Abstract
Electrical conductivities of dilute aqueous solutions of lithium, sodium, potassium, cesium, rubidium and ammonium sulfates were determined and analyzed in terms of partially associated electrolytes of the 1:2 type. The conductivities reported here were determined from 15 to 35 °C and are compared with available literature results. Representation of conductances, in a framework of the ion association model, was performed using the Quint–Viallard conductivity equation and the Debye–Hückel expression for activity coefficients. However, the equilibrium constants were considered as adjustable parameters. Specific conductivities in concentrated aqueous solutions of sulfates were fitted to a new empirical equation with only three adjustable parameters. These parameters at constant temperature are much easier to determine from experimental conductivities than the corresponding four parameters in the usually applied Casteel and Amis conductivity equation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The representation of electrical conductance in aqueous solutions, in pure organic solvents and in mixed solvents has been considered in the series of papers written by the present author [1,2,3,4,5]. Various types of electrolytes were discussed and they included the symmetrical electrolytes of the type 2:2 and 3:3 (alkali earth sulfates, transition metal sulfates, rare earth hexacyanoferrates(III) and hexacyanocobaltates(III)) and the unsymmetrical electrolytes of the type 3:1, 1:3, 3:2, 4:1, 4:1, 4:2, 2:4, 1:5, 6:1 and 1:6 (rare earth salts, cyanides, phosphates and various other salts). A new group of unsymmetrical electrolytes of the type 1:2, the aqueous solutions of alkali metal sulfates and of ammonium sulfate, are considered here.
Alkali metal sulfates, which are very soluble in water, can be found in large quantities near the surface of the earth and at least three of them, sodium sulfate, potassium sulfate and ammonium sulfate are produced in large quantities. They serve as main components or additives to fertilizers, Portland cements, glasses, food products, detergents and other manufactured goods. Numerous applications, in the Kraft process of wood pulping to produce paper, in heat storage in passive solar heating systems, in textiles and starch production, in purification of proteins, in promotion of catalytic activity and in many other areas are mentioned in the literature. It is worthwhile to point out that lithium sulfate, like other lithium salts, is employed as a drug to treat people suffering from the bipolar disorder (depression) and cesium sulfate is used to prepare dense aqueous solutions which are applied in density-gradient centrifugation.
Physicochemical properties of aqueous solutions of alkali metal sulfates have been extensively investigated, especially their thermodynamic properties (see for example [6,7,8,9,10,11,12,13,14,15,16]) and mainly these studies were directed to sodium and potassium sulfates. With only few exceptions, considering importance of alkali metal sulfates in chemical industry, determination of electrical conductivities started rather early but practically always measurements were performed in moderately or highly concentrated solutions, at high temperatures and pressures [6, 9, 10, 13, 17,18,19,20,21,22,23,24,25,26,27]. This fact and the absence of conductivity equations to represent dilute solutions of 1:2 type unsymmetrical electrolytes prevented the analysis of conductances in alkali metal sulfate solutions. There was no particular interest in determining conductivities of alkali metal sulfates in dilute aqueous solutions because the limiting conductance at infinite dilution λ 0(Me+) of cations (Me+ = Li+, Na+, K+, Cs+ and Rb+) and the sulfate anion \(\lambda^0({\text{SO}}_4^{2-})\), were well known from measurements in numerous systems with these ions (Table 1). However, it is clear from available data that in dilute aqueous solutions alkali metal sulfates do not always behave as strong 1:2 type electrolytes and therefore they were discussed in terms of the ionic association and changes in the degree of ion hydration [28, 29]. The main attention in analyzing properties of alkali metal sulfate solutions has been directed to concentrated solutions, namely to the importance of the cation hydration numbers and the ion/water ratios associated with the position of maximum values of specific conductivities and their shift as a function of temperature and concentration [23, 26].
Since 1978, with the appearance of the Lee–Wheaton (LW) [30, 31] and the Quint–Viallard (QV) [32,33,34,35] conductivity equations it was possible to represent conductances of unsymmetrical electrolytes in an adequate way, but these equations were rarely applied and never in the case of 1:2 type electrolytes.
In this investigation, the measured conductivities of dilute aqueous solutions of alkali metal sulfates, together with available values from the literature, were analyzed using the Quint–Viallard conductivity equation and the Debye–Hückel expression for activity coefficients. The determined ion association constants K A(T), treated as adjustable parameters, are also reported. The representation of conductivities in concentrated solutions was performed by replacing the usually used Casteel and Amis conductivity equation with its four adjustable parameters by a new conductivity equation. The proposed new equation gives excellent fitting to experimental conductivities, is mathematically simpler than the Casteel and Amis equation and has only three adjustable parameters.
2 Experimental
Sodium sulfate, rubidium sulfate and cesium sulfate (all better than 0.99 mass fraction and lithium sulfate 0.985 mass fraction) were purchased from Sigma Aldrich. Potassium sulfate and ammonium sulfate (all better than 0.99 mass fraction) were from Merck. All sulfates were used without further purification. Since lithium sulfate is hydroscopic, the solution samples of this and other reagents used here were prepared under isopiestic conditions. Salts were dissolved in water in a glove box where the vapor pressure of water was fixed to that of saturated solutions (for details see [36]). Solutions were prepared by weight by dissolving these reagents in double distilled water.
Electrical conductivities of solutions were determined using a glass cell (with a cell constant of 27.4 cm−1) which was immersed in thermostated bath (± 0.01 K). The cell was calibrated with dilute potassium chloride solutions [37]. The resistances were determined with the help of a Wayne–Kerr Universal Bridge, model B211 [38]. Specific conductances κ were corrected by taking into account impurities dissolved in water (specific conductance less than about 2×10−7 S·cm−1). The molar conductances were calculated from Λ = 1000κ·c −1. Conversion from molal m to molar c units in dilute solutions was performed by using densities of pure water [37]. In the case of sodium sulfate solutions, their densities were correlated by d w(T)/(1 − d w(T)f) where d w(T) is density of water at T and f = 0.98871w − 0.28642w 2 where w is the mass fraction of the salt.
Considering the sources of error (calibration, measurements, impurities), the specific conductivities are estimated to be accurate within ± 0.3%.
3 Results and Discussion
3.1 Conductivity Equations
Molar conductance of an electrolyte Λ(c,T) is the sum of ionic contributions λ j (c,T)
where κ is the measured specific conductance, z j are the corresponding charges of the cation and anion (z+ = 1 and z− = −2) and c j are their molar concentrations. The ionic conductances λ j (c,T) are represented by the following equation:
where the coefficients S j , E j , J 1j and J 2j are complex functions of the limiting equivalent ionic conductances λ 0 j , the distance parameters a j and the physical properties of water (dielectric constant D(T) and viscosity η(T)). These coefficients are available from the Quint–Viallard theory [32,33,34,35] (for explicit expressions see also [39]).
Without going into exact details about steps and mechanism of ion pairing process, if electrolytes are assumed to be partially associated, then the formal analytical concentration of solution c can be replaced by cα where α is the fraction of “free” ions and c(1 − α) denotes non-conducting (uncharged) particles. Thus, α = 1 defines the fully dissociated electrolyte (the “strong electrolyte”). Evaluation of α for a given c (the so-called chemical problem) leads to the overall association constant K A, which represents some kind of the apparent thermodynamic equilibrium constant. This constant can determined from the following mass-action equilibrium equation:
where ν + and ν − are the stoichiometric coefficients (in the present case ν + ν − = 2), f j are the activity coefficients of individual ions (f MeY is assumed to be unity) and they are approximated in dilute solutions by the Debye–Hückel expression:
At given absolute temperature T, constants A(T) and B(T) depend on dielectric constant of pure water
Values of the ion size parameters a j in Eq. 4 were recommended by Kielland [40] and they are a(Li+) = 6.0 Å, a(Na+) = a(K+) = 3.0 Å, a(NH4 +) = a(Rb+) = a(Cs+) = 2.5 Å and a \(({\text{SO}}_4^{2-})\) = 4.0 Å. The ion size parameters were assumed to be independent of temperature T. In the conductivity equations, the distance parameters were taken as a half sum of sizes of cation and anion.
At each temperature T, combining the chemical and conductance problems (the mass-action and QV equations), the experimental sets of conductivities can formally be written as (Λ,c) = f(K A, Λ 0, a j , D, η, c) and solved by an optimization procedure to give the K A value that will assure the best fit between the experimental and the calculated conductivities. Iterations start with α = 1 value in solving the quadratic equation:
where values of a j , D(T) and η(Τ) and Λ 0(T) are known. Iterations are stopped when the average standard deviation σ(Λ) has a minimal value:
where N denotes the number of experimental points.
In the present formulation, the mass-action equation takes the simplest mathematical form, but K A is treated as an adjustable parameter. Evidently, if a particular mechanism of ion association is considered, the corresponding mass-action equation will be much more complex than Eq. 3 and the equilibrium constant will have a distinct physical meaning. Thus, in this representation, values of K A give only an indirect indication about the existence of the ion association in electrolyte solutions.
3.2 Lithium Sulfate
Determination of electrical conductances in a few aqueous solutions of lithium sulfate started in 1879 and they are presented in the classical book of Kohlrausch and Holborn from 1898 [17]. More complete measurements were performed by the Jones group in 1912 [18] (determinations were performed by Dr. Jacobson and Dr. West). These measurements covered the 0–65 °C temperature range and the 0.0005 mol·dm−3 to 0.5 mol·dm−3 concentration range. In order to compare these conductances with the modern values they should be multiplied by factor 1.066 [41]. The next determinations were performed only in 1953 by Indelli [21] who reported lithium sulfate conductances at 25 °C from 0.004 mol·dm−3 to 0.66 mol·dm−3 and, in 1970, by Postler [42] from 0.005 mol·dm−3 to 2.5 mol·dm−3. The pressure effect on conductivities up to 2000 atmospheres, in the 0.00012 to 0.005 mol·dm−3 concentration range, was reported by Fisher and Fox [27]. Electrical conductivities of concentrated and saturated lithium sulfate solutions were presented by Maksimova et al. [10] from 20 to 90 °C, Valyashko and Ivanov [23] from 25 to 75 °C and Cartón et al. [25] from 10 to 25 °C. Conductances of aqueous solutions of lithium sulfate at elevated temperatures are reported by Sharygin et al. [24].
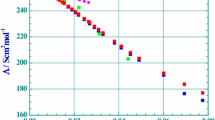
Molar conductances in dilute solutions of lithium sulfate, from 0.00015 to 0.028 mol·dm−3 in the 15 to 35 °C temperature range, were determined in the present investigation and are reported in Table 2. As can be observed in Figs. 1 and 2, there is a reasonable consistency between the literature data in dilute and in concentrated solutions in spite of difficulties to work with hygroscopic lithium sulfate.
Molar conductances of lithium sulfate aqueous solutions at 25.00 °C, Λ(c) as a function of square root of concentration c. pink square, fully dissociated 1:2 electrolyte; dark green square, [18]; blue square, [21]; brown square, [42]; red square, this work; sky blue square, calculated using the Quint–Viallard conductivity equation, this work (Color figure online)
However, there is no doubt (Fig. 1) that all of the available literature conductivities in dilute concentrations at 25 °C differ considerably from those expected from the Onsager equation for strong electrolytes of the 1:2 type [35]:
As can be seen in Fig. 1, the proposed molecular model is valid only for the limited range of concentrations c < 0.005 mol·dm−3 and, in very dilute solutions, c < 0.0001 mol·dm−3, a large scattering of experimental conductances exists. It is observed that the association constant K A which is evaluated from Eq. 3 varies linearly with temperature T
In concentrated solutions of lithium sulfate, the agreement between conductances coming from different investigations is very satisfactory and this is illustrated in Fig. 2 where the specific conductivities κ(c) at 25.0 °C are plotted as a function of square root of concentration c. The form of specific conductivity curves, the ion/water ratios associated with the position of maximum values, and their shift as a function of temperature and concentration is discussed in [23, 26, 43].
3.3 Sodium Sulfate
Electrical conductivities of sodium sulfate aqueous solutions are well documented in the literature [13, 17, 18, 44]. Old determinations of Kohlrausch from 1879, performed at 18 °C are presented in his book [17]. Dr. Winston and Dr. Clover from the Jones group [18] in 1912, measured conductances in the 0–65 °C temperature range and from 0.0002 to 0.25 mol·dm−3. Data for concentrated solutions from the few investigations in the 1879–1921 period are tabulated by Timmermans tables [44]. Actually, only the Bachofner thesis from 1904 is of importance [44], it includes specific conductances in the 0.1 to 1.25 mol·dm−3 concentration range, and from 20 to 80 °C. Modern determinations in dilute solutions, in the 1950 to 1981 period, all at 25 °C only, include investigations of Jenkins and Monk [20] (from 0.00005 to 0.0006 mol·dm−3), Indelli [21] (from 0.0004 to 0.27 mol·dm−3), Broadwater and Evans [22] (from 0.0003 to 0.0022 mol·dm−3, also in D2O and at 10 °C), Fisher and Fox [6] (from 0.00056 to 0.06 mol·dm−3 and at pressures up to 2000 atm) and Vivo et al. [43] (from 0.00045 to 0.0028 mol·dm−3). Our measurements cover dilute and moderately concentrated solutions (from 0.00021 to 1.4 mol·kg−1) in the 15 to 35 °C temperature range (Table 2). Concentrated solutions of sodium sulfate were investigated by Valyashko and Ivanov [23] from 25 to 75 °C, Maksimova et al. [10] from 20 to 90 °C and Isono [9] from 15 to 55 °C.
As can be observed in Fig. 3, there is an excellent agreement between conductivities of sodium sulfate solutions coming from different investigations and in very dilute solutions they nearly behave as strong electrolytes of the 1:2 type. The association constants K A have negligible values for concentrations lower than 0.0064 mol·dm−3 (as expected from the Onsager equation), and this is in an agreement with reported values in the literature [6]. Determined specific conductances in concentrated solutions of sodium sulfate are also in good agreement (Fig. 4).
Molar conductances of sodium sulfate aqueous solutions at 25.00 °C, Λ(c) as a function of square root of concentration c. pink square, fully dissociated 1:2 electrolyte; brown square, [6]; black square, [18]; sky blue square, [20]; orange square, [21]; light green square, [22]; dark green square, [45]; blue square, this work; red square, calculated using the Quint–Viallard conductivity equation, this work (Color figure online)
Specific conductances of sodium sulfate aqueous solutions at 25.00 °C, κ(c) as a function of square root of concentration c. pink square, [6]; light green square, [9]; black square, [18]; sky blue square, [20]; dark green square, [21]; orange square, [22]; red square, [23]; brown square, [45]; blue square, this work (Color figure online)
3.4 Potassium Sulfate
Similar to the situation for sodium sulfate, the conductances of dilute aqueous solutions of potassium sulfate were reported in a number of investigations [17,18,19,20,21,22, 46]. Old measurements were performed by Klein in 1886 [17] and by Dr. West and Dr. Clover in 1912 [18] (in the 0–65 °C temperature range and from 0.00098 to 0.5 mol·dm−3). Modern determinations at 25 °C started with measurements of transference numbers and conductances in 1937 by Hartley and Donaldson [46] (from 0.00048 to 0.0025 mol·dm−3) and in 1941 by Fedoroff [19] (from 0.0001 to 0.65 mol·dm−3). The next series of investigations in the 1950–1977 period includes these of Jenkins and Monk [20] (from 0.000057 to 0.00057 mol·dm−3), Broadwater and Evans [22] (from 0.00031 to 0.0034 mol·dm−3, also in D2O and at 10 °C), Indelli [21] (from 0.0021 to 0.12 mol·dm−3), and Fisher and Fox [26] (from 0.00005 to 0.05 mol·dm−3 and at pressures up to 2000 atm). Sharygin et al. [24] measured conductances of aqueous solutions of potassium sulfate at elevated temperatures. Reported here are new measurements from 15 to 35 °C in the 0.00027 to 0.0007 mol· dm−3 concentration range (Table 2). Concentrated solutions of potassium sulfate were determined only by Valyashko and Ivanov [23] from 25 to 75 °C and Maksimova et al. [10] from 20 to 90 °C. Unfortunately, they can not be compared because they were determined at different temperatures.
Molar conductances of aqueous potassium sulfate coming from different investigations are plotted in Fig. 5. As with sodium sulfate, potassium sulfate in very dilute solutions behaves as a nearly strong electrolyte of the 1:2 type. The association constants K A have negligible values for concentrations lower than 0.0007 mol·dm−3.
Molar conductances of potassium sulfate aqueous solutions at 25.00 °C, Λ(c) as a function of square root of concentration c. Pink square, fully dissociated 1:2 electrolyte; sky blue square, [18]; brown square, [19]; orange square, [20]; red square, [21]; dark green square, [22]; blue square, [26]; black square, [46]; light green square, this work (Color figure online)
3.5 Rubidium Sulfate
Unlike the cases of sodium or potassium sulfates, electrical conductance studies in rubidium sulfate aqueous solutions are rare. In dilute solutions, there is only the investigation of Fisher and Fox [27] (at 25 °C, from 0.0001 to 0.005 mol·dm−3 and at pressures up to 2000 atm). Here, electrical conductivities, from 0.0001 to 0.01 mol·kg−1, in the 15 to 35 °C temperature range are presented in Table 2.
Our measured conductivities of rubidium sulfate solutions at 298.15 K and the corresponding values from the Fisher and Fox investigation [27] are consistent and are plotted in Fig. 6. As can be observed, moderate association exists in rubidium sulfate solutions because Λ(c) values deviate from those predicted for a fully dissociated electrolyte (from Eq. 8). As for lithium sulfate, the association constant K A depends linearly on temperature T
Molar conductances of rubidium sulfate aqueous solutions at 25.00 °C, Λ(c) as a function of square root of concentration c. Pink square, fully dissociated 1:2 electrolyte; light green square, [27]; blue square, this work; red square, calculated using the Quint–Viallard conductivity equation, this work (Color figure online)
It follows from Eq. 10 that, at 15 °C, the association is small but increases strongly with temperature. The proposed model is valid only for a very dilute solutions c < 0.001 mol·dm−3.
Few old measurements in concentration solutions of rubidium sulfate were tabulated in [44] and modern values in a wide temperature and concentration range were reported by Maksimova et al. [10] and Valyashko and Ivanov [23], but once again they can not be compared because their measurements were performed at different temperatures.
3.6 Cesium Sulfate
In dilute aqueous solutions of cesium sulfate, the electrical conductivities were reported only by Fisher and Fox [27] (at 25 °C, from 0.0001 to 0.005 mol·dm−3 and at pressures up to 2000 atm) and in the present investigation (from 0.0004 to 0.01 mol·kg−1, in the 15 to 35 °C temperature range, see Table 2). If these conductivities at 298.15 K are compared with those calculated for the molecular model (Fig. 7) then it is evident that the association effect in aqueous solutions of cesium sulfate exists, but it is rather small. The effect is smaller than for rubidium sulfate, increases with T, but practically is negligible at low temperatures
The proposed molecular model is valid for c < 0.002 mol·dm−3.
Molar conductances of cesium sulfate aqueous solutions at 25.00 °C, Λ(c) as a function of square root of concentration c. Pink square, fully dissociated 1:2 electrolyte; light green square, [27]; blue square, this work; red square, calculated using the Quint–Viallard conductivity equation, this work (Color figure online)
Specific conductivities in concentrated cesium sulfate were determined by Maksimova et al. [10], Valyashko and Ivanov [23] and Shilovskaya et al. [47]. Unfortunately, in the last investigation, they are presented only in graphical form. However, if the Shilovskaya et al. [47] conductivities are converted into a digital set and compared at the same temperatures (in Fig. 8) with those of Maksimova et al. [10], it is clear that both sets of data differ considerably, especially in very concentrated solutions of cesium sulfate.
3.7 Ammonium Sulfate
First measurements of conductances in aqueous solutions of ammonium sulfate were performed 1912 by Dr. Winston and Dr. Clover from the Jones group [18] (in the 0–65 °C temperature range and from 0.0002 to 0.5 mol·dm−3 concentration range). Scatchard and Prentiss [48] in 1932, determined conductances of ammonium sulfate solutions in the 0.0031 to 1.23 mol·kg−1 concentration range but only at 10 °C. In dilute solutions, their results are above the corresponding values calculated for the strong 1:2 type electrolyte (from Eq. 8). The electrical conductivities were reported also by Fisher and Fox [27] (at 25 °C, from 0.0001 to 0.005 mol·dm−3 and at pressures up to 2000 atm). The conductivities presented here (from 0.00025 to 0.01 mol·kg−1, in the 15 to 35 °C temperature range) are given in Table 2. In concentrated solutions, the specific conductivities were determined only by Isono [49] (from 0.05 to 5.0 mol·kg−1, in the 15 to 55 °C temperature range).
The available electrical conductances in dilute aqueous solutions of ammonium sulfate at 25 °C are plotted in Fig. 9. They are nearly consistent with behaviour of strong electrolyte of 1:2 type for c < 0.001 mol·dm−3. Thus, as with sodium sulfate and potassium sulfate, in ammonium sulfate solutions the association effect is very small in dilute solutions..
The association constants K A at 25 °C, determined in this investigation can be arranged in the following series (NH4)2SO4, Na2SO4, K2SO4 ≪ Cs2SO4 < Li2SO4 < Rb2SO4. The position of cesium sulfate in the above series is rather surprising, considering that cesium and rubidium cations have practically the same value of the limiting conductivities, λ 0(Rb+) = 77.81 S·cm2·mol−1 and λ 0(Cs+) = 77.28 S·cm2·mol−1. It is worthwhile noting also the relatively large value of the limiting conductance of the sulfate anion \(\lambda^0(1/2{\text{SO}}_4^{2-})\) = 80.02 S·cm2·mol−1 as compared with other anions. This is interpreted by a special mechanism of charge transfer in the case of sulfate anions [50] and therefore the cation–anion interaction can be different in each case. This is also true if hydration numbers of alkali metal ions are taken into account (Li+ > Na+ > K+ > Cs+ > Rb+ [37]).
Generally, the association constants of alkali metal sulfates and of ammonium sulfate are small, but also the upper limit of concentrations (usually c < 0.001 mol·dm−3) where the applied molecular model can be used is also small. Thus, in dilute solutions of alkali sulfates, association exists, but in a different form then that expressed by Eq. 3. This is clearly indicated by a strong decrease in conductivity Λ(c,T) with increasing c, as compared with that expected for corresponding strong electrolyte. In old papers, the formation of monovalent sulfate ion pairs \(({\text{MeSO}}_4^{-})\) was usually expressed in terms of dissociation constants K d (formally, they are interrelated by 2K A = 1/K d). The available in the literature values of K d were determined by several experimental techniques and they lie in the following range 0.3 < pK d < 1.0 [51, 52]. They indicate only a small association and are consisted with K A values reported here. However, there is not always agreement when pK d series for\(({\text{MeSO}}_4^{-})\) ion pairs are presented. For example, at total molality m = 0.1 mol·kg−1, at 25 °C, Reardon [52] proposed that pK d can be arranged in the following way HS\({\text{O}}_4^{-}\) > NH4S\({\text{O}}_4^{-}\) > NaS\({\text{O}}_4^{-}\) > LiS\({\text{O}}_4^{-}\) > RbS\({\text{O}}_4^{-}\) > CsS\({\text{O}}_4^{-}\). Righellato and Davies [51] at 18 °C found that the degree of ion pairing increases for the series LiS\({\text{O}}_4^{-}\) > NaS\({\text{O}}_4^{-}\) > KS\({\text{O}}_4^{-}\), but pK d(RbS\({\text{O}}_4^{-}\)) and pK d(CsS\({\text{O}}_4^{-}\)) are lower than for other sulfates, which gives a rather strange maximum value for KS\({\text{O}}_4^{-}\). Thus, there is no doubt that in aqueous solutions of monovalent sulfates the ion association is small, but for a particular alkali metal ion the exact strength of it continues to be questionable.
4 Concentrated Solutions of Monovalent Sulfates
Representation of the conductivities in dilute alkali metal and ammonium sulfates solutions using the Quint–Viallard conductivity equation is the main subject of this investigation. However, concentrated solutions are also important, especially from a practical point of view considering their industrial applications. Most determinations of specific conductances in concentrated solutions were performed by Valyashko and Ivanov [23], Isono [49] and especially by Maksimova et al. [10], but unfortunately their results are not easily accessible in the literature. Considering these circumstances, it is worthwhile presenting the specific conductances reported by above authors in a simple mathematical form.
The representation of specific conductivities of electrolytes in pure or mixed solvents is usually performed using the empirical equation proposed in 1972 by Casteel and Amis [53, 54]
At constant temperature T, the above equation includes four adjustable parameters a, b, m max and κ(m max) and these parameters have no physical meaning. The last two parameters are not actual values of the maximum of specific conductivity and the corresponding concentration at which the maximum is situated. In many cases maxima do not exist at all or a broad maximum κ(m max) is observed, if the solubility is high enough to reach it. Thus, values of m max and κ(m max) should also be determined by fitting procedures. Different concentration units can be used in the Casteel and Amis equation, molalities m can be replaced by molarities c or by mass fractions w (0 < w < 1).
The main disadvantage of the Casteel and Amis equation is that adjustable parameters must be derived using specially prepared computer programs. Unfortunately, an easy to use multivariate least-square method (e.g. Linest program in Excel) can not be applied. Thus, the Casteel and Amis equation or its logarithmic form cannot be reduced to the form
where f i (w) are some known functions and a i are adjustable parameters. Since the Casteel and Amis equation is a purely empirical equation, there is no reason not to use such functions f i (w) which are convenient in performing mathematical operations. Evidently, the simplest way to fit κ(w) is to use polynomials of w with the imposed condition that κ(w) = 0 at w = 0
or in the form
However, there is a second possibility to replace the Casteel and Amis equation by applying the semi-theoretical approach in an interpretation of the temperature dependence of transport properties in glass-forming liquids and fused salts. Such an equation has the form of the modified Arrhenius equation (the Vogel–Fulcher–Tammann type equation [54])
where A and k are constants and T 0 is the glass-transition temperature. This equation was originally derived from the free volume theory of viscous liquids and is adapted to represent transport properties of concentrated electrolyte solutions.. At constant temperature T, Angell [55,56,57] suggested that A and k are independent of composition and T 0(w) can be related in electrolyte solutions with the help of a polynomial [54, 55,56,57]
Actually, Angell used only the first two terms in the representation of electrical conductivities in concentrated solutions of electrolytes [56, 57].
Considering that T 0/T < 1 and (T 0/T)2 ≪ 1, the argument of the exponent can be written in the following form:
and Eq. 16 becomes:
and finally, if the exponent is also expanded, this equation takes the simple form given by Eq. 14. If no exact physical meaning or values are associated with adjustable parameters in Eq. 18, then the representation of specific conductivities at constant T can be reduced to a simple equation having the form:
Thus, the final new equation for electrical conductivities in concentrated solutions has only three A, B and C adjustable parameters
This equation gives an excellent representation of specific conductances and their parameters are easily available using its logarithmic form (Eq. 19). If the specific conductivity has a maximum,
then the maximum appears at the mass fraction given by
In Table 3 are presented A, B and C parameters for the monovalent cation sulfates considered here, w* denotes the concentration region, w max value (if it exists for the investigated sulfate) is calculated from Eq. 22 and finally the standard deviation of specific conductance σ(κ(w)), which gives an indication how the fit of experimental results by using Eq. 21 is good.
5 Conclusions
Electrical conductivities of dilute aqueous solutions of lithium, sodium, potassium, cesium, rubidium and ammonium sulfates were determined from 15 to 35 °C. The literature conductivities and those reported here are consistent. It was observed that sulfates can be represented in dilute solutions as strong electrolytes or partially associated electrolytes of the 1:2 type. Conductances were analyzed using an ion association model, which included the Quint–Viallard conductivity equation and the Debye–Hückel expression for activity coefficients. In this model, the calculated equilibrium constants were considered as adjustable parameters and can be arranged in the following series (NH4)2SO4, Na2SO4, Na2SO4 ≪ Cs2SO4 < Li2SO4 < Rb2SO4.
It was demonstrated that specific conductivities in concentrated aqueous solutions of alkali metal sulfates can be fitted to a new empirical equation with only three adjustable parameters. The proposed conductivity equation for specific conductivities is superior to the usually used Casteel and Amis equation with four adjustable parameters.
References
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 1. Symmetrical 2:2 type electrolytes. Dilute aqueous solutions of alkaline earth metal sulfates and transition metal sulfates. J. Solution Chem. 40, 1209–1233 (2011)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 2. Symmetrical 3:3 type electrolytes. Dilute aqueous solutions of rare earth hexacyanoferrates(III) and hexacyanocobaltates(III). J. Solution Chem. 40, 1724–1734 (2011)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 3. Unsymmetrical 3:1, 1:3, 3:2, 4:1, 1:4, 4:2, 2:4, 1:5 1:6 and 6:1 type electrolytes. Dilute aqueous solutions of rare earth salts, various cyanides and other salts. J. Solution Chem. 40, 1291–1316 (2011)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 4. Symmetrical 2:2, 3:3 and unsymmetrical 2:1, 3:1 and 1:3 type electrolytes in pure organic solvents. J. Solution Chem. 40, 1234–1257 (2011)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Universal curves of limiting conductances and Walden products of electrolytes in mixed solvents. Part 5. Symmetrical 2:2, 3:3 and unsymmetrical 1:2, 2:1 and 1:3 type electrolytes. J. Solution Chem. 40, 1544–1562 (2011)
Fisher, F.H., Fox, A.P.: NaS\({\text{O}}_4^{-}\) ion pair in aqueous solutions at pressures up to 2000 atm. J. Solution Chem. 4, 225–235 (1975)
Chen, C.T.A., Chen, J.H., Millero, F.J.: Densities of sodium chloride, magnesium chloride, sodium sulfate, and magnesium sulfate aqueous solutions at 1 atm. From 0 to 50° and from 0.001 to 1.5 m. J. Chem. Eng. Data 25, 307–310 (1980)
Bhatnagar, O.N., Campbell, A.N.: Osmotic and activity coefficients of sodium sulphate in water from 150 to 250 °C. Can. J. Chem. 60, 1754–1758 (1982)
Isono, T.: Density, viscosity, and electrical conductivity of concentrated aqueous electrolyte solutions at several temperatures. Alkaline-earth chlorides, LaCl3, Na2SO4, NaNO3, NaBr, KNO3, KBr and Cd(NO3)2. J. Chem. Eng. Data 29, 45–52 (1984)
Maksimova, I.N., Pak, C.S., Pravdin, N.N., Razuvaev, V.E., Sergeev, S.V., Ustinov, Y.N.: Physicochemical properties of electrolyte solutions over a wide range of temperatures and concentrations. Deposited Doc. VINITI 4113–4184 (1984)
Phutela, R.C., Pitzer, K.S.: Densities and apparent molar volumes of aqueous magnesium sulfate and sodium sulfate to 473 K and 100 bar. J. Chem. Eng. Data 31, 320–327 (1986)
Holmes, H.F., Mesmer, R.E.: Thermodynamics of aqueous solutions of the alkali metal sulfates. J. Solution Chem. 15, 495–518 (1986)
Lobo, V.M.M.: Electrolyte Solutions. Literature Data on Thermodynamic and Transport Properties. Physical Science Data Series 41. Elsevier, Amsterdam (1990)
Obšil, M., Majer, V., Hefter, G.T., Hynek, V.: Densities and apparent molar volumes of Na2SO4(aq) and K2SO4(aq) at temperatures from 298 K to 573 K and at pressures up to 30 MPa. J. Chem. Eng. Data 42, 137–142 (1997)
Hnedkovsky, L., Wood, R.H., Balashov, V.N.: Electrical conductances of aqueous Na2SO4, H2SO4 and their mixtures. Limiting equivalent ion conductances, dissociation constants, and speciation to 673 K and 28 MPa. J. Phys. Chem. B 109, 9034–9046 (2005)
Mantegazzi, D., Sanchez-Valle, C., Reusser, E., Driesner, T.: Thermodynamic properties of aqueous sodium sulfate solutions to 773 K and 3 GPa derived from acoustic velocity measurements in the diamond anvil cell. J. Chem. Phys. 137, 224501–2245012 (2012)
Kohlrausch, F., Holborn, L.: Das Leitvermögen der Elektolyte. Insbesondere der Lösungen. Methoden, Resultate und Chemische Anwendungen. Druck und Verlag von B.G. Teubner, Leipzig, (1889)
Jones, H.C.: The electrical conductivity, dissociation and temperature coefficient of conductivity (from zero to sixty-five degrees) of aqueous solutions of a number of salts and organic acids. Carnegie Institution of Washington, Publ. No. 170, pp. 48–49 (1912)
Fedoroff, B.: Contribution a l’étude des sulfates simple et double de quelques métaux de la série magnésienne. Ann. Chim. Appl. 16, 155–214 (1941)
Jenkins, I.L., Monk, C.B.: The conductance of sodium, potassium and lanthanum sulfates at 25 °C. J. Am. Chem. Soc. 72, 2695–2698 (1950)
Indelli, A.: Misure crioscopiche sulle soluzioni acquose dei sulfati di litio, sodio e potassio. Ric. Sci. 23, 2258–2266 (1953)
Broadwater, T.L., Evans, D.F.: The conductance of divalent ions in H2O at 10 and 25 °C and in D2O. J. Solution Chem. 3, 757–769 (1974)
Valyashko, V.M., Ivanov, A.A.: Electrical conductivity of alkali metal sulfates at 75 °C. Zhurn. Neorg. Khim. 19, 2978–2983 (1974)
Sharygin, A.V., Grafton, B.K., Xiao, C., Wood, R.H.: Conductance of aqueous solutions of potassium sulfate and lithium sulfate at elevated temperatures. In: Tremaine, P.R., Hill, P.G., Irish, D.E., Balakrishnan, P. (eds.) Steam, Water, and Hydrothermal Systems. NRC Research Press, Ottawa (2000)
Cartón, A., Sobrón, F., Bolado, S., Gerbolés, J.L.: Density, viscosity, and electrical conductivity of aqueous solutions of lithium sulfate. J. Chem. Eng. Data 40, 987–991 (1995)
Fisher, F.H., Fox, A.P.: KS\({\text{O}}_4^{-}\), Na\({\text{O}}_4^{-}\), and MgCl+ ion pairs in aqueous solutions up to 2000 atm. J. Solution Chem. 6, 641–649 (1977)
Fisher, F.H., Fox, A.P.: LiS\({\text{O}}_4^{-}\), RbS\({\text{O}}_4^{-}\), CsSO\({\text{O}}_4^{-}\), and (NH4)S\({\text{O}}_4^{-}\) ion pairs in aqueous solutions up to 2000 atm. J. Solution Chem. 7, 561–570 (1978)
Vasin, S.K., Aleshko-Ozhevskii, Y.P., Klement’eva, I.I.: Ionic association in aqueous solutions of alkali metal sulfates III. M2SO4 + H2O solutions. Zhurn. Fiz. Khim. 54, 1884–1888 (1980)
Pak, C.S., Maksimova, I.N.: Some regularities in changes in density, viscosity and electrical conductivity of solutions of alkali metal salts. Zhurn. Fiz. Khim. 59, 1265–1269 (1985)
Lee, W.H., Wheaton, R.J.: Conductance of symmetrical, unsymmetrical and mixed electrolytes. Part 1.—Relaxation terms. J. Chem. Soc., Faraday Trans. 2 74, 743–766 (1978)
Lee, W.H., Wheaton, R.J.: Conductance of symmetrical, unsymmetrical and mixed electrolytes. Part 2.—Hydrodynamic terms and complete conductance equation. J. Chem. Soc., Faraday Trans. 2 74, 1456–1482 (1978)
Quint, J., Viallard, A.: Relaxation field for the general case of electrolyte mixtures. J. Solution Chem. 7, 137–153 (1978)
Quint, J., Viallard, A.: The electrophoretic effect for the case of electrolyte mixtures. J. Solution Chem. 7, 525–531 (1978)
Quint, J., Viallard, A.: Electrical conductance of electrolyte mixtures of any type. J. Solution Chem. 7, 533–548 (1978)
Quint, J.: Contribution a l’étude de la conductibilité électrique de mélanges d’électrolytes. PhD. Thesis, University of Clermont-Ferrand, April (1976)
Apelblat, A., Weintraub, R., Tamir, A.: Enthalpy of solution of lithium chloride and of lithium chloride monohydrate in water. J. Chem. Thermodyn. 116, 935–941 (1984)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd edn, pp. 425–429. Butterworths, London (1965)
Orekhova, Z., Ben-Hamo, M., Manzurola, E., Apelblat, A.: Electrical conductance and volumetric studies in aqueous solutions of nicotinic acid. J. Solution Chem. 34, 688–700 (2005)
Apelblat, A., Neueder, R., Barthel, J.: Electrolyte Data Collection. Electrolyte Conductivities and Dissociation Constants of Aqueous Solution of Organic Dibasic and Tribasic Acids, vol. XII, Part 4c. Dechema Chemistry Data Series. Frankfurt (2006)
Kielland, J.: Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 59, 1675–1678 (1937)
Apelblat, A.: Dissociation constants and limiting conductances of organic acids in water. J. Mol. Liquids 95, 99–145 (2002)
Postler, M.: Conductance of concentrated aqueous solutions of electrolytes. Coll. Czech. Chem. Commun 33, 2244–2249 (1970)
Pavlova, V.V., Poluden, R.I., Tolsaya, M.A.: Study of the electrical conductivity of concentrated solutions of some alkali metal carbonates and sulfates in the 20–80 °C range. Izv. Vyss. Uczeb. Zaved., Khim. Khim Tekhnol. 19, 862–864 (1976)
Timmermans, J.: The Physico-chemical Constants of Binary Systems in Concentrated Solutions, vol. 3. Systems with Metallic Compounds. Interscience Publ. Inc., New York (1960)
Vivo, A., Esteso, M.A., Llorente, M.L.: Electrolytic conductance of ethanol–water mixtures. III. Limiting conductivity of sodium sulphate at mixtures. Ann. Quimica 77, 204–208 (1981)
Hartley, G.S., Donaldson, G.W.: Transport numbers of unsymmetrical electrolytes and a simplified moving boundary apparatus. Trans. Faraday Soc. 33, 457–469 (1937)
Shilovskaya, M.E., Poluden, R.I., Lenkova, V.I.: Determination of the electrical conductivity of concentrated solutions of some salts in the 20–80 °C range. Teploenergetica (Moscow) 3, 92–93 (1974)
Scatchard, G., Prentiss, S.S.: The freezing points of aqueous solutions. III Ammonium chloride, bromide, iodide, nitrate and sulfate. J. Am. Chem. Soc. 54, 2696–2705 (1932)
Isono, T.: Densities, viscosities, and electrical conductivities of concentrated aqueous solutions of 31 solutes in the temperature range 15–55 °C and empirical equations for the relative viscosity. Rikagaku Kenkyusho Hokoku 61, 53–79 (1985)
Kortüm, G. Treatise on Electrochemistry. Sec. Rev. Ed., Elsevier Publ. Co., Amsterdam (1965)
Righellato, E.C., Davies, C.W.: The extent of dissociation of salts. Part II. Uni-bivalent salts. Trans. Faraday Soc. 26, 592–600 (1930)
Reardon, E.R.: Dissociation constants of some monovalent sulfate ion pairs at 25 °C from stoichiometric activity coefficients. J. Phys. Chem. 79, 422–425 (1975)
Casteel, J.F., Amis, E.S.: Specific conductances of concentrated solutions of magnesium salts in water–ethanol system. J. Chem. Eng. Data 17, 55–59 (1972)
Barthel, J.M.G., Krienke, H., Kunz, W.: Physical Chemistry of Electrolyte Solutions. Modern Aspects. Springer, Darmstadt (1998)
Angel, C.A.: On the importance of metastable liquid state and glass-transition phenomenon to transport and structure studies in ionic liquids. I. Transport properties. J. Phys. Chem. 70, 2793–2803 (1966)
Angel, C.A.: Free volume–entropy interpretation of the electrical conductance of aqueous electrolyte solutions in the concentration range 2–20 N. J. Phys. Chem. 70, 3988–3998 (1966)
Angel, C.A.: Fluidity and conductance in aqueous solutions. An approach from the glassy state and high-concentration limit. I. Ca(NO3)2. J. Phys. Chem. 76, 3244–3253 (1972)
Acknowledgements
Technical assistance of Paulina Veinner is very much appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apelblat, A. The Representation of Electrical Conductances for Polyvalent Electrolytes by the Quint–Viallard Conductivity Equation. J Solution Chem 46, 103–123 (2017). https://doi.org/10.1007/s10953-016-0556-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0556-9