Abstract
The adsorption efficiency of Opuntia ficus indica fibres regarding the removal of hexavalent uranium [U(VI)] from aqueous solutions has been investigated prior and after the chemical treatment (e.g. phosphorylation and MnO2-coating) of the biomass. The separation/removal efficiency has been studied as a function of pH, uranium concentration, adsorbent mass, ionic strength, temperature and contact time. Evaluation of the experimental data shows that biosorption is strongly pH-depended and that the MnO2-coated product presents the highest adsorption capacity followed by the phosphorylated and non-treated material. Experiments with varying ionic strength/salinity don’t show any significant effect on the adsorption efficiency, indicating the formation of inner-sphere surface complexes. The adsorption reactions are in all cases exothermic and relatively fast, particularly regarding the adsorption on the MnO2-coated product. The results of the present study indicate that adsorption of uranium from waters is very effective by cactus fibres and particularly the modified treated fibres. The increased adsorption efficiency of the cactus fibres is attributed to their primary and secondary fibrillar structure, which result in a relative relative high specific surface available for sorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a ubiquitous natural element with an average concentration in earth’s crust of about 3 ppm and constant uranium concentration of about 3 ppm in oceans [1]. Nevertheless, pollution of the environment with uranium and associated health effects to human have recently become of major concern, particularly due to the use of depleted uranium in armour-breaking bullets [2]. Indeed, uranium in environmentally significant concentrations is found near to uranium mining and processing facilities and usually involves large volumes of wastewater. Removal of uranium from such large volumes of wastewaters will require a cost effective remediation technology. Conventional technologies relying on mineral adsorbents or chemical flocculating agents are relatively expensive. Recently, biosorption is presented as an alternative method for removing toxic metals from wastewater [3]. On the other hand, there is great interest in the development of technologies for uranium recovery from seawater, which would improve the uranium resource availability and sustain long-term fuel supply for nuclear power reactors [4, 5].
Biosorption technologies in which living or dead biomass is used to accumulate heavy metals are methods that often replace conventional processes for remediating metal pollution in wastewaters. However, the major drawback of these microbial systems is cost of growing a sufficient quantity of bacterial or algae biomass. Alternatively, the removal of dissolved metals by plant tissues has been studied using a variety of biomasses that represent by-products from other commercial processes with little commercial value and thus representing good candidates for the development of inexpensive biosorption processes. Natural adsorbents, such as agricultural wastes are inexhaustible, low cost and non-hazardous adsorbent materials and easily disposed by incineration [3, 6, 7]. Opuntia ficus is a long domesticated and the most widespread cactus species, and economically important crop throughout arid and semiarid parts of the world (e.g. Central America, North Africa and Mediterranean countries) [8]. Besides fruit production, Opuntia ficus plants can be used to prevent soil erosion, as fences and windbreaks in horticulture, and animal feed [8, 9]. On the other hand, dead plants could be used as source for biomass, which could act as efficient adsorbents for metal ions [10]. Uranium adsorption on minerals [11] and biomasses is extensively investigated [12] and there are several studies dealing with uranium adsorption on non-treated [6, 13] and chemically modified biomass by-products, particularly with respect to the selective separation of uranium from seawater [5].
The present study deals with the adsorption of uranium by non-treated and chemically modified Opuntia ficus indica fibres. Chemical modification, such as phosphorylation and metal (manganese) oxide coating of the biomass by-product is expected to result in novel derivatives with increased separation efficiency and selectivity for metal ions [7]. The main goal of the study is the investigation of various parameters (e.g. pH, uranium concentration, mass of the adsorbent, ionic strength, temperature and contact time) affecting the biosorption performance and the determination of thermodynamic parameters (e.g. K d, ΔG, ΔH and ΔS), which are of fundamental importance for both the assessment of the chemical behaviour of uranium in heterogeneous aquatic systems and the development of water treatment technologies related to (radio)toxic metal ion removal.
Experimental
All experiments were performed at room temperature (23 ± 2 °C) under normal atmospheric conditions in aqueous solutions, without background electrolyte added, except those related to the ionic strength effects. Generally, the experiments were performed in duplicate and the mean values have been used for data evaluation. The relative deviation of the corresponding data was always below 5 %, which within the relative uncertainty given for the concentration values determined by photometry. The preparation of the test solutions was carried out by dissolution in the desired aqueous solution the appropriate amount of a U(VI) stock solution (0.1 M) prepared by dissolution of UO2(NO3)2 × 6H2O in distilled water. pH measurements were performed by a commercial glass electrode (Sentek), which was calibrated prior and after each experiment using a series of buffer solutions (pH 2, 4, 7 and 10, Scharlau).
Opuntia ficus fibres
Opuntia ficus cladodes were collected from plants used as effective living fence in a suburb of Nicosia, Cyprus. The cladodes, which had been broken off the plants, were extensively sun-dried and partially disintegrated. The cladodes were carefully hand-peeled and thoroughly washed by distilled water and the wooden residuals (e.g. primary and secondary fibres) were dehydrated at 70 °C for 15 h and ground for a few minutes in a coffee grinder. The grinded product was sieved and the particle fraction between 200 and 500 microns was selected for the adsorption experiments. Specific surface area measurements based on the Brunauer–Emmett–Teller-theory (BET measurements) by means of N2-adsorption (ASAP 2000, micromeritics) have shown that the material has not internal surface.
Phosphorylation of the cactus fibres
The preparation of the phosphorylated material was performed by addition of the fibres (5 g) in 500 ml 1.5 M Η3PO4 solution (Aldrich Chemical Company) and continuous stirring of the suspension for 24 h at room temperature [14]. Following, the solid phase was separated by filtration and washed thoroughly with distilled water until the pH of the filtrates was neutral. The phosphorylated biomass was dried for several days in a desiccator and then stored for later use.
MnO2-coating of the cactus fibres
The preparation of the MnO2-coated material was performed using the reductive precipitation of MnO2 oxide onto the biomass substrate, by addition of the fibres (10 g) in 100 ml 2 % (w/v) ΚΜnO4 solution (Fisons Scientific Equipment) and continuous stirring of the suspension for 1 h at 50 °C [15]. Following, the solid phase was separated by filtration, washed thoroughly with distilled water and dried for several days in a desiccator.
Characterization of the non treated and chemically modified biomass by-products
The characterization of the cactus fibres (CF) and the products obtained after chemical treatment was performed by Fourier Transform Infra Red Spectroscopy (FTIR spectrometer 8900, Shimadzu), and acid/base titrations. For the titrations, alkaline biosorbent suspensions (pH 12) of each of the three products (0.25 g in 15 ml of deionized water) were titrated by 0.1 M HClO4 standard solutions (BDH Laboratory Supplies) under continuous magnetic stirring. Acid/base titrations were also performed by stepwise adding of 0.1 M HClO4 or 0.1 M NaOH to biosorbent suspensions. After each addition of the titrant, the pH was allowed to reach equilibrium and finally measured by means of a pH-meter (Hanna Instruments). FTIR measurements were performed by means of prepare translucent KBr disks including finely ground biomass was encapsulated at a 10:1 mass ratio.
Adsorption measurements
In order to investigate the effect of various parameters (pH, uranium concentration, mass of the adsorbent, ionic strength, temperature and contact time) on the U(VI) adsorption on non-treated and chemically modified CF, different classes of experiments (e.g. the effect of pH, ionic strength etc.) were conducted. In these experiments the parameter under investigation was varied, while other experimental parameters were kept constant. The effect of pH was studied in an adsorption system: (0.01 g adsorbent and 15 ml of the test solution: [U(VI)] = 1 × 10−5 M) in which pH was varied between 2 and 8.5 by addition of HClO4 or NaOH. All test solutions were prepared using 0.1 M NaClO4 (Aldrich Co) as the background electrolyte. The effect of the ionic strength (salinity) was performed by addition of NaClO4 solution of various concentrations (0.001, 0.01, 0.1, 0.5, 0.7 and 1 M) at constant adsorbent amount (0.01 g), total uranium concentration (1 × 10−5 M) and the optimum pH value for each adsorbent. For studying the effect of initial uranium concentration, the latter was varied between 9 × 10−6 and 9 × 10−4 M, at a prefixed amount of adsorbent (adsorbent dosage = 0.01 g and the optimum pH value for each adsorbent). The effect of the amount of the CF by-products was investigated by adding different amounts of the adsorbents (between 0.005 and 0.2 g) in 15 ml test solutions of constant uranium concentration (1 × 10−5 M). The effect of temperature was studied between 10 and 70 °C at the same conditions that are described above. For kinetic studies certain amount of the biomasses (0.04 g) were mixed with uranium solution (1 × 10−5 M). Samples withdrawn at time intervals (usually after an equilibration time of 24 h) were centrifuged and filtered with membrane filters (pore size: 450 nm). Finally, the uranium concentration was determined spectrophotometrically (UV 2401 PC Shimadzu) by means of arsenazo-III, according to a previously described method [16]. For each test solution, a corresponding reference solution was prepared and was similar to the test solution expect that it didn’t contain the adsorbent material. The relative amount of U(VI) adsorbed was determined using the following equations:
where \( [ {\text{U(VI)]}}_{ 0} = \) the total uranium concentration (mol l−1) in the system or in the reference solution, \( [ {\text{U(VI)]}}_{\text{aq}} = \) uranium concentration (mol l−1) in the test solution, V (l) is the volume of the test solution and m (kg) is the mass of the adsorbent.
Results and discussion
Preparation and characterization of the chemically treated cactus fibres
The main constituents of the CF obtained are polysaccharides including cellulose and therefore the adsorption of toxic metal ions is basically attributed to R–CH2–OH alcohol group at the glucose units of the biopolymers [10]. Phosphorylation of the CF leads to the conversion of the hydroxyl groups to phosphate groups upon esterification of the alcoholic groups by phosphoric acid according to the following Eq. (3):
As a result, the almost neutral surface of the CF becomes acidic, as indicated by the corresponding acid/base titration curve in Fig. 1. The average pK value of the surface groups is about 3, which is characteristic for phosphorylated surfaces [17]. In comparison, the inflection point of the titration curve before phosphorylation is close to pH 7, because of the low acidity of the alcoholic hydroxyl groups (pK > 15). According to the corresponding titration curve even in the strong alkaline pH (pH 11) region no deprotonation occurs, whereas proton consumption observed in the acidic pH region is attributed to the rapid protonation of the glycosidic oxygen atom, which finally results in the acid-catalysed hydrolysis of cellulose [18]. The increased surface acidity upon phosphorylation is of particular importance, because the surface can be deprotonated and become negatively charged even at pH < 4. Hence, the phosphorylated biomass becomes more attractive for positively charged metal ions, even in the acidic pH region and the surface can bind metal ions specifically with higher affinity to phosphates.
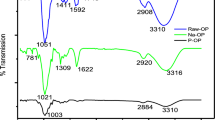
The FTIR spectra prior and after phosphorylation of the CF are shown in Fig. 2. The broad peak at 3,430 cm−1 is attributed to hydroxyl stretching vibrations, while the peaks at 2,850–2,900 cm−1 are assigned to aliphatic C–H groups [7, 13]. Although the spectra corresponding to chemically treated and non-treated CF differ from one another (different relative intensities), they don’t show any distinct/characteristic peaks, which could be attributed to chemical modification (phosphorylation and manganese oxide coating) most probably because of the broad absorption bands in the spectra and the relative low ratio of inorganic to organic material.
Manganese oxide has been widely used for the separation of radiotoxic metal ions from aqueous solutions [7, 15, 19]. The MnO2-coating of the CF has been performed to enhance the adsorption capacity and selectivity of the by-product, because of the dramatic modification of the surface (organic to inorganic) and the fact that MnO2 presents extraordinary affinity for cations [7, 19]. The MnO2 coating of the CF particles has been performed by reduction of MnO4 − by reductive groups on the CF surface (e.g. R–OH) and subsequent deposition of the resulting MnO2 on the particle surface, which is indicated by textural changes (change of color to black and higher density) of the material. Upon coating of the CF the surface charge of the MnO2-coated material is determined by the MnO2 surface properties. MnO2 forms a colloidal coating on the CF. Hydrous MnO2 gains surface charge through protonation and deprotonation according to reactions described by Eqs. (4) and (5).
The pH value at which colloidal MnO2 dispersed in an electrolytic solution exhibits zero net electrical charge on the surface corresponds to the point of zero charge value and is expected to be similar to other oxides of the MO2 type reported in the literature (e.g. SiO2: pH 2, ZrO2: pH 4, SnO2: pH 4.5, TiO2: pH 6) [19]. Hence within the pH range of interest colloidal MnO2 is most probably neutral or negatively charged. Acid/base titration curves (Fig. 1) of the MnO2-coated material clearly indicate the successful preparation of the desired product. However, the corresponding FTIR spectra of the chemically treated and non-treated CF (Fig. 2), although different, don’t show any distinct/characteristic peaks, most probably because of the broad absorption bands and the relative low ratio of inorganic to organic phase. The non-treated and chemically modified CF samples have been used as adsorbents for U(VI) from aqueous solutions. The advantage of using biomass by-products as adsorbents is of particular interest because of their availability and low-cost. The latter compensates the limitations in recycling the biomass by-products and allows thermal treatment (e.g. burning) of the organic material, which results in significant reduction of waste material and recovery of the metals.
Adsorption experiments
pH effect
The relative adsorption is related to the chemical affinity of the surface for the adsorbate, which depends on both the chemical behaviour of U(VI) in solution and the surface charge of the adsorbent. Hence, the solution pH is one of the most important parameters affecting adsorption on surfaces, because pH may govern both the chemical behavior of a metal ion in solution and the surface charge of an adsorbent. To study the effect of pH on the U(VI) adsorption, samples of the three different biomass by-products were conducted with U(VI) solutions at different pH (2 < pH < 9).
The effect of pH on the relative adsorption of U(VI) on the three different materials is shown in Fig. 3. In the case of the non-treated CF the relative adsorption increases with increasing pH and reaches a maximum value (~85 %) at pH > 6. For pH values below 6 the relative adsorption decreases almost linearly with pH and its value becomes almost zero at pH 2. Because pH < 6 U(VI) is exclusively present in the form of positively charged species [e.g. UO2 2+, UO2(OH)+] [1, 20], the adsorption of the metal ion is solely governed by cation exchange reactions and hence the proton concentration in solution. The corresponding cation-exchange reaction between protons and U(VI) cations on the CF surface, is formulated by Eqs. (6) and (7).
Above pH 6.5 the values of the relative adsorption decreases dramatically because of the formation of U(VI)-carbonato species [e.g. UO2(CO3) (2n−2)− n ], which predominate in alkaline solutions and compete the formation of U(VI) adsorbed species.
On the other hand, the phosphorylated CF present even at low pH values (pH ~ 4.5) the highest adsorption capacity (100 %) for U(VI). The shift of more than 2.5 pH units to the acidic area of the maximum values of the relative adsorption is related to lower pK (pH ~ 3) values of the organophosphate moieties present on the CF surface upon phosphorylation. The protonation of the organophosphate moieties results in the gradual decreasing of relative adsorption of U(VI) for pH < 4. The corresponding reactions are described by Eqs. (6) and (7).
Between pH 4 and 6.5 the relative adsorption decreases (~20 %) most probably because of the hydrolysis of UO2 2+ to UO2OH+, which results in lower affinity of the hydrolysed species for the phosphorylated biomass surface. This is expected since electrostatic interactions play a significant role in the formation of surface complexes between cations and the phosphorylated biomass surface [7].
In the case of MnO2-coated CF, the highest value of the relative adsorption (100 %) is observed in the pH range between 4.5 and 7.5. Above pH 7.5 the relative adsorption of U(VI) on the MnO2-coated biomass decreases because of the formation of U(VI)-carbonato species [e.g. UO2(CO3) (2n−2)− n ], which compete with the formation of U(VI) adsorbed species. In the acidic pH region the cation exchange capacity of the hydrous MnO2 decreases significantly with decreasing pH (pH < 4.5) and results in lower relative adsorption of U(VI) (Fig. 3). On the other hand, the adsorbate in the studied pH range is expected to be present predominantly in the form of UO2 2+ aquo-cations [1, 20]. Assuming that only the adsorption of UO2 2+ on the neutral MnO2 surface takes place, the chemical reactions at the solid–liquid interface can be described by Eqs. (9) and (10)
Distribution coefficient (K d) values
The distribution coefficient (K d) is an important parameter in estimating the adsorption potential of dissolved contaminants by adsorbent materials. To investigate the affinity of the non-treated and chemically modified biomass by-product for U(VI) adsorption, experiments have been performed at room temperature (23 ± 2 °C) and I = 0.1 M in which pH was varied between 2 and 8.5. According to Fig. 4 the K d values ([U(VI)]ads/[U(VI)]aq) as a function of pH behave similarly to the relative adsorption values. Moreover, the K d data clearly indicate that the chemically treated products present by far the highest affinity for U(VI) for a wide pH range, especially in the case of the MnO2-coated CF. Specifically, the K d values evaluated at the optimum pH for each CF type are 6,000 l kg−1 (pH 6.0), 13,000 l kg−1 (pH 4.5–5.0) and 15,000 l kg−1 (pH 4.5–7.5) for the non-treated, phosphorylated and MnO2-coated CF, respectively. These values, which are significantly higher than K d values reported in literature for metal ion adsorption on non-treated and chemically modified biomass by-products [5–7, 13], could be attributed to the fine primary and secondary fibres constituting the present biomass and resulting in a relative enormous specific surface available for adsorption. The relatively high specific surface available for adsorption is basically attributed to fibrous character of the adsorbent, because no internal surface (porosity) could be determined. It has to be noted that to our knowledge this is the first time that K d values have been reported for the adsorption of U(VI) on non-treated and chemically modified biomasses, particularly CF. K d values corresponding to U(VI) adsorption on biomass by-product of plant origin are of particular interest for the development of water treatment technologies based on adsorption.
Effect of uranium concentration
In order to evaluate the maximum adsorption capacity (q max) adsorption experiments with varying uranium concentrations have been performed at the optimum pH for each cactus biomass adsorbent. The corresponding isotherms are graphically shown in Fig. 5. The experimental data are well fitted by the Langmuir isotherm (R = 0.95 for CF, R = 0.97 for phosphorylated CF and R = 0.97 for CF coated with MnO2). According to the results, the experimental q max values are ~0.26 mol kg−1 (62 mg g−1), 0.45 mol kg−1 (107 mg g−1) and 0.46 mol kg−1 (110 mg g−1), for non treated CF, phosphorylated CF and CF coated with MnO2, respectively, indicating high adsorption capacities for uranium metal ion. Further, the q max values of the chemically treated adsorbents clearly show that chemical modification of the adsorption material improves its adsorption attributes and MnO2 CF can be used as an alternative for MnO2 resins [15].
Effect of mass adsorbent
The dependence of U(VI) adsorption on dose was studied by varying the amount of adsorbents while keeping the other parameters constant. The relative adsorption of U(VI) on the investigated biomass by-products as a function of mass adsorbent is shown in Fig. 6a, b (expanded scale). The results indicate that the three products behave very different from one another. In the case of the non treated CF, the relative amount of adsorbed U(VI) decreases with increasing biomass dose most probably due to partial aggregation and a screening effect on the biomass surface, which results in decreasing the number of active sites available for U(VI) adsorption and hence in lower uranium uptake per unit mass of biosorbent. On the other hand, the relative adsorption of U(VI) on the modified treated CF increases with increasing the amount of the chemically modified biomasses until U(VI) is quantitatively removed from solution. Evaluation of the maximum biosorption capacity from the experimental data results in values far higher than corresponding values obtained from the previous experiments regarding U(VI)-adsorption on biomass by-products [5–7, 13].
Effect of the adsorbent dose on the U(VI) adsorption onto non-treated (CF), phosphorylated (CF-PO4) and MnO2-coated (CF/MnO2) cactus fibres (0.005–0.2 g of biomass, [U(VI)]tot = 1 × 10−5 mol l−1, pH = optimum pH value, I = 0.1 mol l−1 NaClO4, T = 23 ± 2 °C, 24 h of reaction time). a Full-scale (0–0.25 g) and b expanded scale (0–0.05 g)
The effect of ionic strength/salinity on the adsorption efficiency
Furthermore, the effect of ionic strength ([NaClO4] = 0.001, 0.01, 0.1, 0.5, 0.7 and 1 M) on the relative adsorption of U(VI) was investigated, in order to understand the interaction mechanisms on which U(VI) binding on the three different types of biomass by-products is based. The experimental data obtained from the corresponding experiments are graphically summarized in Fig. 7, showing clearly that the relative adsorption of CF-PO4 (phosphorylated CF) is stronger affected by increasing salinity, as the relative adsorption decreases from 100 to 80 %. This effect indicates that binding between the phosphate moieties on the CF surface and U(VI) is affected by electrostatic interactions.
On the other hand, the relative adsorption of the MnO2-coated cactus fibres (CF-MnO2) is almost not affected by increasing salinity and indicates specific interactions between U(VI) and the hydroxy-groups of the MnO2 surface. The specific interactions could be attributed to the formation of inner-sphere complexes, which are only little affected by salinity changes [7]. Regarding the U(VI) adsorption on the non-treated CF, the relative adsorption increases slightly with increasing ionic strength. This could be attributed to the decrease of the activity coefficient values of the cationic species [e.g. H+ and UO2(OH)+].
The effect of temperature on the adsorption efficiency
The effect of temperature on uranium adsorption on CF was investigated to estimate the corresponding thermodynamic data based on the van’t Hoff equation. Evaluation of the data shows that adsorption of U(VI) on CF is an exothermic, both enthalpy- and entropy-driven spontaneous process. The corresponding values of the thermodynamic parameters, which are summarized in Table 1, indicate clearly that the MnO2 coated product presents the highest chemical affinity (ΔH o = 67.1 kJ mol−1) for U(VI), followed by the non-treated (ΔH o = 31.9 kJ mol−1) and phosphorylated product (ΔH o = 23.1 kJ mol−1). Generally, the values of the thermodynamic parameters evaluated are close to corresponding values reported in literature for similar systems [5–7, 13]
Kinetic measurements
According to Fig. 8, which shows the relative amount of adsorbed U(VI) as a function of time, the adsorption of U(VI) on the three different biomass products is relatively fast but differs from one another. The adsorption on the MnO2-coated biomass seems to be the fastest, whereas the adsorption on the phosphorylated biomass occurs in two steps indicating two different adsorption mechanisms for the U(VI) binding on the phosphorylated biomass (Fig. 9). Evaluation of corresponding data by the first order rate expression described by Lagergren [21] results in values for the reaction rate constant (k ads), which are summarized in Table 2. The k ads for the adsorption of U(VI) on CF are similar to corresponding values reported in literature for similar systems [5–7, 13].
Conclusions
In this study non-treated and chemically modified Opuntia ficus indica CF were used to adsorb U(VI) ions from aqueous solutions. The results indicate that the non-treated opuntia CF show increased affinity for U(VI), which is further improved by chemical modification of the biomasses. U(VI) adsorption is strongly dependent on pH and differs significantly from one product to another, indicating that the MnO2 coated product presents the highest relative adsorption in a wide pH range (4.5 < pH < 7.5), followed by the phosphorylated (pH = 4) and non-teated product (pH = 6.5). Increasing temperature affects positively the adsorption capacity and the corresponding data evaluation indicates that both ΔH and ΔS favor biosorption. The equilibrium data were described well by a Langmuir isotherm, indicating that the adsorption of uranium on biomass is based on specific chemical interactions. The biosorption of U(VI) ions on the non-treated and chemically modified CF follows a pseudo-first-order kinetics. CF are very effective biosorbents basically because of the relative high surface available for sorption because of the primary and secondary fibres constituting this biomaterial.
References
Choppin GR (2007) Actinide speciation in the environment. J Radioanal Nucl Chem 273:695–703
Burkart W, Danesi PR, Hendry JH (2005) Properties, use and health effects of depleted uranium. Int Congr Ser 1276:133–136
Nilanjana D (2012) Review remediation of radionuclide pollutants through biosorption—an overview. Clean Soil Air Water 40(1):16–23
Kanno M (1984) Present status of study on extraction of uranium from sea water. J Nucl Sci Technol 21:1–9
Tamada M (2009) Current status of technology for collection of uranium from seawater. Japan Atomic Energy Agency, Takasaki
Konstantinou M, Pashalidis I (2007) Adsorption of hexavalent uranium on biomass by-product. J Radioanal Nucl Chem 273:2095–2102
Prodromou M, Pashalidis I (2013) Radium removal from aqueous solutions by adsorption on non-treated and chemically modified biomass by-product. J Radioanal Nucl Chem 295:549–552
Barbera G, Carimi F, Inglese P (1992) Past and present role of the Indian-fig prickly-pear (Opuntia ficus-indica (L.) Miller, Cactaceae) in the agriculture of Sicily. Econ Bot 46:10–20
Shedbalkar UU, Adki VS, Jadhav JP, Bapat VA (2010) Opuntia and other cacti: applications and biotechnological insights. Trop Plant Biol 3:136–150
Barrera H, Urena-Nunez F, Bilyeu B, Barrera-Diaz C (2006) Removal of chromium and toxic ions present in mine drainage by Ectodermis of Opuntia. J Hazard Mater B136:846–853
EA Jenne (1998) Adsorption of metals by geomedia (variables, mechanisms and model applications). Academic Press, San Diego
Liao X, Lu Z, Du X, Liu X, Shi B (2004) Environ Sci Technol 38:324–328
Saleem N, Bhatti HN (2011) Adsorptive removal and recovery of U(VI) by citrus waste biomass. BioResources 6:2522–2538
Tatsuya O, Kanya K, Keisuke O, Katsutoshi I, Yoshinari B (2008) Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React Funct Polym 68:376–383
Varga Z (2007) Preparation and characterization of manganese dioxide impregnated resin for radionuclide preconcentration. Appl Radiat Isot 65:1095–1100
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63:1165–1169
Murthy PNP (2007) Identification of inositol phosphates by nuclear magnetic resonance spectroscopy: unravelling structural diversity. In: Turner BL, Richardson AE, Mullaney EJ (eds) Inositol phosphates: linking agriculture and the environment. CAB International, Oxfordshire
Xiang Q, Lee YY, Pettersson PO, Torget RW (2003) Heterogeneous aspects of acid hydrolysis of α-cellulose. Appl Biochem Biotechnol 105–108:505–514
Morgan JJ, Stumm W (1964) Colloid-chemical properties of manganese dioxide. J Colloid Sci 19:347–359
Baes CF, Mesmer RE (1976) The hydrolysis of cations. Wiley, New York
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reaction. Scientometrics 59:171–177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prodromou, M., Pashalidis, I. Uranium adsorption by non-treated and chemically modified cactus fibres in aqueous solutions. J Radioanal Nucl Chem 298, 1587–1595 (2013). https://doi.org/10.1007/s10967-013-2565-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2565-0