Abstract
The biosorption of uranium from aqueous solutions by Dictyopteris polypodioides brown algae was investigated. The effect of pH, uranium concentration, mass of the adsorbent, temperature and contact time on the removal efficiency were studied and the results were simulated by Langmuir, Freundlich and Dubinin–Radushkevich isotherm models. Kinetics data could be satisfactorily reproduced by the pseudo-second order equation and thermodynamic parameters were determined in order to evaluate the uranium uptake behavior. SEM/EDS and FT-IR were additionally used to characterize the algae and comprehend the sorption process which can be described as a combination of several mechanisms, including physical sorption, ion exchange and complexation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a naturally occurring radioactive element with an average concentration in earth’s crust of about 3 ppm and constant uranium concentration of about 3 ppb in oceans. Natural uranium is a mixture of three isotopes: 234U, 235U, and 238U. The most common isotope is 238U; it makes up about 99 % of natural uranium by mass. The EPA classified natural uranium or depleted uranium with respect to carcinogenicity because in general, uranyl is considered to be more chemically toxic as a heavy metal than as a radioelement [1–6].
Uranium, as it is known, is associated with mine and mill sites, also occurs naturally at elevated levels in rock, soil, surface water, and groundwater. On the other hand, the nuclear power production, the utilization of phosphate fertilizers, copper metallurgy, military activities and production of conventional energy have resulted in the generation of significant amounts of radioactive wastewater containing uranium with tremendous environmental impact. Removal of uranium from such large volumes of wastewaters will require a cost effective remediation technology [7–9].
The uranium removal from aqueous media can be performed through various processes, such as ion exchange, complexation, chemical precipitation, membrane separation, adsorption using both natural and synthetic sorbents, bio-accumulation and biosorption [10–15]. Biosorption has attracted a significant focus in recent years and can be regarded as an innovative technology replacing conventional processes for remediating metal pollution in wastewaters, because it offers several advantages, such as low operating cost, high sorption capacity, easy regeneration, high efficiency in dilute effluents detoxification, minimal volume of disposable sludge and high environmental viability [16–18]. Biosorption is a term that describes the removal of heavy metals by the passive binding to non-living biomass from an aqueous solution. This implies that the removal mechanism is not metabolically controlled. Biosorption occurs through interaction of the metal ions with functional groups found in the cell wall biopolymers (e.g. alginate) of either living or non-living organisms. Although both living and non-living forms of biomass exhibit relatively high affinities to heavy metals, the use of dead biomass for metal sequestration and immobilization is more advantageous because there are no toxicity concerns, no requirements of growth media or nutrients and there are easy techniques to desorb contaminants from the biomass and reuse them [19–21].
Many types of biosorbents (i.e. fungi, bacteria, yeasts, sea vegetables) were recently investigated for their ability to sequester heavy metals from diluted aqueous solutions [22–30]. Sea vegetables are algae that mainly come from marine environment. Marine organism, such as seaweed is well known to concentrate metals and in some cases has been used as monitor of seawater pollution. Marine algae proliferate ubiquitously and abundantly in the littoral zones of world oceans, they are rather stable and fast growing [16–18, 31–34].
Dictyopteris polypodioides, which is used as biosorbent in the present work, is a species of brown algae most commonly found at low water and shallow subtidal [35]. It is widely distributed and locally abundant in summer period. The aim of the present work was to investigate the interaction of the brown algal sample Dictyopteris polypodioides with uranium aqueous solutions. Batch experiments have been performed to explore uranium biosorption properties of this brown marine macro algae. Aiming in development of low cost—high efficiency water treatment technologies related to (radio) toxic metal ion removal, various parameters (e.g. pH, uranium concentration, mass of the adsorbent, temperature and contact time) have been studied and thermodynamic parameters (e.g., ΔG°, ΔH° and ΔS°) have been evaluated. The effect of the above parameters on the biosorption is of fundamental importance for the assessment of the chemical behavior of uranium in aquatic systems. Furthermore, emphasis is given to understand the mechanism of the U-biosorption on brown algae by application of complementary characterization techniques (SEM/EDS, FT-IR).
Materials and methods
Materials
All reagents used in the experiments were of analytical grade (AR). Ultra pure water prepared with a water purification system (Millipore Model) including Elix and Mili-Q was used in the experiments. A stock solution of 1000 mg/L of uranium was prepared from [UO2(NO3)2⋅6H2O] (Merck) by dissolving the salt in pure distilled water. Diluted solutions were prepared from the stock solution at room temperature in pure distilled water. The pH of each test solution was adjusted to the required value with 0.1 M HNO3 solution and Na2CO3 powder in the beginning of all experiments.
Dictyopteris polypodioides samples, e.g. brown marine macro algae, were collected by hand at the depth of 1.0–1.5 m of Izmir Bay Aegean Sea (Turkey). For the sorption studies, harvested fresh macro algae samples were rinsed with tap water, washed intensively with distilled water to remove the salts (sodium, potassium, magnesium and calcium) and then dried at 40 °C for 24 h. The dry biomass was grounded and sieved by a British standard mesh (<125 μm) sieve. The sieved biomass sample was then stored in polypropylene container in a desiccator.
Instrumentation
The surface of the biosorbent was examined, before and after the sorption procedure, by scanning electron microscopy (SEM) using the JEOL JSM 840A electron microscope equipped with an energy-dispersive X-ray (EDS) INCA micro-analytical system (operating conditions: accelerating voltage 20 kV, probe current 45 nA and counting time 60 s, with ZAF correction being provided on-line). A JEOL JEE-4X vacuum evaporator was used for coating of the samples with carbon before the examination. A Thermo Scientific Nicolet 6700 FT-IR spectrometer was applied to record the FT-IR spectra. The point of zero charge was also determined using a solution of KNO3 (pH region 2–11).
Uranium (VI) concentration was determined by UV–Vis spectrophotometer (Shimadzu UV–Vis 1601 Model). The biosorption experiments were carried out by batch technique using a thermostatic shaker bath GFL–1083 Model. WTW InoLab series pH–720 Model pH meter was used to adjust and measure the pH of samples. A Binder Model oven was used to dry the samples.
Biosorption experiments
The biosorption of uranium on the brown macro algae was investigated in batch experiments. The effects of the initial pH of the solution (pHinit), initial uranium concentration, biomass dosage, contact time and temperature were studied in order to investigate the removal process of uranium from aqueous solutions. 0.01 g of the biosorbent was contacted in polyethylene flasks in a shaking bath with 10 mL of uranium solution (C init 10–250 mg/L). After separation of the solid and liquid phase by filtration (125 mm Ø), the equilibrium pH was measured and the uranium concentration in the supernatant was photometrically determined using the TOPO-DBM (trioctyl phosphine oxide and di-benzoyl methane)-pyridine method at 405 nm [36]. The amount of sorbed uranium (VI) was calculated from the difference between the initial and the equilibrium concentration of uranium in aqueous solutions. The obtained data were used to calculate the quantity of uranium removed per unit mass of the sorbent in mg/g, as well as the biosorption percentage (%) and distribution coefficient (K d) (mL/g). The uranium uptake data were modeled using the Langmuir, Freundlich and Dubinin–Radushkevich isotherm equations whereas the kinetics data using the pseudo-first and pseudo-second order equations [13, 37–41].
The results were expressed as biosorption percentage (%) and distribution coefficient (K d) (mL/g), which were calculated by using the following equations:
where C i and C e are the initial and equilibrium concentrations of uranium (mg/L) in solutions, respectively. V is the volume of the aqueous phase (mL), and m is the weight of the biosorbent (g). All experiments were conducted in triplicates and the relative errors of the data were less than 5 %. Precipitation or sorption of uranium on the walls of polyethylene flask was found to be negligible as determined by running the blank experiments.
Results and discussion
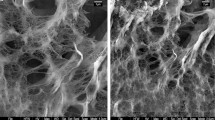
The determination of the point of zero charge (PZC) presented in Fig. 1, showed that the surface of the brown algae was negatively charged in the pH region 5.5–11 (−33 up to −12 mV), indicating its ability to adsorb cations. This is also in agreement with measurements appeared in the literature [35]. Figure 2 presents SEM microphotographs of the brown algae, where the characteristic flattened ultrastructure of cellulose microfibrils can be observed, exhibited random orientation [16]. According to EDS analysis the algae contains mainly oxygen (55–60 %), sulfur (~10 %), calcium (~10 %) as well as small quantities of metals (aluminum, silicon, iron, magnesium, potassium). The FT-IR spectrum of the brown algae is presented in Fig. 3a. According to the literature, the cell walls of Dictyopteris polypodioides, which are responsible for the sorption process, mainly contain cellulose, alginic acid and sulphated polysaccharides. The spectrum of Dictyopteris polypodioides was measured by a FT-IR spectrometer within the range of 550–4000 cm−1 wave number. In the recorded spectrum the band at 3301.6 cm−1 corresponds to O–H, the peaks at 1422.92 cm−1 and 1621.87 cm−1 correspond to asymmetric and symmetric carboxyl (–C=O) stretching groups respectively, whereas the peak at 1031.09 cm−1 indicates the presence of C-O group [16, 25]. In order to characterize the functional groups participating in biosorption, the FT-IR pattern of the U-loaded biomass was recorded and presented in the same figure (Fig. 3b). The comparison of unloaded and U-loaded FT-IR spectra illustrates that the peaks at 3301.60, 1621.87 and 1422.92 cm−1 are shifted to 3404.96, 1636.59 and 1424.86 cm−1, respectively. These shifts indicate that hydroxyl and carboxyl functional groups participate in biosorption process and ion exchange between uranium and hydrogen atom as well as complexation with carboxyl groups, is most probably the dominated mechanism of biosorption of uranium on brown algae. The carboxylic groups are generally the most abundant acidic functional groups in the brown algae and the removal capacity of the algae is directly related to the presence of these sites on the alginate polymer [19, 29].
Effect of pH and adsorbent dose
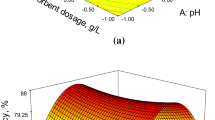
The uranium sorption is a complex phenomenon which is dependent on aqueous chemistry of the element as well as the properties of the sorbent (e.g. chemical composition, particle size). Among the important factors controlling the uranium uptake is the pH, determining its species available in solution and influencing the surface charge of the sorbent material, as well as its concentration. Generally, in acidic solutions the cationic species of uranium are dominant [12, 13]. It was observed that the biosortion process is dependent on the initial pH of the solution. The amount of metal ion adsorbed increased with increase of the pH value and the highest removal efficiency (98 %) was observed at the pH 6. In the acidic region, the metal ion uptake was inhibited and this can be attributed to the presence of H3O+ ions, competing with the uranium species for the same adsorption sites [12]. At low pH values, the uranium is present in the solution mainly in the form of free UO +22 ions, and it was competing with protons for the binding sites on the biomass whereas at higher pH values (around 6) formation of hydroxy-uranyl species is observed as it was revealed by the code MEDUSA for the investigated concentrations region [42]. The equilibrium pH (pHeq) also plays an important role because after the addition of the sorbent the pH is gradually altered. In our case the pHeq values found to be quite similar with the pHinit values. As can be seen in Fig. 4, the biosorption of uranium reaches the maximum value at pH 6 and then decreases to 79.6 % at pH 9. At higher pH values, the speciation of U(VI) is dominated by a series of strong aqueous carbonate complexes which increase the solubility of uranium at these environmental conditions. According to the result, pH 6 was found to be appropriate for maximum biosorption and used in all subsequent experiments. Similar results have been reported in literature for biosorption of uranium [24, 28, 29]. For example, uranium uptake of Bacillus mucilaginosus was studied at pH ranging from 2 to 7 and pH 5 was selected as the best value [24]. Furthermore Bozkurt et al. investigated the biosorption of uranium onto Ulva gigantean (Kutzing) bliding and observed that the adsorption increased with increasing pH from 2 to 3, remained stable until pH 6 and then decreased with increasing pH from 6 to 9 [28].
The influence of ratio solid/liquid phase on the uranium sorption on brown algae was studied, varying the amount of adsorbent from 0.5 to 20 g/L and keeping the other parameters (pH, concentration, and contact time) constant as it is presented in Fig. 5. It was observed that the percent removal of uranium increases from 87.3 to 94.5 % for adsorbent dose 0.5 and 5.0 g/L respectively, due to greater availability of the surface area at higher concentration of the adsorbent. Further increase of the adsorbent dose resulted in slight increase of the % uranium removal. This could be attributed to overlapping of the adsorption sites as a result of the excess number of adsorbent particles.
Effect of initial concentration
The effect of initial uranium solution concentration (in the range 10–250 mg/L) on biosorption was investigated and the results were showed in Fig. 6. From this figure it can be seen that the % removal decreased with the increase of the initial solution concentration. This is probably connected to the fact that at a fixed adsorbent dose, the number of active adsorption sites remained unchanged, while at higher solution concentrations, more adsorbate ions are existing and the removal efficiency is reduced from 100 % for the concentration of 25 mg/L to 89 % for the concentration of 250 mg/L.
The SEM images of the algae after interaction for 24 h with 50 mg/L uranium solution of initial pH 6 are shown in Fig. 7, whereas the presence of different elements as revealed by EDS analysis is given in Fig. 8. According to SEM/EDS examination, there was satisfying uranium distribution on the surface of the material as can be seen in Fig. 7b, c and its concentration was estimated to be between 6 and 20 % in different points. The uranium was uniformly distributed on the surface following the distribution of sulphur and calcium, whereas the distribution of oxygen and silicon is rather oriented as it is illustrated in Fig. 7d–f. The sulfonic acid groups play also a role in metal binding and the presence of other cations existing in the sorbent like Ca2+, K+, Na+ contributes to the ion exchange [7, 19, 29]. The SEM/EDS data in combination with the FT-IR data indicate that the uranium uptake is being mainly extracellular or occurring discretely at the cell wall, as result of different processes (e.g. adsorption, ion exchange, complexation).
The uranium uptake data were modeled using the Langmuir, Freundlich and Dubinin–Radushkevich isotherm equations and the results are presented in Tables 1 and 2, respectively. The determined maximum capacity (in mg/g) describes the total capacity of the biosorbent for uranium and the R L values were calculated within the range of 0–1 confirming the favorable character of the uptake of the uranium by brown algae. As seen from the Table 2, Freundlich model is very suitable for describing the biosorption equilibrium of uranium by the algae and indicates that sorption process occurs on heterogeneous surface.
The data of the maximum uptake capacity are reported in Table 2 and are comparable with uptake values available in the literature under experimental conditions similar to those adopted in this work. For example Aytas et al. have found uptake values up to 50 mg/g using a biocomposite absorbent of marine algae and yeast. Srivastava et al. have reported uptake values up to 75 mg/g using a biomass of plants and Zou et al. values up to 60 mg/g using a biosorbent of pine sawdust [21, 26, 29]. On the other hand, higher uptake values (300–500 mg/g) were reported by Ghasemi et al., Khani and Moghaddam et al., using treated algae biomass as biosorbents [16–18, 31].
Effect of contact time and temperature
The effect of contact time on uranium biosorption on algae was studied using solutions of initial concentration 50 mg/L at pHinit 6. Figure 9 shows the effect of contact time on the uptake for a time period up to 180 min at the temperatures 20, 30, 40 and 50 °C. It is obvious that the adsorption percentage increases slightly (from 95 at 20 °C to 97 at 50 °C) with the time and the state of equilibrium is reached faster when the temperature is increased (from 90 min at 20 °C to 15 min at 50 °C). This behavior usually occurs in the case of physical adsorption.
Kinetic modelling and thermodynamic parameters
To define the sorption kinetics of U(VI) onto the brown algae, the kinetic parameters of the sorption process by the pseudo-first order and pseudo-second order equations were investigated.
The pseudo-first order equation:
and the pseudo-second order equation:
where k 1 and k 2 are the rate constants for the pseudo-first and the pseudo-second order kinetic equations and q e and q t the uranium mass retained per weight unit of the sorbent at equilibrium and at time t, respectively. The parameters obtained by the fitting of all experimental data using the above mentioned kinetic equations are given in Table 3.
As it is shown in Fig. 10 and Table 3, the kinetics of the removal of uranium by brown algae seems to be described by the pseudo second order model, giving a straight line in the plot t/Q t for all investigated temperatures (20, 30, 40 and 50 °C). Constructing the plot of logk 2 as a function of 1/T it was possible to calculate the activation energy (E a) of the uptake process using the Arrhenius equation
where, k is the rate coefficient, A is a constant, E a is the activation energy, R is the universal gas constant and T is the temperature (in Kelvin). The obtained activation energy (E a) was 18.43 kJ/mol (R 2 = 0.953). According to the literature, the reactions controlled by diffusion exhibit activation energy values in the range of 5–20 kJ/mol [41].
Using the equations below, Gibbs free energy (ΔG°), entropy (ΔS°) and enthalpy (ΔH°) were also calculated:
The thermodynamic parameters given in Table 4 show that the process of biosorption is endothermic, as indicated by the positive ΔH° value and spontaneous in nature due to the negative ΔG° values. The positive value of ΔS° indicates increased randomness at the solid/solution interface during the biosorption of uranium onto brown algae. The magnitude of the sorption energy characterizes an ion exchange type reaction [11].
Conclusions
In this study, the biosorption of U(VI) ions was studied on Dictyopteris polypodioides (brown algae) using batch technique under different experimental conditions. Biosorption efficiency was found to reach the value of 94 %, under the optimized experimental conditions (pH 6, Ci: 50 mg U/L, contact time: 120 min, biomass dossage: 5 g/L, temperature: 303 K). The kinetic experiments were carried out at different temperatures and the results have shown that the sorption process fits well into a pseudo-second order mechanism. The present results demonstrate that the Freundlich and Langmuir model fit better than the Dubinin–Radushkevich model for the biosorption equilibrium data. Calculation of the thermodynamic parameters characterizing the biosorption process assumed variation of temperature. The positive value of ∆H° shows that the biosorption of uranium on Dictyopteris polypodioides is an endothermic process whereas the negative values for ΔG° suggest feasibility and spontaneous sorption. A rapid sorption in the initial stages of equilibration was observed and the calculated activation energy (18.4 kJ/mol) is characteristic of an ion exchange type adsorption but the overall observations suggest that the biosorption process was administrated by combination of several mechanisms, such as physical sorption, ion exchange and complexation. Based on this work, brown algae Dictyopteris polypodioides can effectively remove the uranium ions from dilute aqueous solutions and it is a suitable biosorbent with low cost, non-toxic, biocompatible, environmental friendly and biodegradable nature.
References
Toxicological profile for uranium (2011) U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp150.pdf. Accessed 19 Jan 2015
Donat R, Esen K, Cetisli H, Aytas S (2009) Adsorption of uranium (VI) onto Ulva sp.-sepiolite composite. J Radioanal Nucl Chem 279:253–261
Lawrence DG (2004) Uranium toxicity literature with commentaries, Department of Chemistry and Biochemistry, Long Island University, Brooklyn. http://myweb.brooklyn.liu.edu/lawrence/duproject/litsum3.pdf. Accessed 19 Jan 2015
Craig DK (2001) Chemical and radiological toxicity of uranium and its compounds, WSRC-TR-2001-00331, Westinghouse Savannah River Company, Aiken. http://www.osti.gov/bridge. Accessed 19 Jan 2015
U.S. E.P.A. (2000) National primary drinking water regulations, Radon-222, Federal Register/vol. 65, No. 236 Rules and Regulations. http://rais.ornl.gov/epa/fr07de00R.pdf. Accessed 19 Jan 2015
WHO (2012) Uranium in drinking-water. http://www.who.int/water_sanitation_health/publications/2012/background_uranium.pdf. Accessed 19 Jan 2015
Gavrilescu M, Pavel LV, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163:475–510
Yusan SD, Erenturk SA (2011) Sorption behaviors of uranium (VI) ions on α-FeOOH. Desalination 269(1–3):58–66
Buema G, Noli F, Misaelides P, Sutiman DM, Cretescu I, Hatja M (2014) Uranium removal from aqueous solutions by raw and modified thermal power plant ash. J Radioanal Nucl Chem 299:381–386
Nilchi A, Dehaghan TS, Garmarodi SR (2013) Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 321:67–71
Akkaya R (2013) Removal of radioactive elements from aqueous solutions by adsorption onto polyacrylamide-expanded perlite: equilibrium, kinetic, and thermodynamic study. Desalination 321:3–8
Bampaiti A, Misaelides P, Noli F (2015) Uranium removal from aqueous solutions using a raw and HDTMA-modified phillipsite-bearing tuff. J Radioanal Nucl Chem 303(3):2233–2241
Warchoł J, Matłok M, Misaelides P, Noli F, Zamboulis D, Godelitsas A (2012) Interaction of U VIaq with CHA-type zeolitic materials. Microporous Mesoporous Mater 153:63–69
Yusan S, Erenturk SA (2010) Adsorption equilibrium and kinetics of U(VI) on beta type of akaganeite. Desalination 263(1–3):233–239
Sarri S, Misaelides P, Papanikolaou M, Zamboulis D (2009) Uranium removal from acidic aqueous solutions by Saccharomyces cerevisiae, Debaryomyces hansenii, Kluyveromyces marxianus and Candida colliculosa. J Radioanal Nucl Chem 279:709–711
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SL (2011) Biosorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: breakthrough curves studies and modeling. J Hazard Mater 189:141–149
Bhat SV, Melo JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Khani MH (2011) Uranium biosorption by Padina sp. algae biomass: kinetics and thermodynamics. Environ Sci Pollut Res 18:1593–1605
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Gok C, Aytas S (2014) Chapter 16—biosorption of uranium and thorium by biopolymers. In: The role of colloidal systems in environmental protection, Vol. 16. Elsevier, Amsterdam, pp. 363–395
Aytas S, Turkozu DA, Gok C (2011) Biosorption of uranium(VI) by bi-functionalized low cost biocomposite adsorbent. Desalination 280:354–362
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Anirudhan TS, Rijith S (2012) Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J Environ Radioact 106:8–19
Cecal A, Humelnicu D, Rudic V, Cepoi L, Ganju D, Cojocari A (2012) Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour Technol 118:19–23
Zou W, Zhao L (2012) Removal of uranium (VI) from aqueous solution using citric acid modified pine sawdust: batch and column studies. J Radioanal Nucl Chem 292:585–595
Konstantinou M, Pashalidis I (2007) Adsorption of hexavalent uranium on biomass by-product. J Radioanal Nucl Chem 273(3):549–552
Yi Z, Lian B (2012) Adsorption of U(VI) by Bacillus mucilaginosus. J Radioanal Nucl Chem 293:321–329
Bozkurt SS, Molu ZB, Cavas L, Merdivan M (2011) Biosorption of uranium (VI) and thorium (IV) onto Ulva gigantean (Kützing) bliding: discussion of adsorption isotherms, kinetics and thermodynamic. J Radioanal Nucl Chem 288:867–874
Srivastava S, Bhainsa KC, D’Souza SF (2010) Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresour Technol 101:2573–2579
Prodromou M, Pashalidis I (2013) Uranium adsorption by non-treated and chemically modified cactus fibres in aqueous solutions. J Radioanal Nucl Chem 298:1587–1595
Moghaddam MR, Fatemi S, Keshtkar A (2013) Adsorption of lead (Pb2+) and uranium (UO2 2+) cations by brown algae; experimental and thermodynamic modeling. Chem Eng J 231:294–303
Pennesi C, Vegliò F, Totti C, Romagnoli T, Beolchini F (2012) Nonliving biomass of marine macrophytes as arsenic(V) biosorbents. J Appl Phycol 24:1495–1502
Aytas S, Gunduz E, Gok C (2014) Biosorption of Uranium Ions by Marine Macroalga Padina pavonia. Clean Soil Air Water 42(4):498–506
Singhal RK, Basu H, Pimple MV, Manisha V, Basan MKT, Reddy AVR (2013) Spectroscopic determination of U(VI) species sorbed by the Chlorella (Chlorella pyrenoidosa) fresh water algae. J Radioanal Nucl Chem 298:587–592
Sokolova RV, Ermakova SP, Awada SM, Zvyagintseva TN, Kanaan HM (2011) Composition, structural characteristics and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem Nat Compd 47(3):329–334
Francais CA (1958) Rapid spectrophotometric determination of submilligram quantities of uranium. Anal Chem 30:50–54
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I: solids. J Am Chem Soc 38:2221–2295
Freundlich HF (1906) Adsorption in solution. Phys Chem Soc 40:1361–1368
Lagergren S (1898) Zur theorie der sogenannten adsorption geloester stoffe. Handl 24(4):1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Ho YS, Ng JCY, McKay G (2001) Removal of lead(II) from effluents by sorption on peat using second-order kinetics. Sep Sci Technol 36(2):241–261
Puigdomènech (1983) INPUT, SED, and PREDOM: Computer programs drawing equilibrium diagrams, Report TRITA-OOK-3010, Royal Institute of Technology (KTH), Department of Inorganic Chemistry, Stockholm
Acknowledgments
The work for this paper was carried out at the Institute of Nuclear Sciences, Ege University, Bornova-Izmir, within the frame of ERASMUS Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bampaiti, A., Yusan, S., Aytas, S. et al. Investigation of uranium biosorption from aqueous solutions by Dictyopteris polypodioides brown algae. J Radioanal Nucl Chem 307, 1335–1343 (2016). https://doi.org/10.1007/s10967-015-4289-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4289-9