Abstract
The uranium contamination is a major environmental problem. Biosorption is a potentially important pathway for immobilization of uranyl cations (UO2 2+). This study investigated the potentiality of utilization of Bacillus mucilaginosus as a biosorbent for U(VI) removal from aqueous solutions. Batch experiments were conducted to examine U(VI) adsorption to B. mucilaginosus when pH, sorption time, reaction temperature, biosorbent dosage, initial U(VI) concentration were independently changed. The Freundlich and Langmuir adsorption models were used for the mathematical description of the adsorption equilibrium. The accumulation process was highly pH dependent within the pH range between 2.0–7.0. An initial solution pH of 5.5 was most favorable for U(VI) removal. Temperature over the range 25–45 °C had no effect on the U(VI) biosorption. The U(VI) uptake was rapid within the first 30 min and equilibrium was reached at 1 h. The U(VI) removal efficiency increased concomitantly with increasing biomass dosage, while the biosorption capacity decreased. The biomass had an observed maximum U(VI) biosorption capacity of 172 mg/g dry weight of biomass. The biosorption process could be well defined by Langmuir isotherms. The adsorption kinetics data were fitted very well by the pseudo first-order rate model. The X-ray photoelectron spectroscopy analysis confirmed that uranium in the solution was immobilized onto the biomass during the course of biosorption. The present results suggest that B. mucilaginosus can be used as a biosorbent for an efficient removal of U(VI) from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amounts of uranium-bearing wastewater are generated every year during the mining, extraction, and processing of uranium for nuclear fuel and weapons. In particular, radioactive uranium commonly as one of the major toxic contaminants released into the environment during nuclear-related activities, predominates in the hexavalent form ‘UO2 2+’, which is highly soluble and mobile under oxidizing conditions, thereby posing a great human health risk. Therefore, the elimination of uranium from wastewater is essential to uranium pollution control.
Traditional physical or chemical remediation approaches, such as lime neutralization, anion exchange and activated aluminum, are not only prohibitively expensive but can also be limited by poor extraction efficiency, inhibitory competing ions and massive waste production. Considering the high cost and technical limitations of conventional approaches, there is a great need for cost-effective alternatives to remove U(VI) contamination. It should be pointed out that adsorption as a promising innovative technology has been paid considerable attention in recent years. Numerous experimental investigations have been reported on the adsorption of U(VI) by minerals, vegetations, and microorganisms [1–12]. Especially, many researchers have been studying the biosorptive removal of U(VI) using various species of microorganisms, such as actinomycetes, bacteria, fungi, and yeasts [13–18].
Bacillus mucilaginosus, also called silicate bacteria, is a most common soil microorganism. Because it is capable of degrading insoluble soil mineral compounds (silicates, apatites, and phosphorites) with the release of mobile potassium and water-soluble phosphorous, plus its capability to fixing nitrogen, it is widely used as a bio-fertilizer stimulating plant growth. Much research has been conducted concerning this bacterium on potassium releasing from soil minerals as well as its application as a microbial fertilizer [19–24]. In recent years, it has been found that B. mucilaginosus can be employed in wastewater treatment to remove various contaminants [25–30]. Their investigations suggested that it was an excellent bacterium strain with good adsorption effect and application prospect in wastewater treatment. Most researchers deemed that B. mucilaginosus can secrete extracellular polysaccharides with particular chemical structures during their vital activity. These substances are always biologically active and have excellent flocculating activity. However, to the best of our knowledge, the biosorption of U(VI) by B. mucilaginosus has been rarely reported. The main focus of the present work was to investigate the potential ability of U(VI) biosorption by B. mucilaginosus. Of various factors affecting the adsorption, pH, contact time, temperature, initial U(VI) concentration and biosorbent dosage were examined. Moreover, equilibrium models were used to fit experimental data. The goal of this study is to evaluate the feasibility of its use as a U(VI) biosorbent.
Materials and methods
Microorganism, chemicals and U(VI) stock solution
The dry B. mucilaginosus Krassilnikov (strain no. ACCC10012) powder which contains ca. 1.2 × 108 living cells per gram dry weight was purchased from Baolvyuan Biotechnology Company, Beijing, China. The dry powder with size smaller than 200 mesh was selected for subsequent biosorption experiment without further treatment.
A stock solution of U(VI) (1 mg/mL) was prepared by dissolving U3O8 in a mixture of HCl, H2O2 and HNO3. The other concentrations were obtained from the stock solutions by appropriate dilution. The U3O8 was supplied by School of Nuclear Resources and Nuclear Fuel Engineering, University of South China. The other chemicals were purchased from Sinopharm Chemical Reagent Company, Shanghai, China. All chemicals used in this study were of analytical grade. All experimental solutions were prepared using distilled water.
Biosorption experiments
For all the biosorption experiments, 50 mL U(VI) solution was mixed with a known amount of dry B. mucilaginosus powder in a series of 250 mL conical flasks. The pH of the U(VI) solution was adjusted as required using 1.0 mol/L NaOH and 1.0 mol/L HNO3 before mixing with the biomass. Then the experiments continued on a rotary shaker (140 r/min). A sample of solution was withdrawn at suitable time intervals, centrifuged at 12,000×g for 15 min and U(VI) was determined in the supernatant. The U(VI) removal efficiency and adsorption capacity of U(VI) onto the biomass were obtained using the following equations:
where Q (mg/g) is the adsorption capacity of U(VI) onto the biomass, C 0 and C (mg/L) are the concentrations of the U(VI) in the solution before and after biosorption, respectively, V (L) is the volume of the aqueous solution and M (g) is the dry weight of the biosorbent. Uranium-free and biosorbent-free blanks were used as controls. Each of the experiment was repeated thrice and the average values were obtained. The U(VI) concentrations in samples were determined using a standard method given by Xie et al. [31].
The batch biosorption data were fitted with Langmuir and Freundlich isotherms. The relative parameters were worked out by linear regression analysis using Origin 8.0 software. The linearized Langmuir isotherm can be expressed as,
The linearized Freundlich model can be expressed as,
where Q eq (mg/g) denotes the equilibrium adsorption capacity, Q max (mg/g) the maximum monolayer adsorption capacity, C eq (mg/g) the residual U(VI) concentration in the solution at equilibrium, b (L/mg) the Langmuir constant related to the energy of adsorption, K F (mg/g) the Freundlich constant related to the adsorption capacity of biosorbent, n the Freundlich exponent related to adsorption intensity (dimensionless).
Kinetic modeling
In order to examine the kinetics of U(VI) uptake on B. mucilaginosus biomass, the pseudo first-, second-order kinetic models were used for analysis of adsorption kinetics. The pseudo first-order equation, based on solid capacity, is generally expressed as follows:
The integrated form of above equation becomes,
where Q eq and Q t (mg/g) are the amounts of adsorbed metal per unit cell mass at equilibrium and after a contact time t (min), respectively and k 1 (min−1) is the rate constant of pseudo first-order sorption. The value of the rate constant (k 1) and Q eq for the pseudo first-order sorption reaction can be obtained by plotting Q t versus t as well as further nonlinear regression analysis.
The pseudo second-order rate of Lagergren can be expressed as follows [8]:
The integrated linear form of Eq. 7 can be expressed as follows:
where k 2 (g/(mg min)) is the rate constant for the pseudo second-order sorption. The value of the rate constant (k 2) and Q eq for the pseudo second-order sorption reaction can be worked out by plotting t/Q t versus t as well as further linear regression analysis.
X-ray photoelectron spectroscopy analysis (XPS)
The sample of B. mucilaginosus biomass incubated with 100 mg/L U(VI) solution for 1 h was centrifuged (10,000 rpm, 10 min) to remove all supernatants. Then the precipitation was further dehydrated by vacuum drying. The dry and dehydrated samples before and after uranium biosorption were characterized using XPS system (Thermo ESCALAB 250, USA) with a monochromatic Al Kα X-ray beam (energy = 1486.5 eV and power = 150 W). The XPS spectra were recorded in the fixed analyzer transmission mode with a pass energy of 20 eV and with energy steps of 0.1 eV. This technique was employed to elucidate the surface characteristics. A description of this technique and its interpretation were reported by Briggs [32].
Results and discussion
Effect of pH
The solution pH is one of the most critical variable parameters controlling the adsorption process as it influences the dissociation state of biosorbent site as well as the water chemistry of soluble U(VI) cations [33]. In order to search for the optimum pH for the biosorption process as well as to find out whether the biomass was able to show a good U(VI) uptake at extreme pH values, metal uptake was studied at pH ranging from 2.0 to 7.0. As shown in Fig. 1, initial solution pH significantly affected the equilibrium U(VI) sorption capacity. Over the range tested, extreme acid condition (pH 2.0) did not favor biosorption of U(VI). As the pH increased, sorption of U(VI) increased and the maximum loading for U(VI) was observed at pH 5.5. An increase in pH beyond the optimum caused decline in sorption of U(VI).
The reduced sorption at low pH could be attributed to: (i) uranium mainly exists in the form of the simple uranyl cations (UO2 2+) in an acid medium, which have high solubility and are unreadily to be sequestrated; (ii) large quantities of protons compete with U(VI) cations for the adsorption sites resulting in the reduced uptake of U(VI) [5]. As the solution pH increases (up to 5.5), the formation of dominant monovalent hydrolyzed U(VI) species, such as UO2OH+, (UO2)3(OH) +5 reduced its solubility and prompted binding affinity towards the bacterial surface, hence adsorption capacity increases with increasing pH to a certain limit [34]. On the other side, along with the increasing pH, gradual dissociation of protons from functional groups on the cell wall make the adsorbent surface more negatively charged, therefore the adsorption of positively charged species is more favorable. At higher pH (pH ≥ 5.5), schoepite precipitation (4UO3·9H2O) occurs which decreases the dissolved U(VI) concentration in solution, and consequently leads to the reduced availability of U(VI) sorption onto the biomass [35]. Based on this result, all subsequent experiments were carried out at pH 5.5.
Effect of contact time and temperature
The contact time between the adsorbate and adsorbent is an important parameter for designed adsorption process. As shown in Fig. 2, the U(VI) removal efficiency increased with time elapsed at early stage. The rate of metal uptake during the entire period of biosorption was found to be independent of initial U(VI) concentrations used. A larger amount of U(VI) was removed rapidly in the first 30 min during which about 90% of the total U(VI) was removed. Subsequently the U(VI) sorption rate became slowly. The sorption process reached an equilibrium state within 60 min after which no more metal were adsorbed by the biomass. The above phenomena is probably due to the larger surface area of bacteria at the beginning of the adsorption and the exhaustion of surface adsorption sites at late stage. The fast biosorption kinetics observed initially is typical for biosorption process involving no energy-mediated reactions and metal removal from solution is due to purely physico-chemical interactions between biomass and metal solution. The incubation time of 1 h was adopted as the equilibrium time for all of the other experiments.
It appears that temperature affects biosorption of metal ions, yet this is only for energy dependent mechanisms. To investigate the effect of this parameter on the kinetics of U(VI) biosorption, the following four temperatures were chosen: 25, 35, 45 and 55 °C. As shown in Fig. 3, no significant difference in U(VI) uptake was detected. This phenomenon indicated that this process is temperature independent, which is consistent with previous result while studying uranium sorption by Pseudomonas sp. [36]. For many biosorption processes, metal uptake is influenced to a limited extent within a certain range of temperature because physico-chemical processes such as ion exchange exists largely in biosorption. Consequently, temperature was found to have minor effect on the accumulation of U(VI) in our experiment.
Effect of cell dosage
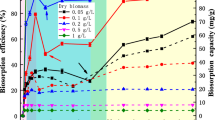
The percentage removal and adsorption capacity of U(VI) by B. mucilaginosus at different biosorbent dosages (0.2–2.0 g/L) for the initial U(VI) concentration 200 mg/L is shown in Fig. 4. It was found that the percentage of uranium removal increased concurrently with increasing biomass dosage. When the biosorbent dosage increased from 0.2 to 1.2 g/L, the percentage removal changed from 16.1 to 87.5%. This phenomena can be explained that the number of available adsorption sites rise accordingly, resulting in an increase of total adsorbed U(VI) concentration. Further increase in biomass dosage from 1.2 to 2.0 g/L was unable to produce significant U(VI) removal due to the low uranyl level in solution.
However, the adsorption capacity kept constant near a maximum value (ca. 172 mg/g dry weight) at a lower cell dosage ranging from 0.2 to 1.0 g/L (Fig. 4). This result could be due to the saturation of sorption sites in the bacterial surface. On the contrary, at a higher cell dosage greater than 1.0 g/L, the adsorption capacity decreased with increasing cell dosage (Fig. 4). This decrease in specific uptake values with increase in biomass dosage has been explained by other researchers hypothesizing that high biomass dosage causes formation of cell aggregates, thereby reducing the effective biosorption area [37], or an increase in biomass dosage leads to interference between binding sites [38]. Similar results on the influence of the biomass dosage on the U(VI) biosorption have been reported for other microorganisms.
Effect of initial uranium concentration
The percentage removal and equilibrium adsorption capacity at different U(VI) concentrations (50–400 mg/L) are presented in Fig. 5. When the initial U(VI) concentration increased from 25 to 150 mg/L, the percentage removal of U(VI) changed little keeping ca. 88%. However, the U(VI) removal efficiency decreased slightly at lower U(VI) concentrations less than 150 mg/L, whereas decreased sharply at higher U(VI) levels greater than 150 mg/L.
As shown in Fig. 5, the adsorption capacity of U(VI) by B. mucilaginosus increased with increasing U(VI) concentration. This increase may be due to higher probability of collision between the metal ions and biosorbent particles, which is driven by concentration gradients with the increase of the initial U(VI) concentration. In other words, the initial concentration gave a key driving force to conquer all mass transfer resistance of uranium between the liquid and solid phase. Therefore a relatively higher initial concentration of U(VI) will promote the adsorption process. When the U(VI) concentration was greater than 250 mg/L, the adsorption capacity changed little, reaching a maximum U(VI) uptake capacity ca. 172 mg/g. This phenomenon can be interpreted that the adsorption sites were saturate since only a finite number of surface adsorption sites were provided at higher U(VI) concentrations.
Adsorption isotherm
There are several isothermal equations used in the literature to describe the adsorption characteristics of an biosorbent. In this study, two typical equilibrium isotherms, the Langmuir and Freundlich models, were chosen to identify which isotherm could better describe the equilibrium adsorption of U(VI) onto the biomass. The Langmuir model presumes that the biosorption process occurs on a surface composed of a fixed number of adsorption sites of equal energy, with one molecule adsorbed per adsorption site until a monolayer coverage is obtained. Unlike the Langmuir isotherm, the Freundlich equation presumes a heterogeneous surface and deems that molecules binding on a surface site will affect the adjacent sites.
Figures 6 and 7 reflect the linearized Langmuir and Freundlich adsorption isotherms of U(VI) at different initial uranium concentrations as well as at different cell dosages, respectively. The calculated results of the Langmuir, Freundlich isotherm constants are given in Table 1. The correlation coefficient values of Langmuir isotherm (R 2 L = 0.997 at different initial uranium concentrations; R 2 L = 0.979 at different initial cell dosages) are nearer to 1.0 than those of Freundlich isotherm (R 2 F = 0.825 at different initial uranium concentrations; R 2 F = 0.417 at different initial cell dosages), so the former isotherm is more significant than the latter isotherm. This suggests that monolayer sorption is dominant for U(VI) sorbed on cells in this study.
The maximum uptake capacity Q max is an important parameter evaluating the ability of microbial biomass to accumulate metals from aqueous solutions. The Q max value for uranium uptake by B. mucilaginosus reported here was 172.7 ± 1.8 mg/g dry wt. Previous studies have reported on different biosorbents with different U(VI) adsorption capacities, such as 198.0 mg/g dry wt by Cystoseria indica algae [9], 124.0 mg/g dry wt by Synechococcus elongates [10] and 112.2 mg/g dry wt by Rhizopus arrhizus [12]. Hence, we can conclude that the U(VI) adsorption capacity of B. mucilaginosus is comparable with some biosorbents mentioned previously. In the case of Langmuir isotherm model, the higher value of Q max and b also suggested that B. mucilaginosus was effective for the removal of U(VI) from aqueous solution.
Kinetic modeling
Generally speaking, the reaction order of biosorption depend upon the characteristics of the metals as well as the nature of the sorption sites available on the biosorbent. Figures 8 and 9 show the plots of nonlinearized form of the pseudo first-order (Q t versus t) and linearized pseudo second-order (t/Q t versus t) kinetic models, respectively. The parameters, including adsorption kinetic constants, correlation coefficients and Q eq values, were obtained using linear or nonlinear regression analysis and presented in Table 2. Although the correlation coefficients of two models were both greater than 0.99 exhibiting good correlations, the first-order kinetic model fits the experimental data better than second-order kinetic model on the basis of correlation Q eq and rate constant k. On the one hand, the calculated Q eq2 values of second-order kinetic model (49.09 and 92.51 mg/L) did not give reasonable values, which were too greater compared with experimental Q exp values; whereas Q eq1 values of first-order kinetic model (45.78 and 87.39 mg/L) were much nearer to experimental Q exp values. In other words, the theoretical values of the pseudo first-order rate kinetics (Q eq1) are also consistent with the experimental ones. On the other side, taking rate constant k into consideration, the first-order kinetic model is also more applicable. The fact that the calculated k values of second-order kinetic model at different U(VI) concentrations without changing temperature varied greatly is obviously unreasonable (Table 2). The reason is that rate constant is only a function of temperature and should keep constant at a fixed temperature. In summary, U(VI) biosorption by B. mucilaginosus followed the pseudo first-order kinetic model. Our result differs from most previous reports on the U(VI) biosorption by other microorganisms, such as Aspergillus fumigatus, Trichoderma harzianum, Pseudomonas aeruginosa, which all agreed well with pseudo second-order reaction [12, 15, 39].
XPS evaluation
The XPS is basically a powerful surface analysis tool. In this study, the uranium phase associated with B. mucilaginosus after adsorption was analyzed by XPS. High-resolution U4f XPS spectra of the raw B. mucilaginosus sample as well as after U(VI) uptake are shown in Fig. 10. Compared with raw B. mucilaginosus sample, there are two new energy peaks for B. mucilaginosus after U(VI) uptake, at 382.0 and 392.8 eV appearing in the curves of binding energy, which correspond to those reported for 4f7/2 and 4f5/2 orbital binding energy respectively in uranium binding to oxygen-containing groups [40]. Table 3 listed the atomic concentrations of relevant chemical elements in B. mucilaginosus sample before and after U(VI) uptake according to the XPS spectra analysis. The XPS results showed that after the biosorption process, the biomass was loaded with uranium.
Additionally, after U(VI) uptake, there were almost no changes in the atomic concentration of in C, O, N, which belongs to the chemical composition of a bacterial cell. Nevertheless, significant changes in the atomic concentrations of K, P and Si were noted after U(VI) uptake. It was observed that element such as K, P and Si were simultaneously released into the bulk solution during U(VI) biosorption. This phenomenon can be explained that the experimental bacteria B. mucilaginosus itself is a microorganism capable of mobilizing phosphate and potassium. The fact that U(VI) binding to the bacterial surface accompanied by the release of K, P and Si implied the involvement of ion exchange mechanism in radionuclide uptake.
Conclusions
The U(VI) adsorption behavior by B. mucilaginosus was investigated. It was found that although temperature over the range 25–45 °C had no effect on the U(VI) biosorption, the sorption process was affected by experimental conditions such as pH, sorption time, biosorbent dosage, initial U(VI) concentration. The best pH for U(VI) adsorption is 5.0. The U(VI) uptake reached an equilibrium state after 1 h. The U(VI) removal efficiency increased concurrently with increasing biomass dosage, yet the adsorption capacity declined. The biomass had an observed maximum U(VI) adsorption capacity of 172 mg/g dry weight of biomass. The adsorption data fitted very well to Langmuir adsorption model as well as pseudo first-order model. The XPS spectrum verified that uranium was fixed onto the biomass during the course of biosorption. The results indicated that B. mucilaginosus can be applied as an effective biosorbent for U(VI) removal from aqueous solution.
References
Morrison SJ, Spangler RR, Tripathi VS (1995) Adsorption of uranium(VI) on amorphous ferric oxyhydroxide at high concentrations of dissolved carbon(IV) and sulfur(VI). J Contam Hydrol 17:333–346
Zhang XZ, Luo SG, Yang Q, Zhang HL, Li JY (1997) Accumulation of uranium at low concentration by the green alga Scenedesmus obliquus 34. J Appl Phycol 9:65–71
Liao XP, Lu ZB, Du X, Liu X, Shi B (2004) Collagen fiber immobilized Myrica rubra tannin and its adsorption to UO2 2+. Environ Sci Technol 38:324–328
Ohnuki T, Yoshida T, Ozakia T, Samadfam M, Kozai N, Yubuta K, Mitsugashira T, Kasama T, Arokiasamy FJ (2005) Interactions of uranium with bacteria and kaolinite clay. Chem Geol 220:237–243
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96:1241–1248
Bayramoglu G, Celik G, Arica MY (2006) Studies on accumulation of uranium by fungus Lentinus sajor-caju. J Hazard Mater 136:345–353
Chen BD, Zhu YG, Smith FA (2006) Effects of arbuscular mycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pteris vittata L.) from a uranium mining-impacted soil. Chemosphere 62:1464–1473
Bhat SV, Meloa JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Khani MH, Keshtkar AR, Ghanadi M, Pahlavanzadeh H (2008) Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J Hazard Mater 150:612–618
Acharya C, Joseph D, Apte SK (2009) Uranium sequestration by a marine cyanobacterium, Synechococcus elongatus strain BDU/75042. Bioresour Technol 100:2176–2181
Chabalala S, Chirwa EMN (2010) Removal of uranium(VI) under aerobic and anaerobic conditions using an indigenous mine consortium. Miner Eng 23:526–531
Wang JS, Hu XJ, Liu YG, Xie SB, Bao ZL (2010) Biosorption of uranium(VI) by immobilized Aspergillus fumigatus beads. J Environ Radioact 101:504–508
Li PF, Mao ZY, Rao XJ, Wang XM, Min MZ, Qiu LW, Liu ZL (2004) Biosorption of uranium by lake-harvested biomass from a cyanobacterium bloom. Bioresour Technol 94:193–195
Gorman LD, Elias PE, Fein JB (2005) Adsorption of aqueous uranyl complexes onto Bacillus subtilis cells. Environ Sci Technol 39:4906–4912
Akhtar K, Akhtar MW, Khalid AM (2007) Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res 41:1366–1378
Merroun ML, Selenska-Pobell S (2008) Bacterial interactions with uranium: an environmental perspective. J Contam Hydrol 102:285–295
Xie SB, Yang J, Chen C, Zhang XJ, Wang QL, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp., mechanism and chemical characterization. J Hazard Mater 163:65–72
Lian B, Souleimanov A, Zhou X, Smith DL (2002) In vitro induction of lipo-chitooligosaccharide production in Bradyrhizobium japonicum cultures by root extracts from non-leguminous plants. Microbiol Res 157:157–160
Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trail. Geoderma 25:155–166
Han HS, Lee KD (2005) Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res J Agric Biol Sci 1:176–180
Li X, Wu ZQ, Li WD, Yan RX, Li L, Li J, Li YH, Li MG (2007) Growth promoting effect of a transgenic Bacillus mucilaginosus on tobacco planting. Appl Microbiol Biotechnol 74:1120–1125
Chen S, Lian B, Liu CQ (2008) The role of a strain of Bacillus mucilaginosus on weathering of phosphorite rock under experimental conditions. Acta Miner Sin 28:77–83 (in Chinese with English abstract)
Basak BB, Biswas DR (2009) Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by Sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soils 317:235–255
Zhao HX, Lian B, Xie ZH, Chen Y, Zhu LJ (2008) Water quality analysis and microbial treatment of the colliery area of Kaili in Guizhou province China. Acta Miner Sin 28:71–76 (in Chinese with English abstract)
Deng SB, Bai RB, Hu XM, Luo Q (2003) Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl Microbiol Biotechnol 60:588–593
Chen Y, Lian B (2005) Study on the flocculability of chromium ion by Bcillus mucilaginosus GY03 strain. Pedosphere 15:225–231
Lian B, Chen Y, Zhao J, Teng HH, Zhu L, Yuan S (2008) Microbial flocculation by Bacillus mucilaginosus: applications and mechanisms. Bioresour Technol 99:4825–4831
Hao JC, Deng YN, Cao WC, Lian B, Liu CQ (2011) Removal of Fe3+ in simulated wastewater by Bacillus mucilaginosus, Asperillus niger, zeolite and their different combination. Chin J Environ Eng 5:1507–1512
Mo BB, Lian B (2011) Hg(II) adsorption by Bacillus mucilaginosus: mechanism and equilibrium parameters. World J Microbiol Biotechnol 27:1063–1070
Xie SB, Zhang C, Zhou XH, Yang J, Zhang XJ, Wang JS (2009) Removal of uranium(VI) from aqueous solution by adsorption of hematite. J Environ Radioact 100:162–166
Briggs D (1990) Applications of XPS in polymer technology. In: Briggs D, Seah MP (eds) Practical surface analysis—Auger and X-ray Photoelectron Spectroscopy, 2nd edn. Wiley Interscience, New York
Uslu G, Tanyol M (2006) Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead(II) and copper(II) ions onto Pseudomonas putida: effect of temperature. J Hazard Mater 135:87–93
Sar P, Kazy SK, D’Souza SF (2004) Radionuclide remediation using a bacterial biosorbent. Int Biodeterior Biodegrad 54:193–202
Saxena S, Prasad M, D’Souza SF (2006) Radionuclide sorption onto low-cost mineral adsorbent. Ind Eng Chem Res 45:9122–9128
Marques AM, Roca X, Simon-Pujol MD, Fuste MC, Congregado F (1991) Uranium accumulation by Pseudomonas sp. EPS-5028. Appl Microbiol Biotechnol 35:406–410
Aksu Z (2001) Equilibrium and kinetic modeling of cadmium(II) biosorption by C. vulgaris in a batch system, effect of temperature. Sep Purif Technol 21:285–294
Gadd GM, White C (1989) Removal of thorium from simulated acid process streams by fungal biomass. Biotechnol Bioeng 33:592–597
Kazy SK, Sar P, D’Souza SF (2008) Studies on uranium removal by the extracellular polysaccharide of a Pseudomonas aeruginosa strain. Bioremediat J 12:47–57
Nancharaiah YV, Joshi HM, Mohan TVK, Venugopalan VP, Narasimhan SV (2006) Aerobic granular biomass: a novel biomaterial for efficient uranium removal. Curr Sci 91:503–509
Acknowledgments
This work was supported by National Science Fund for Creative Research Groups (Grant no. 41021062) as well as National Natural Science Foundation of China (Grant no. 40773069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, Z., Lian, B. Adsorption of U(VI) by Bacillus mucilaginosus . J Radioanal Nucl Chem 293, 321–329 (2012). https://doi.org/10.1007/s10967-012-1702-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1702-5