluble fluoride in soils by means of an ion-selective electrode. Fresenius Z Anal Chem 316:299–301. doi:10.1007/BF00468924
Ponikvar M, Stibilj V, Žemva B (2007) Daily dietary intake of fluoride by Slovenian Military based on analysis of total fluorine in total diet samples using fluoride ion selective electrode. Food Chem 103:369–374. doi:10.1016/j.foodchem.2006.07.032
Saha JK, Kundu S (2003) Determination of fluoride in soil water extract through ion chromatography. Commun Soil Sci Plant Anal 34:181–188. doi:10.1081/CSS-120017424
Räisänen J (2009) “Particle induced gamma emission: PIGE” (Chapter 7). In: Wang Y, Nastasi M (eds) Handbook of modern ion beam materials analysis. Materials Research Society, Warrendale, pp 147–176
Srivastava A, Chhillar S, Singh D et al (2014) Determination of fluorine concentrations in soil samples using proton induced gamma-ray emission. J Radioanal Nucl Chem 302:1461–1464. doi:10.1007/s10967-014-3661-5
Chhillar S, Acharya R, Pai RV et al (2012) A simple and sensitive particle induced gamma-ray emission method for non-destructive quantification of lithium in lithium doped Nd2Ti2O7 ceramic sample. J Radioanal Nucl Chem 293:437–441. doi:10.1007/s10967-012-1764-4
Chhillar S, Acharya R, Vittal Rao TV et al (2013) Non-destructive compositional analysis of sol–gel synthesized lithium titanate (Li2TiO3) by particle induced gamma-ray emission and instrumental neutron activation analysis. J Radioanal Nucl Chem 298:1597–1603. doi:10.1007/s10967-013-2609-5
Chhillar S, Acharya R, Tripathi R, Sodaye S, Sudarshan K, Rout PC, Mukerjee SK, Pujari PK (2015) Compositional characterization of lithium titanate ceramic samples by determining Li, Ti and O concentrations simultaneously using PIGE at 8 MeV proton beam. J Radioanal Nucl Chem 305:463–467. doi:10.1007/s10967-015-4037-1
Roelandts I, Robaye G, Weber G, Delbrouck-Habaru JM (1986) The application of proton-induced gamma-ray emission (PIGE) analysis to the rapid determination of fluorine in geological materials. Chem Geol 54:35–42
Samudralwar DL, Robertson JD (1993) Determination of major and trace elements in bones by simultaneous PIXE/PIGE analysis. J Radioanal Nucl Chem 169:259–267
Savidou A, Aslanoglou X, Paradellis T, Pilakouta M (1999) Proton induced thick target γ-ray yields of light nuclei at the energy region Ep = 1.0−4.1 MeV. Nucl Instrum Methods B 152:12–18
Nsouli B, Bejjani A, Della Negra S et al (2010) Ion beam analysis and PD-MS as new analytical tools for quality control of pharmaceuticals: comparative study from fluphenazine in solid dosage forms. Anal Chem 82:7309–7318
Chhillar S, Acharya R, Sodaye S et al (2012) Application of particle induced gamma-ray emission for non-destructive determination of fluorine in barium borosilicate glass samples. J Radioanal Nucl Chem 294:115–119. doi:10.1007/s10967-011-1525-9
Elekes Z, Uzonyi I, Gratuze B et al (2000) Contribution of PIGE technique to the study of obsidian glasses. Nucl Instrum Methods B 161–163:836–841
Mosbah M, Metrich N, Massiot P (1991) PIGME fluorine determination using a nuclear microprobe with application to glass inclusions. Nucl Instrum Methods B 58:227–231
Venkatesh K, Chhillar S, Kamble GS et al (2014) Determination of boron concentration in borosilicate glass, boron carbide and graphite samples by conventional wet-chemical and nuclear analytical methods. J Radioanal Nucl Chem 302:1425–1428. doi:10.1007/s10967-014-3552-9
Macias ES, Radcliffe CD (1978) Proton induced γ-ray analysis of atmospheric aerosols for carbon, nitrogen, and sulfur composition. Anal Chem 50:1120–1124
Valkovic O, Jaksic M, Fazinic S et al (1995) Quality control of PIXE and PIGE nuclear analytical techniques in geological and environmental applications. Nucl Instrum Methods B 99:372–375
Roelandts I, Robaye G, Weber G et al (1987) Determination of fluorine by proton-induced gamma-ray emission (PIGE) spectrometry in igneous and metamorphic charnockitic rocks from Rogaland (S. W. Norway). J Radioanal Nucl Chem 112:453–460. doi:10.1007/BF02132377
Farooqi AS, Arshed W, Akanle OA et al (1992) Fluorine determination in diet samples using cyclic INAA and PIGE analysis. J Radioanal Nucl Chem 161:71–78
Carvalho ML, Karydas AG, Casaca C et al (2001) Fluorine determination in human healthy and carious teeth using the PIGE technique. Nucl Instrum Methds B 179:561–567
Sastri CS, Banerjee A, Sauvage T et al (2013) Fluorine determination in different types of ivory by PIGE technique. J Radioanal Nucl Chem 298:311–315. doi:10.1007/s10967-012-2410-x
Roelandts I (1985) Determination of fluorine in eighty international geochemical reference samples by proton induced gamma ray emision spectrometry (PIGE). Geostand Newsl 9:191–197
Barthos R, Méhn D, Demortier A et al (2005) Functionalization of single-walled carbon nanotubes by using alkyl-halides. Carbon 43:321–325. doi:10.1016/j.carbon.2004.09.018
Xie ZM, Ye ZH, Wong MH (2001) Distribution characteristics of fluoride and aluminum in soil profiles of an abandoned tea plantation and their uptake by six woody species. Environ Int 26:341–346. doi:10.1016/S0160-4120(01)00010-1
Borges AR, François LL, Welz B et al (2014) Determination of fluorine in plant materials via calcium mono-fluoride using high-resolution graphite furnace molecular absorption spectrometry with direct solid sample introduction. J Anal At Spectrom 29:1564–1569. doi:10.1039/c4ja00067f
Tripathi R, Sodaye S, Tomar BS (2004) Oxygen determination in metal foils using backscattering spectrometry. Nucl Instrum Methods A 533:282–286. doi:10.1016/j.nima.2004.06.173
Mukhopadhyay PK (2001) The operating software of the PHAST PC-MCA Card. Proceedings of symposium on intelligent nuclear instrumentation-2001, Bhabha Atomic Research Centre, Mumbai
Gurkan R, Altunay N, Korkmaz S (2015) A new preconcentration procedure to quantify total acid hydrolyzed fluoride in selected beverages and food by spectrophotometry. Anal Methods 7:5081–5091. doi:10.1039/c5ay00672d
Acknowledgements
The authors from Pune University thank Board of Research in Nuclear Sciences (BRNS), DAE, Government of India for the financial assistance for the Project under BARC-SP Pune University MoU. Authors thank Head, IADD, Mr. S. K. Gupta, Mr. A. Agarwal and FOTIA staff of IADD, BARC for their help during PIGE experiment. Authors also thank Prof. S. Panda, Director, IOP, Prof. P. V. Satyam, Mr. A. K. Behera, Dr. D. K. Ray, IOP and IBL, IOP operation crew members for their help and support. We thank Dr. K. B. Dasari, Ex-Ph.D Scholar, BARC for his help in this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dhorge, P.S., Acharya, R., Rajurkar, N.S. et al. Quantification of trace fluorine concentrations in soil and food samples from fluoride affected region by in situ current normalized particle induced gamma-ray emission method. J Radioanal Nucl Chem 311, 1803–1809 (2017). https://doi.org/10.1007/s10967-016-5118-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5118-5