Abstract
The contamination caused by plastic is an environmental problem due to its high production and inadequate final disposal. Besides, plastic has low or null biodegradation capacity, which poses an alarming issue. So, looking for new income to produce plastics such as films is necessary for some applications. This study aimed to produce and characterize biodegradable films using gelatin, cellulose, glycerol, and Furan-Phenol Conjugates (FPC) from thermally modified Agave vinasses (concentrated in furans, phenols, and sugars). Conjugates were added in 1, 2, and 3% to produce gelatin-cellulose films. Chemical characterization using Fourier transform infrared spectroscopy (FTIR) and mechanical properties were measured by texturing equipment, thermal capacity with differential scanning calorimetry (DSC), and morphological characterization was used by environmental scanning electron microscope (ESEM). The biodegradability of films was determined by weight loss. The films showed characteristic peaks for phenolic compounds and furans such as 5-hydroxymethyl furfural (5-HMF), respectively, with signals at 2930 cm−1 and 1648 cm−1. Mechanical tests indicated that adding FPC improved the mechanical properties of the films. Besides, they increased the melting temperature in all samples. After 30 days of soil burial test, the films showed a weight loss of 95.1% for FPC-1, 87.9% for FPC-2, and 82.73% for FPC-3. Using residual waste as vinasses as an improver of the properties of biodegradable films could be the first step toward a circular economy for residues from distilleries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic and microplastic pollution are a global threat affecting all ecosystems. The increase in consumption of petroleum derivatives (plastic, oil, gasoline, among others) has been related significantly to the increase of greenhouse gas (GHG) and climate change [1]. It requires urgent management to mitigate the problems of final disposal [2]. It is estimated that 275 million metric tons of plastic are produced worldwide annually, and from 4.8 to 12.7 million metric tons are deposited in the oceans. Single-use plastics such as plastic bags and microbeads are the primary contributors to this pollution [3, 4]. In the soil, plastics have significantly impacted agriculture; the principal effects are modifying the microbiota and the mobility of other contaminants that alter the soil ecosystem functionality [5]. For this reason, alternatives have been sought to help mitigate the environmental problems generated by synthetic polymers due to their low or null degradability.
The circular economy based on biological sources requires the correct use of resources [6]. Many commercial products, such as paper, biofuels, and bioplastics, are industrially produced. However, obtaining biopolymers from crops of economic importance increases prices and limits access to these products [7]. For this reason, there is a growing interest in using non-conventional waste, such as those generated in industries and agriculture. The starch, cellulose, pectin, and protein, among other biopolymers, can be isolated from biomass from agro-industrial residues [8]. These biopolymers present mechanical, physicochemical, thermal, and structural properties that make them very similar to those obtained from synthetic plastics [9].
Plastic films or bioplastics can be synthesized from natural organic materials such as polysaccharides, proteins, and lipids, and their biodegradability capacity makes them an alternative to reduce the large volumes of synthetic materials produced annually derived from oil [10].
The proteins used as biopolymers provide multiple molecular interactions (covalent, ionic, and hydrogen bonds) with the biopolymers present in the mixtures, creating materials with improved mechanical properties than bioplastics produced from polysaccharides and lipids [11, 12]. It is necessary to search for biomolecules that can provide firmness and rigidity to biomaterials and offer similar characteristics to plastic made from petroleum derivatives. Some authors have reported the development of biodegradable films using proteins and reinforcing with cellulose nanofiber [13], cellulose nanocrystals [14], and microcrystalline cellulose [15]. However, other compounds could give new alternatives to biomaterial, and they could be found in several agricultural wastes such as 5-HMF, present in the vinasses, a waste of ethanol, and spirit beverage industries.
The ethanol industry generates 10 to 15 L of vinasses per liter of ethanol processed [16]. Similar vinasses are produced in the state of Jalisco, Mexico, from the tequila factories [17]. The Tequila Regulatory Council (TRC) estimates 2288 tons of Agave tequilana Weber var. Azul was used in 2023, generating approximately 5.9 × 109 L of vinasses [18].
The composition of vinasses is complex, presenting a high percentage of water, inulin, fermentable sugars, organic acids, esters, alcohols, lignocellulosic compounds, 5-HMF, furfural and phenolic compounds [19]. Its high organic matter content generates severe problems in agricultural soils due to its irrigation use or inadequate disposal into water bodies [20]. In addition, the treatment for their correct disposal is expensive and not complete by physical, chemical, biological, or combinations [21].
FPCs are mainly composed of furans as 5-HMF, phenols (caffeic, ferulic, 3-hydroxybenzoic, chlorogenic, vanillic, syringic, sinapic, 2,4-di-ter-buthylphenol, salicylic, and cumaric acid) and sugars (xylose, glucose, fructose, and sucrose) from Agave vinasses after two thermal treatments using H2O2 as catalyst [22]. Some molecules in vinasses are precursors of compounds of economic importance due to their ability to form materials with characteristics like synthetic plastics. The 5-HMF is included in the list of the twelve most promising biobased molecules in forming materials biofuels, and as a quality indicator of food, it could be harmful if consumed in excess [23, 24]. Some foodstuffs that contain high concentrations of 5-HMF include coffee (900–1200 mg/kg), breakfast cereals (15–717 mg/kg), corn syrups (47–1655 mg/L), honey (2–232 mg/L), among others [24]. The 5-HMF synthesizes polyethylene furanoate, a material with characteristics like polyethylene terephthalate [7, 25, 26]. Their chemical structure with furan ring and functional groups makes it a material promising for various chemical transformations [27] such as fuels, organic solvents, biologically active molecules, and obtaining plastics [28].
On the other hand, adding inulin to films gelatin-base and cellulose increases the physicochemical properties, lowers water vapor permeability, and improves mechanical properties [29, 30]. The phenolic compounds are considered additives, and their use in forming films could contribute as natural antioxidants [31, 32]. In addition, the development of active, edible, and biodegradable films and coatings has emerged as one of the most significant trends in medicine and food science over the last few decades.
This study proposes an alternative to add value to ethanol distilleries' residues. To our knowledge, this is the first instance where biodegradable films have been developed with the addition of Agave vinasses conjugated (FPC). This study aimed to fabricate a material with good biodegradable characteristics and mechanical, chemical, and structural properties like commercial plastics.
Materials and Methods
Materials
The biopolymers used were gelatin (Duche, Mexico) with 300 Bloom, glycerol (Jalmex, Mexico) used as a plasticizer, and micro cellulose (Sigma-Aldrich™). Dowex Optipore® supplied the SD2 resinore® and the furfural, 5-HMF, xylose, glucose, fructose, sucrose, hydrogen peroxide, sodium chloride, caffeic acid, ferulic acid, 3-hydroxybenzoic acid, chlorogenic acid, vanillic acid, syringic acid, sinapic acid, 2,4-di-ter-buthylphenol acid, salicylic acid and cumaric acid were supplied by Sigma-Aldrich™.
Characterization of Agave Vinasses
Tequila factories provided the vinasses with an artisanal distillery process in Arenal, Municipality of Jalisco, Mexico. The composition of the vinasse original and FPC is shown in Table 1. Analytic methods determined the characterization of vinasses as UPLC-MS (XEVO TQS Micro) (Waters, Milford, USA) for furans, phenolic compounds, glucose, fructose, and xylose, and HPLC-SEC (1220 Infinity LC System HPLC) (Agilent, Alpharetta GA, USA) was used to determine the concentration of inulin according to methods reported by Lorenzo-Santiago et al. [22]. The compounds were quantified using standard reactive with curves in 1, 2.5, 5,12.5, 25 and 50 mg/L for furans, 0.1, 0.5, 1, 2.5 and 5 mg/L for phenolic compounds, 5, 10, 15, 20, 25, and 50 mg/L to xylose, sucrose, glucose and fructose, and 1, 2, 4, 6, 8, 10, 16, and 20 g/L for inulin. The mass spectrometric conditions are shown in Table 2.
Chemical Oxygen Demand (COD) was determined using vials (20–1500 mg/L) (Hach, Mississauga, ON, Canada). The samples desorbed were prepared at a 1:100 dilution, and 2 mL of sample was added to the vials. A blank sample with deionized water was calibrated to zero the absorbance on the spectrophotometer at 550 nm. They are stirred and placed in the digester at 150 °C for 2 h. Subsequently, they were allowed to cool down and analyzed in the spectrophotometer DR 5000™ UV–Vis (Hach, Mississauga, ON, Canada) were used [33].
Thermal Modification of vinasses Agave to Obtain FPC
The vinasses were used to pH 2.4 (natural acidity) and adjusted to pH 7.0 using NaOH to 0.5 N. The experiment was performed in triplicate for each sample and thermal treatment. The Resin SD2 was previously suspended in water. The resin was eluted from ethanol with 140 mL of 4% HCl, followed by distilled water until the pH of the eluent became neutral. In 500 mL Erlenmeyer flasks, 10 g of resin was combined with 100 mL vinasse. Batch adsorption occurred at 25 °C with agitation at 45 rpm for 24 h. For desorption testing, the supernatant was removed by settling, and the resins were hydrated with 50 mL using 95% ethanol: water (v/v). The desorbed effluents underwent two thermal treatments with 0.5% (v/v) of hydrogen peroxide (H2O2) as a catalyst, heating to 130 ºC for 40 min in an autoclave at 34 psi. The conjugates obtained were denominated FPC, as was reported by Lorenzo-Santiago et al. [22] in previous work.
Formulation of Biodegradable Films
Biodegradable films were developed by applying modifications to the methods described by Rendón-Villalobos et al. [34] and Jafari et al. [35] particularly in the gelatinization temperature and concentration of biopolymers. The formulation was prepared from gelatin (3% w/v), cellulose (0.1% w/v), glycerol (0.9% w/v), FPC (1, 2, and 3% w/v), and distilled water (96, 95, 94 and 93% v/v). The proposed formulations and the codes utilized for each film are shown in Table 3. The gelatin was hydrated with distilled water and kept at 60 °C for 10 min. Glycerol, cellulose, and FPC were applied until it reached 65 °C for 10 min. The filmogenic solution was cast onto the Petri dishes (90 mm in diameter) and dried at 40 °C for 24 h. The films obtained were peeled off and stored in a desiccator at 25 °C with a relative humidity of 50% provided by a saturated solution of NaCl following the ASTM F1927-20 method [36] up to characterization analysis. Some biopolymers can absorb or lose moisture depending on the surrounding humidity [37]. This can lead to changes in their flexibility, tensile strength and their biodegradability capacity [38, 39]. By conditioning at a standard relative humity of 50%, we ensure that the material reaches a moisture equilibrium, thereby preventing any fluctuations in film properties that could affect test results. In addition, all films were management at same relative humidity condition.
Water Solubility
The films were cut into squares (20 mm × 20 mm). Subsequently, they were subjected to drying for seven days in a desiccator at room temperature. After that, the samples are weighed and placed in a 50 mL falcon tube with 30 mL of distilled water. The tubes were shaken for 48 h at 200 rpm at room temperature. The tube contents were filtered and dried at 110 °C for 24 h. The percentage of soluble matter was calculated using Eq. 1 [40]. The tests were carried out in triplicate in all formulations and compared with the commercial polymer plastic used as control.
Determination of Mechanical Properties
The films were analyzed following the ASTM D-882-02 method [41]. Films of each formulation with FPC (1–3%), control (gelatin-cellulose), and commercial plastic film (low-density polyethylene (LDPE) bag) were cut (100 mm long by 10 mm wide). The deformation rate used was 30 mm/min until it was broken, with a gap between heads of 50 mm. The mechanical properties analyzed were tensile strength (TS), elongation (%E), and Young's modulus (Ym). These were determined using a BlueHill Lite from INSTRON Model 2519–107 (Massachusetts, USA).
Thermal Characterization by DSC
Thermal characterization was realized using a DSC, TA Instruments DSC 2010 (New Castle, USA). The equipment was calibrated using indium with a melting point of 156.4 °C and an enthalpy of 6.8 cal/g. Each sample (FPC 1–3% and control (gelatin-cellulose)) was carefully weighed between 5 and 10 mg. The sample was used for a heating program that ranged from 20 to 300 °C, with a heating rate of 10 °C/min. During the measurements, 50 mL/min of nitrogen gas (99.9% purity was circulated) was used to maintain an inert atmosphere.
Biodegradability in Soil
Biodegradability analysis by weight loss was performed as per ASTM D5988-12 [42]. Films with FPC (1–3%) and control (gelatin-cellulose) were cut into squares (40 mm × 40 mm), weighed, and deposited in a Petri dish containing 20 g of agricultural soil adjusted to 25% moisture and were incubated at 30 °C. The soil was characterized by texture [43] USDA modified soil texture triangle, pH in water [44], organic C content [45], total N [46], electrical conductivity [47], and total phosphorous [48]. The soil characterization is shown in Table 4.
The film samples were weighed at 0, 5, 10, 15, 20, and 30 days of soil exposition. A negative control was included in the tests using a commercial plastic, such as a low-density polyethylene (LDPE) bag [34]. The remaining films were cleaned using distilled water until all soil was removed, and they were dried for 24 h at 40 °C. Finally, their weight was recorded. The biodegradability percentage was calculated from Eq. 2. Photographic monitoring was done at 0, 5, 10, 15, 20, and 30 days of soil exposition. Also, a surface analysis was realized by ESEM and structural composition by FTIR at 0 and 15 days, described below.
Structural Characterization by ESEM
Films were adhered to a double-adhesion carbon conductive tape. The characterization was realized by ESEM Carl Zeiss EVO LS 10 (Munich, Germany). Subsequently, they were observed at a voltage of 15 and 30 kV, with a resolution of 3─10 nm; the micrographs were taken in surface and cross-sectional at 100×and 500×magnifications [49].
Chemical Composition by FTIR
The biodegradable films (FPC 1–3%), control (gelatin-cellulose), and commercial plastic from 0 and 15 days (from biodegradability assay) were previously cut into dimensions of 10 mm2. FTIR was employed for the structural characterization of films, utilizing a Perkin Elmer Spectrum 100/100 N model (Shelton, CT, USA). The FTIR analysis was conducted in the range of 4000–650 cm−1 in the transmittance mode, with a resolution of 16 cm−1, and eight scans were performed [19].
Statistical Analysis and Software Used
Statistical analyses were performed with Minitab 20.3 software. All results obtained were subjected to a variance analysis (ANOVA) and post hoc Tukey test with a probability of 5% (p < 0.05) with a confidence level of 95% to analyze the differences between the concentration FPC added in films and the capacity of biodegradation in soil. The abstract graphic for this manuscript was done with BioRender [50].
Results and Discussions
Characterization of Furan-Phenol Conjugates (FPC)
The thermal treatment of residual vinasses, catalyzed by H2O2 and adjusted to a pH of 7.0, significantly reduced the concentration of Chemical Oxygen Demand (COD) by 47%. The treatment also led to a reduction in the concentration of total sugars (58.7%), sucrose (94.62%), fructose (75.37%), glucose (84.79%), and xylose by 40.39%, compared to the original vinasse (Table 1). Notably, the concentration of 5-HMF increased 13-fold, and the phenolic compounds showed a significant increase, i.e., the 3-hydroxybenzoic acid (512-folds), 2,4-di-tert-butylphenol acid (290-folds), salicylic acid (77-folds) and syringic acid (56-fold).
The decrease in sugar concentration is likely due to the increase in 5-HMF, with fructose being the primary promoter of furan formation [51]. The reaction mechanisms of sugars from Agave vinasses in the conversion to furans and phenolic compounds have been previously reported [19].
Water Solubility
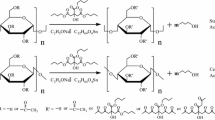
Solubility in water is an important parameter to consider, depending on the application of materials. A high solubility is undesirable when the product will be used with food or comes into direct contact with liquids, such as in packaging, bags, plastics for agricultural use, and other applications [52, 53]. However, there are different applications where high solubility might be necessary, such as in edible coatings for fruits and vegetables [54]. Table 5 shows the water solubility of all films evaluated. The positive control presented the highest solubility (48.92%), attributed to gelatin and glycerol's hydrophilic nature[55]. It is necessary to cross-link polymeric chains or use two or more polymers to increase the solubility [56]. The proposed reaction between compounds is illustrated in Fig. 1.
Adding FPC decreased water solubility in this study. The FPC-3 film showed the lowest solubility at 39.89%. This decrease could be attributed to the formulation's higher content of 5-HMF. 5-HMF can undergo oxidation to form 2,5-furandicarboxylic acid (FCDA) or hydrogenation to form 2,5-dimethylfuran (DMF); both are used in the production of chemicals and fuels [57,58,59]. These compounds may contribute to higher stability in the materials and lower solubility. All films showed significantly higher water solubility than the commercial plastic (Table 5).
These results are consistent with those reported by Almeida Soares et al. [60] for films made from chitosan adding pomegranate (Punica granatum L.) peel extract. The films showed a solubility of 20% for the lowest concentration of extract added in the formulation and 10% for the highest concentration. The solubility in these films decreased when the concentration of bioactive matter was increased in the formulation. The decreased solubility in films made from biopolymers combined with conjugates could suggest a potential to replace conventional plastics, and FPC could improve mechanical properties.
Mechanical Properties
Table 5 displays the mechanical properties of biodegradable films supplemented with conjugates from vinasses and commercial plastic film. The gelatin-cellulose film (control) exhibited a lower elongation (%E) and Young's modulus than all other films. However, there was no significant difference in tensile strength (TS) between the control and FPC-1, FPC-2, and FPC-3 films. These properties could be attributed to the addition of cellulose in the formulation of the control sample. Cellulose is often used as a reinforcement material due to its mechanical and thermal properties, particularly when the polymer matrix cannot form strong bonds or requires additional stability [15, 49].
The sample FPC-3 presented a significative higher (%E) and Young's modulus than FPC-1, FPC-2, and control films but significantly lower with commercial plastic (Table 5). The FPC-3 film increased 2.14 folds the elongation percentage (63.15%) and 3.62 folds Young´s modulus (82.92 MPa), compared to the control film, suggesting that the addition of conjugates provides a high elasticity and resistance into the films. Using a 3% concentration of FPC resulted in significantly higher elongation values and Young’s modulus in the films. This behavior can be attributed to cellulose, inulin, glycerol, and 5-HMF interaction with the gelatin matrix. These compounds are known to form hydrogen bonds with the amine (NH2) and carboxyl (COOH─) groups of gelatin, thereby enhancing the mechanical properties of the films (Fig. 1) [61, 62].
Some authors propose the addition of high-density or low-density polyethylene with modified composites to improve the mechanical capacities [63,64,65]. However, the degradation time increases, and residual microparticles are not degradable in the environmental matrixes after degradation.
Harussani et al. [66] reported tensile properties and Young´s modulus in cornstarch films added 45 to 60% of glycerol and sorbitol, obtaining 4.49 to 13.61 MPa in tensile strength and Young´s modulus from 11.88 to 61.7 MPa, values like showing in this work. It was reported that adding 30% compound plastifiers (such as glycerol and sorbitol) to films could generate less rigid films [66]. Choi et al. [67] prepared films with possible uses in the food industry using turmeric and gelatin added with tannic acid, caffeic acid, and green tea extract. They reported that low concentrations of phenolic compounds cause a reticulation effect, modifying the chemical properties of biopolymers and forming more resistant materials.
Thermal Characterization by DSC
This study used DSC to investigate the thermal capacity of films with gelatin base and added to Agave vinasse conjugate. All films showed endothermic peaks at various temperature ranges (Fig. 2). The control film showed an endothermic peak that occurred at 115.8 °C. After the addition of conjugates, the thermal capacity of the FPC-1, FPC-2, and FPC-3 films increased by 19.5, 21, and 21.3 °C, respectively, compared to the first endothermic peak of the control sample. According to the report by Nunes et al. [68], the peaks that oscillate at 100 °C represent the evaporation of water. Al-saidi et al. [69] reported that this peak could be related to amine, carboxylic, and hydroxyl groups present in the samples or the evaporation of the free water molecules from the hydrophilic components of the polymers [70].
In this work, the melting temperature (Tm) increases with the addition of conjugates, and the enthalpy decreased at 71.5, 99.5, and 91.7 J/g for FPC-1, FPC-2 and FPC-3, respectively, compared to of the control film (263.1 J/g). These results may be attributed to the stability of hydrogen bond chains formed within the polymeric material (Fig. 1) [71]. Additionally, Andrade et al. [66] report that adding phenolic compounds, such as ferulic acid, could promote crosslinking, enhance crystallinity, and increase film thermal capacity.
The second peak of the control film was presented at 218.1 °C with an enthalpy of 7.3 J/g. This enthalpy could be attributed to the addition of cellulose in the formulation [49]. The thermogram showed melting temperatures at 229.8, 235, and 239.2 °C, related to thermal decomposition of gelatin. Similar results were reported by El-Meligy et al. [72] showing a degradation temperature at 230 °C in bovine type B gelatin. The enthalpies were 12.7, 9.5, and 13.8 J/g for FPC-1, FPC-2, and FPC-3, respectively. According to a report by Lorenzo-Santiago et al. [19], the vinasses could be composed of lignocellulosic compounds such as hemicellulose, lignin, and cellulose. Among these, cellulose exhibits a crystalline structure that can enhance the thermal stability of materials [73]. High thermal stability could help produce biodegradable films from the casting or extrusion process [74].
Surface Morphology by ESEM
The morphology from the film's surface and cross-sectional were observed in Figs. 3a and 3b, respectively. Micrographs illustrate the morphology of the films at the initial stage (before degradation in soil). The control film presented a corrugated surface, revealing the structure of the gelatin and glycerol, which were not well distributed. The cross-sectional view displayed low integration and fractures, with porosity visible on all surfaces.
ESEM Micrograph of (a) surfaces (100×) (red circle indicates the lack of homogeneity on the surface of the films), and (b) cross Sects. (500×) from control, FPC-1, FPC-2 and FPC-3 films at 0 days (red circle indicate presence of pores and blue arrow the integration of materials), and (c) surface ESEM images (500×) of films after of 15 days of biodegradation test (red circle indicate presence of pores and blue arrows the presence of microorganisms)
The film FPC-1 displayed varying coloration and an irregular surface, which may be attributed to uneven dispersion of the conjugates within the matrix, leading to discontinuous regions or cavities in the film. However, the cross-sectional view revealed a reduction in porosity. The films FPC-2 and FPC-3 presented a compact surface, smooth and flat, suggesting that films with a higher content of conjugates can achieve better dispersion and compatibility within the gelatin-cellulose matrix. Also, it is attributed to the presence of the carboxyl and amine groups and the formation of hydrogen bonds between the compounds, which aligns with the observed results for the mechanical and thermal properties in films (Fig. 1). Similar results were reported for pea starch/pulp cellulose nanofiber composite biodegradable films added with apple polyphenols [75].
Chemical Characterization by FTIR
The films were compared with the structural composition of a plastic bag and a film without conjugates (Control gelatin-cellulose) (Fig. 4a).
The polymer plastic spectrum displayed absorption bands at 2920 and 2845 cm−1, indicating C–H stretching. Another peak was observed at 1740 cm−1, corresponding to C = O stretching, while peaks at 1460 cm−1 and 720 cm−1 were attributed to CH2 bending and CH2 rocking, respectively, characteristics of high-density polyethylene [76]. The FPC 1–3% of conjugate and control film presented a strong signal to 3280 cm−1, corresponding to the O–H stretching vibration. This peak is associated with water molecules, alcohols, carboxylic acids, sugars such as inulin, and other organic compounds [77]. The increase of this peak was related to the increase in vinasse conjugate, with the film FPC-3 having the most extended peak.
Other characteristic peaks were phenolic compounds. The signal 2930 cm−1 indicated the asymmetric vibration of the aliphatic structures, and 1446 cm−1 the antisymmetric bending in the CH3 bond [78]. On the other hand, the signals for 5-HMF were observed in 1648 cm−1, a region that expresses the vibrations stretching of the aldehyde group (C = O), and in 1522 cm−1 the stretching vibrations of the ring (C = C) [79, 80].
The range 1084 to 800 cm−1 region has been assigned to the C–O stretching vibration, CH2–OH and C–O–C. Functional groups in this region are associated with the compounds with structures, typical structures of carbohydrates such as glucose, xylose, fructose, sucrose, and inulin [77, 80, 81].
Biodegradability of Films by Weight Loss in Soil
The weight loss methodology is the most utilized index for elucidating the degradation rate across varying treatments [34, 79, 82]. The biodegradation process of all samples in the soil can be observed through photographic analysis (Fig. 5), and the percentage of biodegradability by weight loss from films is shown in Fig. 6.
Films biodegradability percentage recorded in soil. Different capital letters indicate significant differences between films on the same day, while different lowercase letters indicate significant differences in the same film between all times. Tukey's test was used with a significance level of p < 0.05. Bars represent the standard error
The polymeric plastic does not present changes after 30 days in soil. The exposition in the soil is not sufficient to present a structural modification or weight loss in polymeric plastic.
Huang et al. [83] reported that the exposition of polyethylene films in the soil for 50 to 100 days could change the microbiota in the soil, but the biodegradability is not representative.
At 5 days in soil, all samples presented a biodegradation lower than 17%, with the FPC-1 (16.2%) film having the highest biodegradability, followed by the control (15.9%) (Fig. 5). However, there are no significant differences between both.
At 10 and 15 days in soil, the control film exhibited the highest biodegradation, reaching 45% and 63%, respectively, while the FPC-1 films showed 29.3% and 51.7%. Similar results were reported by Susmitha et al. [84] with a degradation of 50% after 15 days of exposition in the soil of edible films of corn starch-gelatin integrated with mango and pineapple. In contrast, FPC-2 and FPC-3 demonstrated lower biodegradation, with 16.3% (10 days) and 33.9% (15 days) and 16.5% (10 days) and 35.5% (15 days), respectively (p > 0.05). Both films presented significative differences (p < 0.05) with FPC-1 and control films.
The control film exhibited the most significant weight loss after 30 days in soil, demonstrating a 98% biodegradability. In contrast, FPC-1 showed a slightly lower rate of 95.1%, while FPC-2 and FPC-3 reported 87.9% and 82.7%, respectively, indicating less degradation in films formulated with 2% and 3% concentrations of Agave vinasse conjugates. Statistically significant differences were observed between the four samples. These findings align with previous research on biodegradability, such as a study on a fiber-reinforced starch biocomposite film that achieved complete biodegradability within 30 days [85], as well as a study on a corn starch active biocomposite film used in bread packaging, which demonstrated 100% biodegradability within 35 days in soil [86].
Figure 3c shows the surface characteristics of all samples after 15 days of exposure to soil, as observed through ESEM micrographs. The control film exhibited a high porosity level, with visible fibers on the surface. This surface structure was attracted by microorganisms from the soil. FPC-1 showed the growth of septate mycelium and porosity on the surface. In contrast, FPC-2 exhibited septate and non-septate mycelium, bacteria (coccus), and tiny pores on the surface. Finally, the surface of FPC-3 displayed minimal mycelium and did not present porosity. The presence of phenolic compounds by Agave conjugates in the biodegradable films could potentially inhibit the adaptability of microorganisms [87].
Figure 4b shows the spectrum of all films after 15 days of exposition in soil. The spectrum showed a reduction in the signal at 3280 cm−1 compared to films in the soil at 0 days (Fig. 4a). This change could be due to dehydration during soil exposition and the degradation of organic matter. The same occurs with the signal at 2930 cm−1, peak characteristics by phenolic compound [78].
The peak at 1648 cm−1, which indicates the stretching vibrations of the aldehyde group (C = O) of 5-HMF, significantly reduces in intensity. This reduction in intensity could indicate the degradation of 5-HMF by the soil microorganisms that use HMF as a carbon source [79, 88].
The region between 1084 cm−1 and 800 cm−1, corresponding to carbohydrates, exhibits low signals, indicating the sugars’ degradation in the conjugates [77, 80, 81]. These results suggest that after 15 days in soil, fungi, and bacteria colonized the surface and thoroughly adapted to compounds in the films. In FPC-3 less microbial presence was observed on surface films, possibly by high amounts of phenols and 5-HMF, which done slow biodegradation due to their antimicrobial properties. After 30 days, the samples reach their highest biodegradability in soil (Figs. 5, 6). The properties reported here are better or comparable to the yields obtained from other films using gelatin-cellulose as a base for forming biodegradable materials (Table 6).
The search for materials with biodegradable characteristics becomes a primary need due to pollution problems from the high volumes of single-use plastic waste generated annually.
Conclusions
The films enhanced with conjugates from Agave vinasses could be used in various industries as an alternative to reducing the production of single-use plastics. The increase in thermal capacity, improvement in mechanical properties, and natural antimicrobials such as phenols and furans make them suitable for packaging perishable fruits, as agricultural coatings in postharvest applications, and as biodegradable mulches in agriculture. However, it is necessary to conduct microbial inhibition analyses on these materials using microorganisms that negatively impact the food and crops of economic importance. Additionally, other applications for these films should be researched.
Data availability
All datasheets would be available at the request of any reader.
References
Shen M, Huang W, Chen M et al (2020) (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J Clean Prod 254:120138. https://doi.org/10.1016/j.jclepro.2020.120138
Gallo F, Fossi C, Weber R et al (2018) Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ Sci Eur 30:13. https://doi.org/10.1186/s12302-018-0139-z
Jambeck JR, Geyer R, Wilcox C et al (1979) (2015) Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Xanthos D, Walker TR (2017) International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar Pollut Bull 118:17–26. https://doi.org/10.1016/j.marpolbul.2017.02.048
Yu J, Adingo S, Liu X et al (2022) Micro plastics in soil ecosystem—a review of sources, fate, and ecological impact. Plant Soil Environ 68:1–17. https://doi.org/10.17221/242/2021-PSE
Sherwood J (2020) The significance of biomass in a circular economy. Bioresour Technol 300:122755. https://doi.org/10.1016/j.biortech.2020.122755
Brodin M, Vallejos M, Opedal MT et al (2017) Lignocellulosics as sustainable resources for production of bioplastics—a review. J Clean Prod 162:646–664. https://doi.org/10.1016/j.jclepro.2017.05.209
Ortega F, Versino F, López OV, García MA (2022) Biobased composites from agro-industrial wastes and by-products. Emergent Mater 5:873–921. https://doi.org/10.1007/s42247-021-00319-x
Muneer F, Nadeem H, Arif A, Zaheer W (2021) Bioplastics from biopolymers: an eco-friendly and sustainable solution of plastic pollution. Polym Sci Ser C 63:47–63. https://doi.org/10.1134/S1811238221010057
Shafqat A, Tahir A, Mahmood A et al (2020) A review on environmental significance carbon foot prints of starch based bio-plastic: a substitute of conventional plastics. Biocatal Agric Biotechnol 27:101540. https://doi.org/10.1016/j.bcab.2020.101540
Arfat YA, Ahmed J, Hiremath N et al (2017) Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll 62:191–202. https://doi.org/10.1016/j.foodhyd.2016.08.009
Omrani-Fard H, Abbaspour-Fard MH, Khojastehpour M, Dashti A (2020) Gelatin/Whey protein—potato flour bioplastics: fabrication and evaluation. J Polym Environ 28:2029–2038. https://doi.org/10.1007/s10924-020-01748-1
González A, Gastelú G, Barrera GN et al (2019) Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll 89:758–764. https://doi.org/10.1016/j.foodhyd.2018.11.051
Huang S, Tao R, Ismail A, Wang Y (2020) Cellulose nanocrystals derived from textile waste through acid hydrolysis and oxidation as reinforcing agent of soy protein film. Polymers 12:958. https://doi.org/10.3390/polym12040958
Pan L, Li P, Tao Y (2020) Preparation and properties of microcrystalline cellulose/fish gelatin composite film. Materials 13:4370. https://doi.org/10.3390/ma13194370
Sica P, Carvalho R, Das KC, Baptista AS (2020) Biogas and biofertilizer from vinasse: making sugarcane ethanol even more sustainable. J Mater Cycles Waste Manag 22:1427–1433. https://doi.org/10.1007/s10163-020-01029-y
Díaz-Vázquez D, Carrillo-Nieves D, Orozco-Nunnelly DA et al (2021) An integrated approach for the assessment of environmental sustainability in agro-industrial waste management practices: the case of the tequila industry. Front Environ Sci. https://doi.org/10.3389/fenvs.2021.682093
Tequila Regulatory Council (2024) Producción Total de Tequila y Tequila 100%. https://www.crt.org.mx/EstadisticasCRTweb/. Accessed 13 Feb 2024
Lorenzo-Santiago MA, Rodríguez-Campos J, Rendón-Villalobos R et al (2023) Thermal Treatment to obtain 5-hydroxymethyl furfural (5-HMF), furfural and phenolic compounds from vinasse waste from agave. Molecules 28:1063. https://doi.org/10.3390/molecules28031063
Rojas Álvarez OE, Nicolás Vázquez MI, Oñate-Garzón J, Arango CA (2021) Validation by molecular dynamics of the major components of sugarcane vinasse, on a surface of calcium carbonate (Calcite). Molecules 26:2353. https://doi.org/10.3390/molecules26082353
Tóth AJ, Fózer D, Mizsey P et al (2023) Physicochemical methods for process wastewater treatment: powerful tools for circular economy in the chemical industry. Rev Chem Eng 39:1123–1151. https://doi.org/10.1515/revce-2021-0094
Lorenzo-Santiago MA, Camacho-Ruíz RM, García-Hernández E et al (2023) Conversion of residual sugars from vinasses to 5-Hydroxymethyl furfural (5-HMF) and phenolic compounds using ion exchange resins and thermal treatment. Environ Technol Innov 32:103354. https://doi.org/10.1016/j.eti.2023.103354
Jiang Z, Zeng Y, Hu D et al (2023) Chemical transformations of 5-hydroxymethylfurfural into highly added value products: present and future. Green Chem 25:871–892. https://doi.org/10.1039/D2GC03444A
Martins FCOL, Alcantara GMRN, Silva AFS et al (2022) The role of 5-hydroxymethylfurfural in food and recent advances in analytical methods. Food Chem 395:133539. https://doi.org/10.1016/j.foodchem.2022.133539
Paone E, Mauriello F (2022) From bio-based furanics to biodegradable plastics. Chem 8:897–899. https://doi.org/10.1016/j.chempr.2022.03.004
Espro C, Paone E, Mauriello F et al (2021) Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem Soc Rev 50:11191–11207. https://doi.org/10.1039/D1CS00524C
Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM (2011) 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem 13:754. https://doi.org/10.1039/c0gc00401d
Kucherov FA, Romashov LV, Galkin KI, Ananikov VP (2018) Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, materials science, and synthetic building blocks. ACS Sustain Chem Eng 6:8064–8092. https://doi.org/10.1021/acssuschemeng.8b00971
Zabihollahi N, Alizadeh A, Almasi H et al (2020) Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int J Biol Macromol 160:409–417. https://doi.org/10.1016/j.ijbiomac.2020.05.066
Nikoukheslat HD, Alizadeh A, Roufegarinejad L, Hanifian S (2022) Characterization of physicochemical and antibacterial properties of gelatin and inulin nanobiocomposite films containing crystalline nanocellulose and Malva sylvestris extract. J Polym Environ 30:3078–3090. https://doi.org/10.1007/s10924-022-02398-1
Santos LS, Fernandes CC, Santos LS et al (2021) Ethanolic extract from Capsicum chinense Jacq. ripe fruits: phenolic compounds, antioxidant activity and development of biodegradable films. Food Sci Technol 41:497–504. https://doi.org/10.1590/fst.08220
de Aguiar AC, da Fonseca Machado AP, Figueiredo Angolini CF et al (2019) Sequential high-pressure extraction to obtain capsinoids and phenolic compounds from biquinho pepper (Capsicum chinense). J Supercrit Fluids 150:112–121. https://doi.org/10.1016/j.supflu.2019.04.016
Cazaudehore G, Schraauwers B, Peyrelasse C et al (2019) Determination of chemical oxygen demand of agricultural wastes by combining acid hydrolysis and commercial COD kit analysis. J Environ Manage 250:109464. https://doi.org/10.1016/j.jenvman.2019.109464
Rendón-Villalobos R, Lorenzo-Santiago MA, Olvera-Guerra R, Trujillo-Hernández CA (2022) Bioplastic composed of starch and micro-cellulose from waste mango: mechanical properties and biodegradation. Polímeros. https://doi.org/10.1590/0104-1428.20210031
Jafari R, Zandi M, Ganjloo A (2023) Characterization of alginate-gelatin edible film containing anise (Pimpinellaanisum L.) essential oil. J Polym Environ 31:1568–1583. https://doi.org/10.1007/s10924-022-02707-8
ASTM F1927–20 (2020) Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity Through Barrier Materials Using a Coulometric Detector.
Aaliya B, Sunooj KV, Lackner M (2021) Biopolymer composites: a review. Int J Biobased Plast 3:40–84. https://doi.org/10.1080/24759651.2021.1881214
Yu M, Zheng Y, Tian J (2020) Study on the biodegradability of modified starch/polylactic acid (PLA) composite materials. RSC Adv 10:26298–26307. https://doi.org/10.1039/D0RA00274G
Szewczyk PK, Stachewicz U (2020) The impact of relative humidity on electrospun polymer fibers: from structural changes to fiber morphology. Adv Colloid Interface Sci 286:102315. https://doi.org/10.1016/j.cis.2020.102315
Gómez-Aldapa CA, Velazquez G, Gutierrez MC et al (2020) Characterization of functional properties of biodegradable films based on starches from different botanical sources. Starch Stärke 72:1900282. https://doi.org/10.1002/star.201900282
ASTM D882 02 (2002) Standard Test Method for tensile Properties of Thin PlasticSheeting
ASTM D5988–12 (2012) Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil
Gee GW, Bauder JW (1986) Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods. In: Klute A. (ed) 2nd ed. Soil Health and Sustainable Agriculture, Brazil
ASTM D1293–18 Standard Test Methods for pH of Water. In: 2018. https://www.astm.org/d1293-18.html. Accessed 19 Apr 2024
Amato M (1983) Determination of carbon 12C and 14C in plant and soil. Soil Biol Biochem 15:611–612. https://doi.org/10.1016/0038-0717(83)90059-7
Bremner JM (1996) Methods of Soil Analysis: Part 3 Chemical Methods. In: Spark D.L., Page A.L., Helmke P.A., et al (eds) 1st ed. Soil Health and Sustainable Agriculture. Brazil
Simón M, Garcı́a I (1999) Physico-chemical properties of the soil-saturation extracts: estimation from electrical conductivity. Geoderma 90:99–109. https://doi.org/10.1016/S0016-7061(98)00098-6
NOM-021-SEMARNAT-2000. (2000) Norma Oficial Mexicana que establece las especificaciones de fertilidad, salinidad y clasificación de suelos, estudio, muestreo y análisis. https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2280n.pdf. Accessed 24 Apr 2024
Lorenzo-Santiago MA, Rendón-Villalobos R (2020) Isolation and characterization of micro cellulose obtained from waste mango. Polimeros. https://doi.org/10.1590/0104-1428.09119
BioRender Biorender.. https://biorender.com/. Accessed 2 Mar 2024
Chinnappan A, Baskar S, Baskar C (2017) Conversion of sugars into 5-HMF. Pattimura Conf Proc Sci Technol. https://doi.org/10.30598/PattimuraSci.2017.ICBS3.123-130
Gómez-Aldapa CA, Velazquez G, Gutierrez MC et al (2020) Characterization of functional properties of biodegradable films based on starches from different botanical sources. Starch Stärke. https://doi.org/10.1002/star.201900282
Li X, Zheng G, Li Z, Fu P (2024) Formulation, performance and environmental/agricultural benefit analysis of biomass-based biodegradable mulch films: a review. Eur Polym J 203:112663. https://doi.org/10.1016/j.eurpolymj.2023.112663
Moreira BR, Pereira-Júnior MA, Fernandes KF, Batista KA (2020) An ecofriendly edible coating using cashew gum polysaccharide and polyvinyl alcohol. Food Biosci 37:100722. https://doi.org/10.1016/j.fbio.2020.100722
de Fernandes G et al (2022) Effect of polyvinyl alcohol and carboxymethylcellulose on the technological properties of fish gelatin films. Sci Rep 12:10497. https://doi.org/10.1038/s41598-022-14258-y
Sachin M, Surendra P, Nayaku C (2016) Effect of chemical crosslinking on properties of polymer microbeads: a review. Can Chem Trans. https://doi.org/10.13179/canchemtrans.2015.03.04.0245
Werpy T, Petersen G (2004) Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Golden, CO (United States)
Gallo JMR, Alonso DM, Mellmer MA, Dumesic JA (2013) Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem 15:85–90. https://doi.org/10.1039/C2GC36536G
Mäki-Arvela P, Ruiz D, Murzin DYu (2021) Catalytic hydrogenation/hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran. Chemsuschem 14:150–168. https://doi.org/10.1002/cssc.202001927
de Almeida SL, de Aquino Santana LCL (2024) Physicochemical characterization, antioxidant and antimicrobial potential of biodegradable chitosan-based films containing pomegranate (Punicagranatum L.) peel extract. J Polym Environ 32:1729–1740. https://doi.org/10.1007/s10924-023-03063-x
Łupina K, Kowalczyk D, Zięba E et al (2019) Edible films made from blends of gelatin and polysaccharide-based emulsifiers—a comparative study. Food Hydrocoll 96:555–567. https://doi.org/10.1016/j.foodhyd.2019.05.053
Haghighi H, Biard S, Bigi F et al (2019) Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll 95:33–42. https://doi.org/10.1016/j.foodhyd.2019.04.019
Chen RS, Chai YH, Olugu EU et al (2021) Evaluation of mechanical performance and water absorption properties of modified sugarcane bagasse high-density polyethylene plastic bag green composites. Polym Polym Compos 29:S1134–S1143. https://doi.org/10.1177/09673911211049058
Kaboorani A, Gray N, Hamzeh Y et al (2021) Tailoring the low-density polyethylene—thermoplastic starch composites using cellulose nanocrystals and compatibilizer. Polym Test 93:107007. https://doi.org/10.1016/j.polymertesting.2020.107007
Hammache Y, Serier A, Chaoui S (2020) The effect of thermoplastic starch on the properties of polypropylene/high density polyethylene blend reinforced by nano-clay. Mater Res Express 7:025308. https://doi.org/10.1088/2053-1591/ab7270
Harussani MM, Sapuan SM, Firdaus AHM et al (2021) Determination of the tensile properties and biodegradability of cornstarch-based biopolymers plasticized with sorbitol and glycerol. Polymers 13:3709. https://doi.org/10.3390/polym13213709
Choi I, Lee SE, Chang Y et al (2018) Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT 93:427–433. https://doi.org/10.1016/j.lwt.2018.03.065
Nunes JC, Melo PTS, Lorevice MV et al (2021) Effect of green tea extract on gelatin-based films incorporated with lemon essential oil. J Food Sci Technol 58:1–8. https://doi.org/10.1007/s13197-020-04469-4
Al-Saidi G, Rahman MS, Al-Alawi A, Guizani N (2011) Thermal characteristics of gelatin extracted from shaari fish skin. J Therm Anal Calorim 104:593–603. https://doi.org/10.1007/s10973-010-1240-8
Remiš T, Bělský P, Andersen SM et al (2020) Preparation and characterization of poly(Vinyl Alcohol) (PVA)/SiO 2, PVA/Sulfosuccinic acid (SSA) and PVA/SiO 2 /SSA membranes: a comparative study. J Macromol Sci Part B 59:157–181. https://doi.org/10.1080/00222348.2019.1697023
Ahuja D, Kumar L, Kaushik A (2021) Thermal stability of starch bionanocomposites films: exploring the role of esterified cellulose nanofibers isolated from crop residue. Carbohydr Polym 255:117466. https://doi.org/10.1016/j.carbpol.2020.117466
El-Meligy MA, Valachová K, Juránek I et al (2022) Preparation and physicochemical characterization of gelatin-aldehyde derivatives. Molecules 27:7003. https://doi.org/10.3390/molecules27207003
Ferfari O, Belaadi A, Bedjaoui A et al (2023) Characterization of a new cellulose fiber extracted from SyagrusRomanzoffiana rachis as a potential reinforcement in biocomposites materials. Mater Today Commun 36:106576. https://doi.org/10.1016/j.mtcomm.2023.106576
La Fuente CIA (2022) Casting and extrusion processes to produce bio-based plastics using cassava starch modified by the dry heat treatment (DHT). Innov Food Sci Emerg Technol 75:102906. https://doi.org/10.1016/j.ifset.2021.102906
Li X, Liu Y, Luo B et al (2024) Effect of apple polyphenols on physicochemical properties of pea starch/pulp cellulose nanofiber composite biodegradable films. Int J Biol Macromol 257:128480. https://doi.org/10.1016/j.ijbiomac.2023.128480
Jung MR, Horgen FD, Orski SV et al (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull 127:704–716. https://doi.org/10.1016/j.marpolbul.2017.12.061
Ignot-Gutiérrez A, Ortiz-Basurto RI, García-Barradas O et al (2020) Physicochemical and functional properties of native and modified agave fructans by acylation. Carbohydr Polym 245:116529. https://doi.org/10.1016/j.carbpol.2020.116529
Amaral VA, de Souza JF, Alves TFR et al (2024) Psidium guajava L. phenolic compound-reinforced lamellar scaffold for tracheal tissue engineering. Drug Deliv Transl Res 14:62–79. https://doi.org/10.1007/s13346-023-01381-0
Tsilomelekis G, Orella MJ, Lin Z et al (2016) Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins. Green Chem 18:1983–1993. https://doi.org/10.1039/C5GC01938A
Morales-Leal FJ, Rivera de la Rosa J, Lucio-Ortiz CJ et al (2019) Dehydration of fructose over thiol—and sulfonic—modified alumina in a continuous reactor for 5–HMF production: Study of catalyst stability by NMR. Appl Catal B 244:250–261. https://doi.org/10.1016/j.apcatb.2018.11.053
Vázquez-Vuelvas OF, Chávez-Camacho FA, Meza-Velázquez JA et al (2020) A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll 103:105642. https://doi.org/10.1016/j.foodhyd.2020.105642
Luo Y, Zhang Y, Xu Y et al (2020) Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties. Sci Total Environ 726:138389. https://doi.org/10.1016/j.scitotenv.2020.138389
Huang D, Xu Y, Lei F et al (2021) Degradation of polyethylene plastic in soil and effects on microbial community composition. J Hazard Mater 416:126173. https://doi.org/10.1016/j.jhazmat.2021.126173
Susmitha A, Sasikumar K, Rajan D et al (2021) Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci 41:100977. https://doi.org/10.1016/j.fbio.2021.100977
Srivastava V, Singh S, Das D (2023) Rice husk fiber-reinforced starch antimicrobial biocomposite film for active food packaging. J Clean Prod 421:138525. https://doi.org/10.1016/j.jclepro.2023.138525
Srivastava V, Singh S, Das D (2024) Development and characterization of peppermint essential oil/rice husk fibre/ corn starch active biocomposite film and its performance on bread preservation. Ind Crops Prod 208:117765. https://doi.org/10.1016/j.indcrop.2023.117765
Takó M, Kerekes EB, Zambrano C et al (2020) Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 9:165. https://doi.org/10.3390/antiox9020165
Tan Z, Li X, Yang C et al (2021) Inhibition and disinhibition of 5-hydroxymethylfurfural in anaerobic fermentation: a review. Chem Eng J 424:130560. https://doi.org/10.1016/j.cej.2021.130560
Muralidharan V, Gochhayat S, Palanivel S, Madhan B (2022) Influence of preparation techniques of cellulose II nanocrystals as reinforcement for tannery solid waste–based gelatin composite films. Environ Sci Pollut Res 30:14284–14303. https://doi.org/10.1007/s11356-022-23058-w
Harrazi N, Özbek HN, Yanık DK et al (2024) Development and characterization of gelatin-based biodegradable films incorporated with pistachio shell hemicellulose. J Food Sci Technol. https://doi.org/10.1007/s13197-024-05968-4
Vargas-Torrico MF, von Borries-Medrano E, Aguilar-Méndez MA (2022) Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int J Biol Macromol 206:1012–1025. https://doi.org/10.1016/j.ijbiomac.2022.03.101
Nur Hazirah MASP, Isa MIN, Sarbon NM (2016) Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag Shelf Life 9:55–63. https://doi.org/10.1016/j.fpsl.2016.05.008
Acknowledgements
The authors acknowledge the resources provided by the National Council of Humanities Science and Technology, Mexico (CONAHCYT) through the postdoctoral fellowship received by Lorenzo-Santiago M.A. to realize this project (658080) and the Jalisco Scientific Development Fund (FODECIJAL) by projects 8138-2019 and 10605-2023.
Funding
No funding was obtained for this work.
Author information
Authors and Affiliations
Contributions
M.A.L-S. processing samples, investigation, writing–original draft, formal analysis and conceptualization, E.G.-H. Validation, methodology, and resources, R.R.-V. Methodology, validation, and resources. J.R–C. validation, methodology, and formal analysis, D.A.T.-P. Processing samples and validation, S.M.C.-R. Project administration, supervision, and writing—review and editing manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lorenzo-Santiago, M.A., García-Hernández, E., Rendón-Villalobos, R. et al. Biodegradable Films Added with Conjugates from Residual Agave vinasses: Chemical and Mechanical Characterization. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03352-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03352-z