Abstract
Electrical conductivities of dilute aqueous solutions of aluminum sulfate were determined and analyzed in terms of a strongly associated electrolyte of the 3:2 type. The conductivities reported here were determined from 15 to 35 °C. Representation of conductances, in the framework of the ion association model, was performed using the Quint–Viallard conductivity equations for highly charged electrolytes and the Debye–Hückel expression for activity coefficients. Determined apparent association constants K a(T) were considered as adjustable parameters. The determined limiting conductances of the trivalent aluminum ion λ0((1/3)Al3+) are considerably higher than those reported in the literature. Available specific conductivities in concentrated aqueous solutions of aluminum sulfate were fitted by a new empirical equation with only three adjustable parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aluminum is the third most abundant element in the Earth’s crust and therefore its salts are of considerable interest in various chemical industries, in geochemistry, in aqueous solution and soil chemistry, in environmental and atmospheric sciences and in many other areas. In particular, aluminum sulfate is used as a flocculating agent in the treatment of drinking water, as a mordant in dyeing and printing textiles and to balance pH in gardens [1,2,3,4]. Aluminum sulfate forms a number of different hydrates in the solid phase and when dissolved in small quantities in water, hydrolyzes to form the precipitate of aluminum hydroxide and a dilute sulfuric acid solution. Besides the hydrolysis, similar to other highly charged electrolytes, aluminum sulfate has a strong tendency towards ion association. Therefore, ionic equilibria and the speciation of hydrated complexes as a function of concentration and temperature in the aluminum sulfate + water or aluminum sulfate + sulfuric acid + water systems were the subjects of many spectral and thermodynamic investigations. [3, 5,6,7,8,9,10]. The interpretation of experimental results in these systems is not easy considering the coexistence in water of highly charged Al3+ and SO4 2− ions when the sulfate anion has a special mechanism of charge transfer [11].
Compared to the symmetrical 1:1 or 2:2 type electrolytes, electrical conductivities of aqueous solutions with electrolytes of the 3:2 type were rarely determined. Only in two groups of such measurements were performed, in rare earth sulfates and in trialkyldiaminecobalt(III) sulfates [12]. Owing to important role played by Al3+ and SO4 2− ions in geochemical, oceanographic and industrial processes, it is rather surprising to find that conductivity determinations in dilute aluminum sulfate solutions were performed only once by the Jones group in 1912. The actual measurements were performed by Shaeffer and Winston in the 0–65 °C temperature range and from 0.0002 to 0.25 mol·dm−3 [13] However, these results have only historical character. In moderately concentrated solutions at 25 °C, McIntyre et al. [14] determined specific conductance of aluminum sulfate solutions in the 0.05–1.11 mol·dm−3 concentration range. There is also set of κ values in the 0.01–0.65 mol·dm−3 range which was derived from dielectric relaxation spectroscopy (DRS) [10]. Specific conductivities in concentrated solutions of aluminum sulfate were presented graphically by Vasil’eva et al. [15], from 0.03 to 0.32 mass fraction, in the 50 to 95 °C temperature range and reported also by Ivanov et al. [16] from 0.03 to 0.26 mass fraction, in the 25–50 °C temperature range. Unfortunately, as already mentioned in the Ivanov et al. investigation [10, 16], these sets of specific conductivities are inconsistent. Unexpectedly, also the electrical conductivities of other aluminum salts are scarce. In the Lobo compilation of electrical conductivities [17] we found only conductances of moderately concentrated solutions of aluminum bromide at 25 °C [18] and of aluminum perchlorate in the 20–30 °C temperature range [17]. Surprisingly, the conductivities of aluminum chloride and aluminum nitrate solutions were never measured. A survey of the literature data revels that reported values of the limiting conductance of aluminum ion λ 0[(1/3)Al3+] are usually lacking [11, 19, 20], they are uncertain and considerably lower than for other trivalent ions [12]. At 25 °C, Parson [21] and Horvath [22] give the value of λ 0[(1/3)Al3+] = 61.0 S·cm2·mol−1 and Milazzo [23] 63.0 S·cm2·mol−1, but all of them without giving references to original investigations. Frink et al. [5] give the lowest value of λ 0[(1/3)Al3+] = 59.7 S·cm2·mol−1. They added HCl to aluminum chloride solutions to suppress the hydrolysis of aluminum ion and extrapolated to zero ionic strength by means of the Onsager equation for mixed strong electrolytes. The extrapolation performed is presented in graphical form and no actual conductivities for the investigated solutions are reported. It was observed that the Walden product of aluminum sulfate is not constant, but linearly depends on T −1 [3].
In this investigation, for the first time, precise electrical conductances of dilute aluminum sulfate aqueous solutions are reported in the 15–35 °C temperature range and from 0.0001 to 0.0142 mol·dm−3. They are analyzed using a simple chemical model with the Quint–Viallard conductivity equation and the Debye–Hückel expression for activity coefficients. Since, in aqueous solutions 3:2 electrolytes undergo rather complex ion association (formation of various kinds of ion pairs, triple ions and other associates) it is assumed that the formal analytical concentration of solution c is replaced in conductivity equations by cα where α is the fraction of “free” ions where c(1 − α) denotes non-conducting (uncharged) particles. Thus, without going into exact details about speciation in the solution, values of cα and c(1 − α) represent the final result of overall ion association process at a given concentration c. Determination of α fractions (so-called chemical problem) leads to the association constant K a(T) which evidently represent some kind of apparent thermodynamic equilibrium constant. These association constants should be treated as adjustable parameters, but indirectly their values characterize the ion association process in solutions. In very dilute solutions of aluminum sulfate, a very sharp increase in conductances is observed due to hydrolytic reactions. Evidently, this complicates the applied chemical problem and therefore these conductivities are omitted in calculations. Besides, the available in the literature specific conductivities of aluminum sulfate in concentrated solutions [10, 14, 15] are fitted using a new correlation equation which was recently proposed by the present author. This equation has only three adjustable parameters and is mathematically simpler than the usually applied Casteel and Amis equation [20].
2 Experimental
Aluminum sulfate hexadecahydrate Al2(SO4)3·16 H2O purum p.a. (better than 0.95 mass fraction) was purchased from Fluka. It was used without further purification, but it was observed, following the Karl Fisher titration, that the amount of water is slightly lower than expected, and this fact was taken into account in calculations of concentrations. That the material contain only 0.95 of the mass fraction composition of aluminum sulfate given by producers is associated with the difficulty in insuring the exact stoichiometric number of water molecules in the nominal hexadecahydrate.
Solutions were prepared by weight by dissolving aluminum sulfate in doubly distilled water. Conversion from molal to molar units was performed by using densities of pure water [19]. Electrical conductivities of solutions were determined using a glass cell (with a cell constant of 27.4 cm−1) which was immersed in thermostated bath (±0.01 K). The cell was calibrated with dilute potassium chloride solutions [19]. The resistances were determined with the help of a Wayne–Kerr Universal Bridge, model B211. Specific conductances κ were corrected by taking into account impurities dissolved in water (specific conductance less than about 2 × 10−7 S·cm−1). The molar conductances were calculated as Λ = 1000 κ/c. Conversion from molal m to molar c units in dilute solutions was performed by using the densities of pure water [19, 24].
3 Results and Discussion
3.1 The Apparent Thermodynamic Equilibrium Constants and Conductivity Equations
The evaluation of “free” ion fractions c for a given c is based on assumption that the overall association constant K a can be determined from the mass-action equation
where ν+ and ν− are the stoichiometric coefficients (in the present case ν+ν− = 6), f j are the activity coefficients of individual ions (the activity coefficient of undissociated salt is assumed to be unity) and are approximated in dilute solutions by the Debye–Hückel expression
At given temperature T, constants A(T) and B(T) depend on the dielectric constant of pure water [19]
Values of the ion size parameters a j in Eq. 4, were recommended by Kielland [25] and they are a(Al3+) = 9.0 Ǻ and \( a\left( {{\text{SO}}_{4}^{2 - } } \right) = 4.0 \, \) Ǻ. The ion size parameters were assumed to be independent of temperature T. In the conductivity equations, the distance parameters were taken as half the sum of sizes of cation and anion.
Using fixed a j values and assuming K a(T), the fraction of conducting ions α can be evaluated, for any given c and T, by solving the quadratic equation
Molar conductance of electrolyte Λ(c,T) is the sum of ionic contributions λ j (c,T)
where κ is the measured specific conductance, z j are the corresponding charges of the cation and anion (z+ = 3 and z− = −2) and c j are their molar concentrations.
Applying the Quint–Viallard theory, which is valid for any kind of symmetrical and unsymmetrical electrolytes, the ionic conductances λ j (c,T) are represented by the following equations
where the coefficients S j , E j , J 1j and J 2j are complex functions of the limiting equivalent ionic conductances, \( \lambda_{j}^{0} \), the distance parameters a j and the physical properties of water [dielectric constant D(T) and viscosity η(T)]. These coefficients are directly available from [25,26,27,28,29] (for explicit expressions see also [30]).
Combining Eqs. 4–6, in an optimization procedure, it is possible to obtain the best values of the limiting conductance of the aluminum cation λ0((1/3)Al3+) and the apparent thermodynamic association constant K a.
3.2 Conductivities of Aluminum Sulfate in Dilute Aqueous Solutions
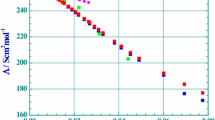
The measured molar conductances of aluminum sulfate in dilute aqueous solutions are presented in Table 1. They can be compared only with an old set of conductances coming from the Jones group [13]. As can be observed in Fig. 1, where values at 25 °C conductances are plotted, the Jones values (in order to compare these conductances with the modern values they should be multiplied by factor 1.066 [31]) are consistent, but systematically lower than those determined here. It is also observed in Fig. 1, that the Λcalc.(c;T) values calculated from the applied chemical-conductivity model differ considerably from the experimental values Λexp.(c;T) in two concentration regions. In very dilute solutions of aluminum sulfate, the conductivity increases strongly due to hydrolysis processes. In moderately concentrated solutions, the high value of the ionic strength (I = 15 cα) makes the Quint–Viallard conductivity equation invalid. However, at each temperature, over the concentration range 0.0002 to 0.003 mol·dm−3, there is excellent agreement between the Λexp.(c;T) and Λcalc.(c;T) values (Table 1). This is expressed by the very low mean standard deviations σ(Λ), and also by values of 100σ(Λ)/<Λ.(c;T)> which lie in the 0.45 to 0.80 range, where <Λ.(c;T)> denotes the average conductivity within the concentration range. It is also clearly evident from Fig. 1, that, due ion association reactions, the conductivities of aluminum sulfate are considerably lower than those expected from the Onsager equation [19] for a fully dissociated 3:2 type electrolyte.
Molar conductances of aqueous solutions of aluminum sulfate at 298.15 K, Λ(c), as a function of square root of concentration c. pink square—fully dissociated 3:2 electrolyte; green square—[13]; blue square—this work; red square—calculated values using the Quint–Viallard conductivity equation for a chosen molecular model, this work (Color figure online)
In the literature, the reported limited conductances of the trivalent aluminum ion λ0((1/3)Al3+) vary from 59.7 to 63.0 S·cm2·mol−1. These values are most likely incorrect because they were derived by an extrapolation from moderately concentrated solutions by assuming that aluminum sulfate is a strong electrolyte. These values are lower by 5–10 S·cm2·mol−1 than those of other trivalent ions [12]. The limiting conductance of λ0((1/3)Al3+) determined in this work, at 298.15 K, is 69.9 ± 1.0 S·cm2·mol−1. It was found, that the change of limiting conductances with temperature is linear for both ions
The temperature dependence of the limiting conductances can also be expressed in terms of the Eyring transition state theory [32]
where d 0(T) is density of pure water and \( \Delta H_{\lambda }^{\dag } (T) \) is the partial molar enthalpy associated with the movement of ions and its value is assumed to be independent of temperature. In the case of investigated ions we have
which gives the partial molar enthalpy, \( \Delta H_{\lambda }^{\dag } \) for the aluminum cation 24.55 ± 0.05 kJ·mol−1 and for sulfate anion 16.74 ± 0.05 kJ·mol−1. The value for Al3+ ion is significantly higher than the corresponding Eyring activation enthalpy for viscous flow of pure water 14.97 kJ·mol−1. This indicates the rearrangement of water molecules in the vicinity of trivalent aluminum ion.
As pointed out by Schrödle et al. [10], the standard thermodynamic association constants for the formation Al(SO4)+ ion at 25 °C vary over the range 1.9 < log10 K 0a < 3.9. If extremal values of K 0a are excluded, its value is 3.4 ± 0.3. In order to compare K 0a with that reported here, the apparent association constants K a (K 0a were determined by different experimental techniques), the stoichiometric factor ν+·ν− = 6 in Eq. 1 should be taken into account, K 0a = ν+·ν− K a. For aluminum sulfate solutions at 25 °C, we have log10[ν+·ν− K a] = 3.6, and this value is very similar to that derived in the literature for the predominant ion-pair, Al(SO4)+. However, it should be taken into account that electrical conductivity Λ(c;T) is an integral property which expresses the overall deviation from expected behaviour of strong electrolytes. Their interpretation depends on the chosen molecular model for solutions with multiply charged ions, on the form of activity coefficients and on validity of conductivity equations. Thus, K a values should be regarded as an additional parameter required to properly represent determined Λ(c;T) curves. Values of the apparent association constants K a have therefore an indicative character and sometimes, as in the present case, they are comparable with thermodynamic results. It was observed that the apparent association constant K a varies linearly with temperature T
3.3 Specific Conductivities of Aluminum Sulfate in Concentrated Aqueous Solutions
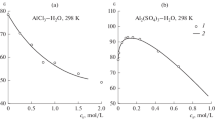
As mentioned above, specific conductivities κ(m,T) in concentrated solutions of aluminum sulfate were systematically determined only by Vasil’eva et al. [15]. They cover a rather higher temperature range, from 50 to 95 °C (in the 0.03 to 0.32 mass fraction concentration range). Unfortunately, these results are presented only in graphical form. At 25 °C, few conductances cover moderate concentrated solutions up to 1.11 mol·kg−1 [10, 14].
Considering the importance of aqueous solutions of aluminum sulfate in many areas of science and technology, it is of interest to correlate the available literature specific conductances. At constant temperature T, specific conductivities of electrolytes in pure or mixed solvents are usually performed using the empirical equation proposed in 1972 by Casteel and Amis [20]
Different concentration units can be used in the Casteel and Amis equation, molalities m can be replaced by molarities c or by mass fractions w. This equation includes four adjustable parameters a, b, m max and κ(m max) and these parameters have no physical meaning. The last two parameters are not actual values of the maximum of specific conductivity and the corresponding concentration at which the maximum is situated. They also should be determined in fitting procedures. In many cases maxima do not exist at all or a broad maximum κ(m max) is observed, if the solubility is high enough to reach it. Recently, the present author proposed replacing Eq. 11 with a new empirical equation which gives an excellent representation of specific conductivities. The advantage of this equation is that at constant T it includes only three adjustable parameters, and in the logarithmic form is [33]
or explicitly
where w is mass fraction of the salt (other concentration units can also be introduced in Eq. 13). As in the Casteel and Amis equation, no physical meaning is associated with A, B and C. These parameters can be easily determined by using Eq. 12. If the maximum of specific conductance exists
then its position can be determined directly from
For example, using the Schrödle et al. [10] and McIntyre et al. [14] results, expressed in molarities, the specific conductances of aluminum sulfate solutions at 25 °C can be represented by
where σ(κ) denotes the mean standard deviation of specific conductivity. Using Eqs. 15 and 16, the maximum values are κ(c max) = 3.352 S·m−1 and c max = 0.52 mol·dm−3.
If specific conductances presented in graphical form in the Vasileva et al. [15] investigation are converted into a digital set, then it is possible to represent them very satisfactorily by Eq. 13. Results of such calculations are summarized in Table 2. It was observed that the position of maximum of specific conductivity can be correlated linearly with temperature T
and this equation is valid in the 50–95 °C temperature range.
4 Conclusions
Electrical conductivities of dilute aqueous solutions of aluminum sulfate were for the first time determined from 15 to 35 °C. These were analyzed using an ion association model, which included the Quint–Viallard conductivity equations for the 3:2 type electrolyte and the Debye–Hückel expression for activity coefficients. Evaluated apparent association constants K a were considered as adjustable parameters. The applied model covers the 0.00026 < c < 0.003 mol·dm−3 concentration range where aluminum sulfate is strongly associated. The calculated limiting conductances of trivalent aluminum ion λ0((1/3)Al3+) are considerably higher than those reported in the literature. It was demonstrated that specific conductivities in concentrated aqueous solutions of aluminum sulfate can be fitted to a new empirical equation with only three adjustable parameters.
References
Rudolph, W.W., Mason, R.: Study of aqueous Al2(SO4)3 solution under hydrothermal conditions: sulfate ion pairing, hydrolysis, and formation of hydronium alunite. J. Solution Chem. 30, 527–548 (2001)
Ridley, M.K., Wesolowski, D.J., Palmer, D.A., Kettler, R.M.: Association quotients of aluminum sulphate complexes in NaCl media from 50 to 125 °C: results of a potentiometric and solubility study. Geochim. Cosmochim. Acta 63, 459–472 (1999)
Baghalha, M., Papangelakis, V.G.: High-temperature conductivity measurements for industrial applications. 2. H2SO4–Al2(SO4)3 solutions. Ind. Eng. Chem. Res. 39, 3646–3652 (2000)
Huang, M., Papangelakis, V.G.: Electrical conductivity of concentrated Al2(SO4)3–MgSO4–H2SO4 aqueous solutions up to 250 °C. Ind. Eng. Chem. Res. 46, 1598–1604 (2007)
Frink, C.R., Peech, M.: Hydrolysis of aluminum ion in dilute aqueous solutions. Inorg. Chem. 2, 473–478 (1963)
Akitt, J.W., Farnsworth, J.A., Letellier, P.: Nuclear magnetic resonance and molar volume studies of the complex formed between aluminium(III) and the sulphate anion. J. Chem. Soc. Faraday Trans. 1(81), 193–205 (1985)
Reardon, E.J.: Ion interaction parameters for aluminum sulfate and application to the prediction of metal sulfate solubility in binary salt systems. J. Phys. Chem. 92, 6231–6426 (1988)
Kaatze, U., Giese, K.: Dielectric spectroscopy on some solutions of 3:2 valent electrolytes. A combined frequency and time domain study. J. Mol. Liq. 36, 15–35 (1987)
Dickson, A.G., Wesolowski, D.J., Palmer, D.A., Mesmer, R.E.: Dissociation constant of bisulfate ion in aqueous sodium chloride solutions to 250 °C. J. Phys. Chem. 94, 7978–7985 (1990)
Schrödle, S., Rudolph, W.W., Hefter, G., Buchner, R.: Ion association and hydration in 3:2 electrolyte solutions by dielectric spectroscopy: aluminum sulfate. Geochim. Cosmochim. Acta 71, 5287–5300 (2007)
Kortüm, G.: Treatise on Electrochemistry. Elsevier, Amsterdam (1965)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 3. Unsymmetrical 3:1, 1:3, 3:2, 4:1, 1:4, 4:2, 2:4, 1:5 1:6 and 6:1 type electrolytes. Dilute aqueous solutions of rare earth salts, various cyanides and other salts. J. Solution Chem. 40, 1291–1316 (2011)
Jones, H.C.: The electrical conductivity, dissociation and temperature coefficient of conductivity (from zero to sixty-five degrees) of aqueous solutions of a number of salts and organic acids. Carnegie Institution of Washington, Washington (1912)
McIntyre, J.F., Foley, R.T., Brown, B.F.: Ultraviolet spectra of aluminum salt solutions. Inorg. Chem. 21, 1167–1672 (1982)
Vasil’eva, L.F., Gitis, E.B., Shmorgun, V.I.: Electric conductivity of aluminum sulfate aqueous solutions. Zhurn. Prikl. Khim. 49, 2539–2541 (1976)
Ivanov, A.A., Kirilenko, I.A., Selin, A.N., Zaitseva, L.A.: Properties of concentrated aqueous solutions of aluminium sulphate. Zh. Neorg. Khim. 32, 1052–1056 (1987)
Lobo, V.M.M.: Electrolyte Solutions Literature Data on Thermodynamic and Transport Properties, Physical Science Data Series 41. Elsevier, Amsterdam (1990)
Canpbell, A.A.: The conductances and viscosities of aqueous solutions of indium tribromide, indium nitrate, and aluminum bromide at 25 °C. Can. J. Chem. 54, 3732–3736 (1976)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd edn. Butterworths, London (1965)
Barthel, J.M.G., Krienke, H., Kunz, W.: Physical Chemistry of Electrolyte Solutions. Modern Aspects. Springer, Darmstadt (1998)
Parson, R.: Handbook of Electrochemical Constants. Butterworths, London (1959)
Horvath, A.L.: Handbook of Aqueous Electrolyte Solutions. Ellis Horwood Limited, Chichester (1985)
Milazzo, G.: Electrochemistry Theoretical Principles and Practical Applications. Elsevier, New York (1963)
Apelblat, A., Azoulay, D., Sahar, A.: Properties of aqueous thorium nitrate solutions I. Densities, viscosities, conductivities, pH, solubility and activity at freezing point. J. Chem. Soc. Faraday Trans. 69, 1618–1623 (1973)
Kielland, J.: Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc 59, 1675–1678 (1937)
Quint, J., Viallard, A.: Relaxation field for the general case of electrolyte mixtures. J. Solution Chem. 7, 137–153 (1978)
Quint, J., Viallard, A.: The electrophoretic effect for the case of electrolyte mixtures. J. Solution Chem. 7, 525–531 (1978)
Quint, J., Viallard, A.: Electrical conductance of electrolyte mixtures of any type. J. Solution Chem. 7, 533–548 (1978)
Quint, J.: Contribution a l’étude de la conductibilité électrique de mélanges d’électrolytes. PhD Thesis, University of Clermont-Ferrand, April (1976)
Apelblat, A., Neueder, R., Barthel, J.: Electrolyte Data Collection. Electrolyte Conductivities and Dissociation Constants of Aqueous Solution of Organic Dibasic and Tribasic Acids, vol. XII, Part 4c. Dechema Chemistry Data Series. Frankfurt (2006)
Apelblat, A.: Dissociation constants and limiting conductances of organic acids in water. J. Mol. Liq. 95, 99–145 (2002)
Brummer, S.B., Hills, G.J.: Kinetics of ionic conductance. Part 1. Energies of activation and the constant volume principle. Trans. Faraday Soc. 57, 1816–1822 (1961)
Apelblat, A.: The representation of electrical conductances for polyvalent electrolytes by the Quint–Viallard conductivity equation. Part 7. Unsymmetrical 1:2 type electrolytes. Alkali metal (Li, Na, K, Rb and Cs) sulfates and ammonium sulfate. J. Solution Chem. 46, 1103–1123 (2017)
Acknowledgements
The author is very much grateful to Professor Richard Buchner, Regensburg University, Germany, to Professor Vladimiros G. Papangelakis, Toronto University, Canada, for valuable comments related to aluminum sulfate solutions, to Professor Marija Bešter-Rogač, Ljubljana University, Slovenia, for converting specific conductivities from graphical to digital form, and finally to Paulina Veinner for her capable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apelblat, A. The Representation of Electrical Conductances for Polyvalent Electrolytes by the Quint–Viallard Conductivity Equation. J Solution Chem 46, 1165–1175 (2017). https://doi.org/10.1007/s10953-017-0630-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0630-y