Abstract
In this work, sorption of cadmium from aqueous solution using a novel sorbent, Hypnea musciformis, was carried out. In order to optimize the operating variables namely, pH (3–6), sorbent dosage (0.5–2.5 g/L) and agitation speed (60–100 rpm), Box–Behnken Design was employed. The optimum conditions for the maximum cadmium percentage removal was 4.6, 1.67 g/L and 81 rpm for pH, sorbent dosage and agitation speed, respectively. At the optimal operating conditions, studies were carried out for effects with respect to various concentrations of cadmium and temperature for isotherm, kinetics and thermodynamic studies. The data obtained have been analysed by isotherm models such as Langmuir, Freundlich, Dubinin-Radushkevich and Temkin isotherms. The most fitted equation for describing the isotherm profile was the Langmuir model (R2 = 0.9990) with the maximum sorption capacity of 105.49 mg/g. The kinetics studies exposed that the process of sorption of cadmium onto red algae has been satisfactorily described for pseudo-second order. The calculated thermodynamic parameters such as ∆G ̊, ∆H ̊ and ∆S ̊ showed that the sorption of cadmium onto red algae was feasible, spontaneous and endothermic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium, one of the high priority toxic heavy metal responsible for polluting the ecosystems with the maximum potential threat to humans and the environment. Cadmium has been incorporated in the red list of priority pollutants by Department of Environment, UK (UK Red List Substances 1991) and in List I (the ‘‘black list”) of Directive 76/464/EEC Council directive 1976). USEPA has also classified cadmium as group B1 carcinogen (USEPA 1999). The industries dealing with electroplating, smelting, paint pigments, batteries, fertilizers, alloy and mining industries (Iqbal and Edyvean 2005) are the major sources of cadmium into the environment through wastewater streams. Cadmium is non-biodegradable (Wu et al. 2012), toxic even at low concentration and enters the food chain (Da Silva et al. 2003) and causes serious human diseases and disorders. The cadmium exposure leads to adverse health effects to human viz. renal dysfunction, liver damage, bone degradation, hypertension and can disrupt protein metabolism (Bernard 2008; Nordberg et al. 1993; Vimala and Das 2011).
Sorption of heavy metals from aqueous solutions has been assured as a very sanguine process in the removal of heavy metal contaminants. Also it proved to be an efficient, competitive, clean and inexpensive technology for the treatment of low-concentration effluents (Volesky et al. 1999; Pagnanelli et al. 2002). The sorption process gives rise to certain potential advantages over conventional treatment methods include : low operating cost, minimization of chemical or biological sludge, highly effective efficiency of heavy metal removal from diluted solutions, regeneration of sorbents, possibility of metal recovery and being eco-friendly (Ahluwalia and Goyal 2007). Lot of research has been undertaken for developing and employing cost effective sorbents for the treatment of wastewaters carrying heavy metal cadmium (Aksu 2001). In this study, a novel sorbent is employed for the removal of Cadmium from aqueous solution. The process variables were optimized using RSM and at the optimized conditions equilibrium, kinetic and thermodynamic studies were carried for the sorption process.
Materials and methods
Preparation of sorbent
The fresh red algal, Hypnea musciformis, was collected from the coats of Mandapam, Ramanathapuram district, Tamilnadu, India. It was washed thoroughly with distilled water to remove sand, salts and other impurities adhered. Then the sorbent was sun dried and further dried in oven at 50 °C for 48 h to remove the moisture. The dried sorbent was then ground using domestic mixer. The particle size of the ground sorbent was determined using Zetasizer (6.12), Nano series, Malvern instruments Ltd, UK. The mean particle diameter of the ground sorbent was found to be 1503 nm.
Preparation of metal ion solution
The stock solution of cadmium (II) of 1000 ppm concentration was prepared by dissolving 2.1 g of analytical grade cadmium nitrate in 1 L of distilled water.
Characterization of sorbent
Scanning electron microscopy (SEM)
The surface morphology of red alage was assessed by scanning electron microscope (JSM-5610 LV, JEOL, USA), with voltage of 10 KV and at high vacuum mode.
Fourier transform infrared (FTIR) spectroscopy
FTIR was used to determine the presence of functional groups on the sorbent surface for both raw and cadmium loaded sorbent. FTIR analyses were conducted by KBr pellets method using FTIR spectrophotometer (Thermo scientific- Nicolet iS5 FTIR, USA) in the wave number region of 4000–400/cm−1 .
BET
The surface area, average pore diameter and pore volume were determined by Brunauer Emmett and Teller method using Micrometrics ASAP 2020 and the observed values are 0.8576 m2/g, 269.24 Ả and 0.0057 cm3/g respectively. Nitrogen was used as adsorptive agent for the algal sample of 0.625 g.
Experimental design
Box- behnken design (BBD) was followed for optimizing the sorption of cadmium by red algae Hypnea musciformis. Batch experiments were conducted base on BBD, to study the effects of three variables including pH, sorbent dosage (g/L) and agitation speed (rpm) on percentage removal of cadmium. The range of independent variables along with the experimental design were given in Table 1. A statistical program package (design expert 7.1.5) was used for regression analysis of the data obtained and to estimate the coefficient of the regression equation. The equation was validated by ANOVA. At the optimal operating conditions, experiments were performed at various concentrations of cadmium and temperature for isotherm, kinetics and thermodynamic studies.
Sorption studies procedure
Based on Box Behnken design (BBD), experiments were conducted in 250 ml Erlenmeyer flasks containing 100 ml of 100 ppm Cadmium solution. The solution was agitated in incubated orbital shaker (Labline instruments, India) for a period of 60 min and 30 °C in order to study the effects of pH, sorbent dosage and agitation speed. After 1 h of agitation, the algae was separated from the aqueous experimental sample solution using whatman filter paper (No. 41). The presence of cadmium concentration in the aqueous solution was analyzed by using ICP-AES (Perkin Elmer, Germany). The percentage of cadmium removal was calculated as
where Co (mg/L) is the initial concentration of cadmium and Ce (mg/L) is the final or equilibrium concentration of cadmium. The amount of adsorbed cadmium per unit mass of adsorbent (qe, mg/ g) was determined according to equation:
Where v is the volume of solution in liter and m is the amount of sorbent in gram.
Sorption and desorption procedure
For desorption studies, 10 mL of 0.1–0.5 M HCl concentration was utilized for the sorption and desorption experiments for ten cycles. One cycle consists of sorption followed by desorption. For the subsequent use of sorbent, they were washed with excess of HCl solution and distilled water, sequentially.
Results and discussion
Box–Behnken design was carried out for studying the effects of 3 independent variables and the results obtained from the BBD experiments, along with the theoretically predicted responses were shown in Table 1. The quadratic regression model was used to explain the mathematical relationship between the independent variables and the dependent responses for cadmium removal in terms of coded factor. It is:
where, Y is the percentage removal of cadmium and A, B and C are coded values of pH, sorbent dosage(g/L) and agitation speed (rpm), respectively.
The ANOVA results for cadmium removal were shown in Table 2. The values of “probability > F” less than 0.05 indicate that the model terms are significant. In this study A, B, AC, A2 and B2 were significant model terms. This implies that the linear and square effects of pH and sorbent dosage were more significant factors. The interactive effect of pH and agitation speed was also significant. The goodness of fit of the model was checked by coefficient of determination (R2). For a good statistical model R2 value should be closed to 1. In our model, R2 was found to be 0.9852, which implies that more than 98% of experimental value was compatible with the predicted value. Adeq precision greater than 4 indicating an adequate signal. The “predicted R2 “of 0.763 was found to be in reasonable agreement with “adjusted R2” of 0.9585.
The interactive effects of operating variables on cadmium removal efficiency are illustrated in three dimensional response surface plots (Fig. 1a, b). The interactive effect of pH and sorbent dosage on the cadmium removal efficiency was studied in the pH range of 3–6 and the results are shown in the Fig. 1a. From the plot, it was observed that the sorption efficiency increases with the increase in pH from 3 to 4.6. This is due to the fact that, as the number of negatively charged sites increased due to deprotonation of the hydrogen ions from the functional group present on the sorbent. At pH 4.6, a maximum cadmium removal efficiency of 84.5% was achieved. However at solution pH less than 3 (highly acidic), the removal efficiency of cadmium was found to be less than 45%. This is because of the competition between hydronium ions (H3O+) and cadmium ions to the few negatively charged sites of the sorbent. Further increase in pH beyond 4.6, the sorption efficiency of cadmium decreases due to the precipitation of cadmium as hydroxide or in the form of Cd (OH)2. Therefore, the pH is pegged at 4.6 for further experimental studies. It can be inferred from the results that the solution pH was one of the most important factor which influences metal sorption and also the surface properties of the sorbent. Similar observations were reported for red sorbent (Wael and Ibrahim 2011).
The effect of sorbent dosage on the cadmium removal efficiency was studied in the range of 0.5–2.5 g/L. The results are shown in Fig. 1a. From the plot, the maximum removal efficiency was observed at 1.67 g/L. The sorption of metal efficiency increases with increase in sorbent from 0.5 to 1.67 g/L and decreases with further increase in sorbent dosage. Partial aggregation of the sorbent which minimizes the effective surface area could be the reason for the decrease in cadmium removal efficiency at higher sorbent dosage (Karthikeyan et al. 2007). Therefore, the optimum dosage of sorbent is 1.67 g/L for further experimental studies.
The effect of agitation speed was studied by changing the agitation speed in the range of 60 to 100 rpm. The results obtained are shown in Fig. 1b. From the plot, it was inferred that maximum sorption of cadmium occurred at 81 rpm. The sorbent accumulates at the bottom of the flask instead of spreading in the sample solution, at low agitation speed. As a result, the lower layer wraps up the active sites of the sorbent within that and hence they are not exposed to the phenomenon of cadmium ion removal while the upper layer active sites of sorbent adsorb the cadmium ion. At higher agitation speed, cadmium removal efficiency decreases, may be due to the desorption tendency of sorbent (Saha and Datta 2009).
Sorption isotherm study
The sorption capacity of a sorbent can be described by equilibrium sorption isotherm, which is characterized by definite constants whose values express the surface properties and affinity of the sorbent. In this study, four sorption isotherm models namely Langmuir, Freundlich, Dubinin-Radushkevich and Temkin were selected to evaluate the batch experimental data.
Langmuir isotherm
The Langmuir isotherm assumes that sorption occurs at specific homogenous sites on the sorbent and is used successfully in many monolayer sorption process. The linear form of Langmuir isotherm (Liu and Liu 2008) is expressed by
where qe is the equilibrium concentration of the metal on the sorbent (mg/g), qmax is the monolayer sorption capacity (mg/g), Ce is the equilibrium concentration of the metal in the solution (mg/ L) and kL is the Langmuir sorption constant (L/mg) related to the free energy of sorption. The plots of \(\frac{1}{{{\text{qe}}}}\) versus \(\frac{1}{{{\text{Ce}}}}\) were drawn as shown in Fig. 2a for six different initial metal concentration at constant temperature. In general, good sorbents have high qmax and a high correlation co-efficient (R2). The values of qmax, R2,and b are tabulated in Table 3. The high correlation co-efficient (R2 = 0.994) indicates that the sorption of the cadmium metal on to the red algae Hypnea musciformis, best suits the Langmuir model. Table 4 presents the comparison of sorption capacity (qmax, mg/g) of various red algae for cadmium.
Freundlich isotherm
The Freundlich isotherm is an empirical equation used to describe the adsorption on heterogenous surfaces (Freundlich 1906) and its linear form is represented by:
where Kf is the Freundlich isotherm indicating adsorption capacity and \(\frac{1}{n}\) is the sorption intensity. The values of Kf and \(\frac{1}{n}\) were calculated from the intercept and slope of the plot between log qe versus log Ce (Fig. 2(b)). The values of Kf ,\(\frac{1}{n}\) and the correlation coefficient (R2) are given in Table 3. Generally, \(\frac{1}{n}\) values between 0 and 1 indicate good sorption (Vasudevan et al. 2009). In this study, \(\frac{1}{n}~\)= 0.336 indicates that the sorption of cadmium onto the red algae was favorable. However, compared to the R2 values, the Langmuir isotherm equation fits the experimental values better than the Freundlich isotherm equation.
Dubinin-Radushkevich (D-R) isotherm
D-R isotherm was applied to determine the nature of sorption processes as physical or chemical using the experimental data and it’s linear form (Dubnin 1960) is :
where, qe is the amount of metal ions adsorbed on per unit weight of algae (mg/g), qm is the maximum sorption capacity (mg/g), β is the activity co- efficient related to mean sorption energy (mol2/ kJ2) and ε is the Polanyi potential described as
where, R is the universal gas constant 8.314 × 10−3 in kJ/mol K, T is the temperature in Kelvin and Ce is the equilibrium concentration of the cadmium in solution (mg/L).
The mean free energy of sorption per molecule of sorbate required to transfer one mole of ion from the infinity in the solution to the surface of sorbent and can be determined by the equation:
Dubinin- Radushkevich (D-R) isotherm constant β, and qm were evaluated from the slope and intercept of plot lnqe versus ε2 (Fig. 2c) and the results are presented in Table 3. The energy value, E = 0.61 kJ/mol, obtained is less than 8 kJ/mol indicates that the adsorption is a physical process. Also the sorption capacity was lower than the Langmuir model.
Temkin isotherm
The Temkin isotherm equation assumes that the heat of absorption of all the molecules in layer decreases linearly with coverage due to adsorbent–adsorbate interactions, and that the adsorption is characterized by a uniform distribution of the bonding energies, up to some maximum binding energy (Temkin and Pyzhev 1940). The Temkin isotherm is represented by the following equation:
Equation (9) can be arranged linearly as
where, T is the temperature in Kelvin, R is the Universal gas constant in kJ/mol K, KT is the equilibrium binding constant in L/mg, b is the variation of sorption energy in J/mol.
\(B{\text{ }}=\frac{{RT}}{b}\), is the Temkin constant related to the heat of sorption.
Figure 2(d) shows the plot of qe versus ln Ce. The isotherm constants were found and given in Table 3. The correlation factors show that the Langmuir model approximation to the experimental values was better than the Temkin model. Consequently, among the four isotherm modes used, the Langmuir model offers the best correlation factor.
Effect of temperature and thermodynamic study
The effect of temperature on the sorption of cadmium ions was studied in the temperature range of 298.15–313.15 K. An increase in temperature from 298.15 to 313.15 K increases the uptake capacity from 51.69 to 57.62 mg/g of cadmium by red algae. This effect is due to the rate of sorbate diffusion across the external boundary layer and in the internal pores of the sorbent particles because, liquid viscosity decreases as the temperature increases. Thermodynamic behavior of the sorption of cadmium onto red algae were calculated from following equation:
Where, R is the universal gas constant (8.314 × 10−3 kJ/mol K), T is the temperature in Kelvin and k is the equilibrium constant, calculated as the surface and solution metal distribution ratio (k = qe/Ce) (Aravindhan et al. 2007), ∆Gº is the Gibbs free energy (kJ/mol), ∆H ͦ is the enthalpy change (kJ/mol) and ∆Sͦ is the entropy change (kJ/mol K).
The thermodynamic feasibility and the nature of the sorption process can be described from the Eq. (15). The intercept and slope of the plot ∆Gº versus T, gives the values of ∆Hº and ∆Sº respectively, for the adsorption process of cadmium using red algae Hypnea musciformis. The calculated thermodynamic parameters are presented in Table 5. The positive values of ∆Hº suggest the endothermic nature of the sorption and the positive values of ∆S ͦ show the increasing randomness at the solid-solution interface during the sorption process (Li et al. 2009). The negative values of ∆G ͦ indicate the spontaneous nature of the sorption process and the higher negative value reflects a more energetically favorable adsorption (Chegrouche et al. 2009).
Sorption kinetics
The sorption kinetics behavior of cadmium ions onto red algae was analyzed to examine the mechanism of the sorption process and to test the experimental data, by adopting three kinetic models: pseudo first order, pseudo second order and intra-particle diffusion. Kinetic data are often used for the scale-up of sorption system.
Pseudo first order model
The linear form of pseudo first order model (Ho 1999) as described by Lagergren is expressed as
Where \({K_1}\) is the rate constant of the first order equation (/min),\({q_t}\) and \({q_e}\)are the amount of the cadmium ions sorbed at given time and equilibrium (mg/g) respectively and t is the time in minutes. The values of K1 and qe were calculated from the slope and intercept, respectively of the plot log (qe– qt) versus t, as shown in Fig. 3a. The values of qe, K1, and the correlation coefficient (R2) are given in Table 6.
Pseudo second order model
Linear form of pseudo second order can be expressed as (Ho and McKay 1999) ;
where \({K_2}\)is the rate constant of second order equation (g/mg min), \({q_t}\) and \({q_e}\) are the amount of the cadmium ions sorbed at given time and equilibrium (mg/g) respectively and t is the time in min. The values of K2 and qe were calculated from the slope and intercept, respectively of the plot \(\frac{t}{{{q_t}}}\) versus t, as shown in Fig. 3 b. The values of qe, K2, and the correlation coefficient (R2) are given in Table 6. When compared with the correlation coefficients, R2 from the Table 6, it is observed that pseudo second order model fits with the experimental data better than the pseudo first order.
Intra particle diffusion model
Intra particle diffusion model, described by Weber and Morris (1963), is tested for the diffusion mechanism and it is given by:
where Kid is the intra particle diffusion rate constant in mg/g/min and C is the intercept which gives intra particle accumulation in the boundary layer.
The values of Kid and t0.5 can be calculated from the plot qt versus t0.5 as shown in Fig. 3 c and reported in Table 6. Results clearly suggest that intra particle diffusion was not only the sole rate controlling factor. However, based on the results from the table pseudo second order model has higher correlation coefficient value indicating that the sorption of cadmium on the red algae follows pseudo second order kinetic model, suggesting chemisorption mechanism. Higher values of R2 show a better fitness of the sorption data (Deng et al. 2007).
Desorption Studies
Desorption of cadmium ion from the Cd loaded red algae was studied using HCl and the results were sown in Fig. 4. From the results, it was inferred that increase in hydrochloric acid concentration upto 0.3 M increases the desorption rate and then became stable. A maximum percentage recovery of 98.62% Cd was achieved with 0.3M HCL solution. The Cd ions adsorbed onto the sorbents were eluted with 0.3M HCL for the resuse of red algae. From the results it was observed that, in the first cycle, a maximum of 98.62% of the adsorbed Cd ions was desorbed from the sorbents. As the number of cycle increases the Cd sorption decreased to 81.55% at the end of tenth cycle. This loss may be because of the amount of sorbent lost during the sorption–desorption process. The results are in well agreement with the literatures (Jayakumar et al. 2015a, b). The results indicate that the red algae can be utilized repeatedly for sorption process with little loss in their initial adsorption capacities.
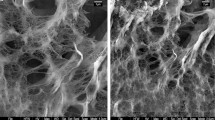
Scanning electron microscope analysis
The morphological changes of sorbent surface before and after cadmium loading was analyzed by the electron microscopic images. Red algae surface cells before cadmium loading were rough and rigid as shown in Fig. 5 a. After cadmium loading the surface becomes smooth and swollen, which can be observed from the Fig. 5b. This confirms the sorption of cadmium onto the surface of the sorbent.
Fourier transform infrared spectrometer (FTIR)
FT-IR spectrum identifies the functional group present in the uptake of cadmium onto red sorbent. Figure 6a, b shows the FTIR spectra of fresh and cadmium loaded red algae in the range of 4000−400/cm. The strong band shift from 3424.05 to 3432.75 cm− 1 corresponds to bonded hydroxyl (0-H) group (Su et al. 2008). The peak shift from 2920.20 to 2926.28/cm can be assigned to C-H stretching of aliphatic group (Vaghetti et al. 2009). Band shift from 1647.33 to 1635.79/cm can be ascribed to C=O stretching of carboxyl group (Sawant et al. 2011). A slight change in the wave number from 1384.17 to 1384.58/cm can be attributed to O–H bending of the phenolic & carboxylic group (Centeno and Shamir 2008). Peak change from 1073.22 to 1032.08 can be assigned to C–O stretching of carboxylic group (Martin et al. 2005). The infrared spectral wavelengths of each sorption peak and the corresponding functional groups were shown in Table 7. Therefore, observations from the FTIR analysis clearly shown that the important functional groups such as hydroxyl, carboxylic, C=O and C–O groups were responsible for the binding of cadmium heavy metal on to the red algae.
Conclusions
In an attempt to explain the effect of main operating parameters such as pH, adsorbent dose and agitation speed and their interactions on the phenomenon of removal of cadmium, the present study of RSM, based on the three-factor-three-level BBD was put into use as a tool for the experimental design. The outcome of RSM indicated that the cadmium removal efficiency was significantly affected by all the variables. The chosen red algae is found to be highly potential and most suitable for an efficient removal of cadmium from solution. According to the ANOVA results, the model presents a high R2 value (0.97) for cadmium removal efficiency. At optimum initial cadmium concentration (100 mg /L), pH (4.6), agitation speed (81 rpm), and sorbent dosage (1.67 g /L), a maximum removal efficiency of 92.65% was achieved. Sorption of Cd was analyzed using Langmuir, Freundlich, Dubinin-Radushkevich,Temkin, isotherm models and found that the Langmuir model presented a better fit. SEM confirmed the presence of Cd ions on the sorbent surface. Temperature affects the sorption process and the thermodynamic parameters show the spontaneous character of the sorption reaction. From the kinetics study, it was found that the sorption follows pseudo second order and intra particle diffusion models. The reusability of the sorbent was good after ten consecutive adsorption–desorption cycles without any considerable loss in sorption capacity.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium(II) biosorption by C. Vulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294
Aravindhan R, Rao JR, Nair BU (2007) Removal of basic yellow dye from aqueous solution by sorption on green alga Caulerpa scalpelliformis. J Hazard Mater 142:68–76
Aravindhan R, Bhaswant M, Sreeram KJ, Raghava Rao J, Unni Nair B (2010) Biosorption of cadmium metal ion from simulated wastewaters using Hypnea valentiae biomass: a kinetic and thermodynamic study. Bioresour Technol 101:1466–1470
Bernard A (2008) Cadmium & its adverse effects on human health. Indian J Med Res 128: 557–564
Centeno SA, Shamir J (2008) Surface enhanced Raman scattering (SERS) and FTIR characterization of the sepia melanin pigment used in works of art. J Mol Struct 873:149–159
Chaisuksant Y (2003) Biosorption of cadmium(II) and copper(II) by pretreated biomass of marine alga Gracilaria fisheri. Environ Technol 24:1501–1508
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Council Directive (1976) 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community
Da Silva JB, Borges DL, Da Veiga MA, Curtius AJ, Welz B (2003) Determination of cadmium in biological samples solubilized with tetramethyl ammonium hydroxide by electro-thermal atomic absorption spectrometry, using ruthenium as permanent modifier. Talanta 60:977–982
Deng L, Su Y, Su H, Wang X, Zhu X (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–266
Freundlich HMF (1906) Over the adsorption in solution. J Physical Chem 57:385–470
Hashim MA, Chu KH (2004) Biosorption of cadmium by brown, green, and red seaweeds. Chem Eng J 97:249–255
Herrero R, Lodeiro P, Rojo R, Ciorba A, Rodriguez P, de Vicente MES (2008) The efficiency of the red alga Mastocarpus stellatus for remediation of Cadmium pollution. Bioresour Technol 99:4138–4146
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater 192:1827–1835
Iqbal M, Edyvean RGJ (2005) Loofah sponge immobilized fungal biosorbent: a robust system for cadmium and other dissolved metal removal from aqueous solution. Chemosphere 61:510–518
Jayakumar R, Rajasimman M, Karthikeyan C (2015a) Sorption and desorption of hexavalent chromium using a novel brown marine algae Sargassum myriocystum. Korean. J Chem Eng 32:2031–2046
Jayakumar R, Rajasimman M, Karthikeyan C (2015b) Optimization, equilibrium, kinetic, thermodynamic and desorption on the sorption of Cu (II) from an aqueous solution using marine greenalgae: Halimeda gracilis. Ecotox Environ Safe 121:199–210
Karthikeyan S, Balasubramanian R. Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455
Li K, Zheng Z, Huang X, Zhao G, Feng J, Zhang J (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. J Hazard Mater 166:213–220
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242
Martín JA, Solla A, Coimbra MA, Gil L (2005) Metabolic distinction of Ulmus minor xylem tissues after inoculation with Ophiostoma novo-ulmi. Phytochemistry 66:2458–2467
Nordberg GF, Herber RFM, Alessio L (1993) Cadmium in the human environment: toxicity and carcinogenicity. IARC Scientific Publications, Lyon
Pagnanelli F. Esposito A, Veglio F (2002) Multi-metallic modelling for biosorption of binary systems. Water Res 36:4095–4105
Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98:3344–3353
Saha P, Datta S (2009) Assessment on thermodynamics and kinetics parameters on reduction of methylene blue dye using flyash. Desalin Water Treat 12:1–3
Sari A, Tuzen M (2008) Biosorption of cadmium(II) from aqueous solution by red algae Ceramium (virgatum):Equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157:448–454
Sawant SD, Baravkar AA, Kale RN (2011) FT-IR spectroscopy: Principle, technique and mathematics. Int J Pharma Bio Sci 2:513–519
Sheng PX, Ting Y-P, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by Marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Coll Interface Sci 275:131–141
Su H, Li J, Tan T (2008) Adsorption mechanism for imprinted ion (Ni2+) of the surface molecular imprinting adsorbent (SMIA). Biochem Eng J 39:503–509
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted Iron catalysts. Acta Physio Chem URSS 12:217–256
UK Red List Substances (1991) Environmental Protection (Prescribed Processes and Substances) Regulations, 1991 (SI 1991/472)
US Environmental Protection Agency (1999) Integrated risk information system (IRIS) on cadmium, national centre for environmental assessment. Office of Research and Development, Washington, DC
Vaghetti JCP, Lima EC, Royer B, da Cunha BM, Cardoso NF, Brasil JL, Dias SLP (2009) Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II)from aqueous solutions. J Hazard Mater 162:270–280
Vasudevan S, Jayaraj J, Lakshmi J, Sozhan G (2009) Removal of iron from drinking water by electro coagulation: adsorption and kinetics studies. Korean J Chem Eng 26:1058–1064
Vimala R, Das N (2011) Mechanisms of Cd (II) adsorption by macrofungus Pleurotusplatypus. J Environ Sci 23:288–293
Volesky B (1999) Biosorption for the next century. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment toward the mining of the 21st century (part B): international biohydrometallurgy symposium-proceedings. Elsevier, Amsterdam, pp 161–170
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div Am Soc Civil Eng 89:31–60
Wu P, Li C, Chen J, Zheng C, Hou X (2012) Determination of cadmium in biological samples: an update from 2006 to 2011. Appl Spectrosc Rev 47:327–370
Yipmantin A, Maldonado HJ, Ly M, Taulemesse JM, Guibal E (2011) Pb(II) Cd(II) biosorption on Chondracanthus chamissoi (a red alga). J Hazard Mater 185:922–929
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jayakumar, V., Govindaradjane, S. & Rajasimman, M. Isotherm and kinetic modeling of sorption of Cadmium onto a novel red algal sorbent, Hypnea musciformis. Model. Earth Syst. Environ. 5, 793–803 (2019). https://doi.org/10.1007/s40808-018-0566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-018-0566-2