Abstract
In this work, the effect of different concentrations of pomegranate peel extract (0.04, 0.07, 0.1 and 0.3%) were evaluated on the physicochemical, morphological and thermal properties, as well as the antioxidant and antimicrobial potential of chitosan films (CHRE-0.04, CHRE-0.07, CHRE-0.1 and CHRE-0.3). The incorporation of the extract did not significantly change the thickness, luminosity or transparency of the films. On the other hand, there was an increase in moisture and water vapor permeability and a decrease in solubility with the increase in the extract concentration added to the films. The films CHRE-0.04 and CHRE-0.07, with lower concentrations of extract, were the ones that demonstrated greater thermal stability. Higher antioxidant activity was obtained by the CHRE-0.3 film, with increases that varied between 5.0 and 36.5 times relative to the film without extract. The film without extract showed greater antimicrobial potential; however, the CHRE-0.3 film was the greatest inhibitor of Escherichia coli (51.6% inhibition). Ecologically friendly chitosan films incorporated with pomegranate peel extract have shown the potential to be used in food preservation as an alternative to conventional packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After the COVID-19 pandemic, there was an increase of more than eight million tons in the production of single-use disposable plastics [1]. Conventional petroleum-based plastic packaging is widely used due to its high resistance, low cost, convenience and versatility; however, it is an environmental problem due to its non-degradability [2]. Seventy-five percent of global production these petroleum-based materials are not degradable, creating an environmental issue [3]. In this sense, the food industry, the largest user of plastic materials, has chosen to change packaging material to sustainable packaging around the world [3].
Chitosan (poly-(1 → 4)N-acetyl-d-glucosamine) is the linear polysaccharide obtained from the deacetylated form of chitin. This polymer possesses useful properties, such as nontoxicity, biodegradability, biofunctionality and being a biocompatible antimicrobial, as well as having antifungal, antitumor and hypocholesterolemic, antioxidant, antacid, colon targeting and analgesic properties [4]. However, low antioxidant and antibacterial activities and low solubility are some disadvantages of chitosan [5]. In this context, the incorporation of extracts, oils and essential or bioactive compounds in chitosan films can improve functionalities such as physical properties, antioxidant, and antimicrobial activity, as well as being a barrier against UV light, due to the gradual release of these active agents [6]. Pomegranate peel extracts have demonstrated antioxidant potential due to the presence of compounds such as punicalagins, punicalins, ellagic acids, epicatechin, catechin and rutin [7, 8]. The presence of polyphenolic compounds also promotes the antimicrobial activity of pomegranate peel extracts against various microorganisms, such as Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella Enteritidis, Aspergillus niger, Saccharomyces cerevisiae, Fusarium sambucinum, Penicillium italicum, Bacillus subtilis, S. epidermidis, Klebsiella pneumoniae, S. Typhi, Yersinia enterocolitica and Candida albicans [8].
Due to its properties, some researchers have incorporated pomegranate peel extracts into biodegradable films. Hanani et al. [9] obtained fish gelatine films incorporating pomegranate peel powder, and Costa et al. [10] elaborated poly(vinyl alcohol), potato starch and poly(acrylic acid) blends incorporating hydroalcoholic extracts of pomegranate peels. Cui et al. [11] obtained zein active film incorporated with pomegranate peel extract (PE) encapsulated in chitosan nanoparticles. Zeng et al. [12] produced films containing the ground pomegranate peel powder and chitosan, and Bertolo et al. [13] obtained chitosan and gelatine films containing pomegranate peel extract in 60% ethanol; Kumar et al. [14] and Yuan et al. [15] incorporated in chitosan films pomegranate peel extract obtained with methanol. Meanwhile, Soltanzadeh et al. [16] obtained films of gelatine and cress seed gum containing chitosan nanoparticles and methanolic extracts of dried peels of pomegranates, and Catti et al. [17] obtained films with cassava starch, glycerol, polybutylene adipate-co-terephthalate, potassium sorbate and pomegranate peel extract in 70% ethanol.
In the works cited above, more than one component is used in addition to chitosan for the preparation of films and concentrations of pomegranate peel extract above 1%. Also in some works, the thermal properties of films were not shown, the antimicrobial activity was performed with a maximum of two bacteria and methanol (a toxic solvent) was used to obtain the extracts. This work aimed to obtain a low-cost film, with low concentrations of ethanolic extract and totally “green”, i.e., environmentally friendly. The films were evaluated for physicochemical, morphological, thermal, antioxidant and antibacterial properties.
Material and Methods
Materials
Pomegranate fruits of the “Wonderful” variety were collected in the rural area of the municipality of Caetité (latitude 14° 10′ 16″ south and longitude 42° 52′ 28″ west), Bahia, Brazil. The chitosan used was produced by the company Polymar (Fortaleza, Ceará, Brazil) with 100 mesh granulometry, pH 7.4, viscosity of 24 cPs and an 82.89% degree of deacetylation. Ethanol, methanol and 2,3,5-triphenyl tetrazolium chloride reagents were purchased from Neon Analytical reagents (São Paulo, Brazil). Glycerine, potassium persulfate, acetic acid and ferric chloride III were purchased from Dinâmica Contemporânea LTDA (São Paulo, Brazil). The 6-hydroxy-2,5,7,8-tetramethylchromo-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline) 6-sulfonic acid (ABTS+) and ferric reduction antioxidant power (FRAP) were acquired from Sigma Aldrich and Fluka Analytica (St Louis, MO, USA). The BHI broth was obtained from Kasvi (Paraná, Brazil), and the acetate was purchased from Vetec Química Fina (Rio de Janeiro, Brazil).
Bacteria Strains
The strains of B. cereus (CBAM 0353), B. subtilis (CBAMd f 0441), Enterococcus faecalis (INCQS 00531), E. coli (CBAM 0002), Pseudomonas aeruginosa (CBAM 0679), S. Enteritidis (INCQS 00258), S. aureus (CBAM 0629), Serratia marcescens (CBAM 0519), and S. Typhimurium (CBAM 0018) were provided by the Oswaldo Cruz Foundation (Manguinhos, Rio de Janeiro, Brazil) and by the Leônidas and Maria Deane Institute (Amazonian bacteria collection) and kept in Brain Heart Infusion (BHI) broth with 20% glycerol at – 80 °C in an ultrafreezer.
Preparation of Extract from Pomegranate Peel
The peel was manually separated from the pulp and seeds and dried at a temperature of 35 °C in a drying oven for 48 h. Next, the peel was crushed in an industrial blender and sieved through a 32-mesh mesh, until obtaining a powder [18]. The extracts were prepared in a 1:5 ratio (powder:solvent), with a 70% aqueous ethanol solution and shaken at 200 rpm at 30 °C for 1 h in an orbital shaker (model TE-420, Tecnal, BR), subsequently filtered on quantitative filter paper and the supernatant used [18].
Preparation of Films
The films were prepared with 2 g of chitosan solubilized in 100 mL of 1% (v/v) acetic acid, remaining at rest for 1 h, followed by stirring at 25 °C for 1 h. Then, 1.5% (w/v) glycerine was added to the filmogenic dispersion and agitated for 30 min. Then, the mixture was filtered, and the pomegranate peel extract was added in the following proportions: 0, 0.04, 0.07, 0.1 and 0.3% (w/w based on dispersion), followed by stirring for 15 min at 25 °C. The dispersions were placed in plastic Petri dishes and dried in an oven at 30 °C for 48 h [19]. The films were named CH (chitosan film without extract), CHRE-0.04 (chitosan film containing 0.04% extract), CHRE-0.07 (chitosan film containing 0.07% extract), CHRE-0.1 (chitosan film containing 0.1% extract) and CHRE-0.3 (chitosan film containing 0.3% extract). The films were produced in duplicate.
Physicochemical Characterization of Films
Thickness
The thickness of the films (mm) was determined in triplicate at six different points using a digital calliper (Pantec - 11112B-150).
Moisture Content and Solubility in Water
To determine the moisture content (in triplicate), 4 × 4 cm pieces of the films were dried in an oven at 105 °C for 24 h, and the results were calculated according to Eq. (1) [20].
where P1 is the weight of the film before drying (g), and P2 is the initial dry mass value and the weight of the film after drying.
For water solubility, 4 × 4 cm pieces of the films were immersed in 30 mL of distilled water for 24 h and dried at 105 °C for 24 h. The experiments were performed in triplicate, and the results were calculated using Eq. (2) [21].
where P1 is the initial weight of the film, and P2 is the final weight of the film after drying.
Water Vapor Permeability (WVP)
To assess PVA, films were placed on the surface of beakers containing silica gel (RH 0%), inside a desiccator containing distilled water (RH 100%) at 25 °C for 48 h (ASTM E96/E 96M-16). The experiments were performed in duplicate, and the WVP was calculated using Eq. (3):
where W is the weight gain (g), L is the average thickness of the films (mm), ΔP is the vapor pressure difference between the two sides of the film (Pa), S is saturation vapor pressure at 25 °C (Pa), t is the total time (s), and A is the permeation area (m2).
Transparency of Film
Rectangular pieces of the films were placed in glass cuvettes, and the transmittance was read at 600 nm in a UV spectrophotometer (KAZUAKI, IL-226-NM, Tokyo, Japan) [21].
The transparency was determined by Eq. (4).
where ε is the film thickness in millimetres.
Colour of Films
The colour was analysed at four random points on the films using a digital colorimeter (Konica Minolta, CR-10, Tokyo, Japan), where the coordinates a* (blue and red), b* (green and yellow) and L* (brightness) were determined.
Morphology
The morphology of the films (pieces of 8 mm) was assessed using a Scanning Electron Microscope (JEOL, JSM-6510LV, Tokyo, Japan). Film samples were immobilized on an aluminum stub with carbon tape under vacum conditions and the images were obtained using with an accelerating voltage of 10 kV and image magnification of × 2000.
Thermogravimetric Analysis
The thermal stability of the films was determined in a thermogravimetric analyser (Hitachi, STA7200RV, Tokyo, Japan), operating in a temperature range from 0 to 600 °C, using a nitrogen atmosphere at a flow rate of 30 mL/min and a heating rate of 10 °C/min. The mass loss thermograms were expressed as a function of the temperature range [22].
Differential Scanning Calorimetry (DSC)
The thermal properties of the films were determined by DSC analysis in TA Instruments equipment, model Q20, under a nitrogen atmosphere at a flow rate of 30 mL/min at 0 to 300 °C, in a heating interval of 10 °C/min [23].
Fourier Transform Infrared Spectroscopy (FT-IR)
The FT-IR spectra of the films were determined between 650 and 4000 cm−1, with 4 cm−1 resolution and 128 scans for all samples using a Cary 630 FTIR spectrometer (Agilent Technologies, Malaysia).
Antioxidant Activity (AA)
First, the films (100 mg) were diluted in 2 mL of pure methanol and stirred for 3 h, filtered through qualitative filter paper, and the supernatant was used for the determination of AA by the ABTS, DPPH and FRAP methods [24]. For analysis by DPPH, 500 µL of the supernatant was mixed with 2 mL of DPPH solution (0.06 mM), vortexed and allowed to stand for 30 min in the dark. Then, the absorbances were read at 517 nm in a UV spectrophotometer (KAZUAKI, IL-226-NM, Tokyo, Japan). Pure methanol (100% v/v) was used as a blank replacing the supernatant [25]. The analysis was in triplicate, and the results were calculated as a percentage of inhibition according to Eq. (5). In the ABTS assay, 1 mL of the ABTS radical was diluted in 55 mL of pure ethanol, to obtain an initial absorbance of the solution at 0.700 (at a wavelength of 734 nm). Then, 2970 µL of the ABTS radical solution was mixed with 30 µL of the supernatant and allowed to stand for 6 min. Pure methanol was used as a blank. The absorbances were read at 734 nm [26] and AA was determined in triplicate by the percentage of inhibition calculated by Eq. (5).
where A0 is the absorbance of the blank and A1 is the absorbance of the solution containing the film supernatant.
For the FRAP method (in triplicate), 150 µL of the supernatant was mixed with 2850 µL of FRAP reagent. The solution was kept at 37 °C for 30 min in a water bath. Then, the absorbance was read at 593 nm, in triplicate, and the results were expressed in μmol Trolox/g [27].
Antibacteria Activity of Films
The antimicrobial activity of the films against nine strains of bacteria was tested in 96-well microplates. First, bacterial suspensions were prepared in BHI broth at a concentration of 1.5 × 108 CFU/mL. Then, 100 µL of bacterial suspension, 100 µL of BHI broth and a piece of film (1 cm2) were added to the wells of the microplates, being kept under incubation at 37 °C for 24 h. The negative control was performed with only the bacterial suspension. The films were removed and the optical density was measured at 600 nm, in duplicate [28]. The percentage of inhibition of the films was calculated according to Eq. (7).
where, A0 corresponds to the absorbance value of the negative control and A1 corresponds to the absorbance value of the samples.
Statistical Analysis
The results were analysed by one-way analysis of variance (ANOVA), and the means were compared by the Tukey test at the 5% significance level (p < 0.05) using the using the Statistica program 10.0.
Results and Discussion
Thickness of Films
The thickness of the films did not change with the addition of different concentrations of extract and did not differ from the film without extract (p > 0.05, Fig. 1A). Kumar et al. [14] also found that the incorporation of pomegranate peel extracts did not significantly affect the thickness of the chitosan-based films. Hanani et al. [9] and Moghadam et al. [29] obtained an increase in thickness with increasing concentration of pomegranate peel extract incorporated into fish gelatine films and bean protein films, respectively. The authors associated this behaviour with the polyphenolic compounds present in the fruit peel forming interactions with the functional groups of the polymer, which may lead to a greater thickness.
Moisture, Solubility and Water Vapour Permeability of Films
The moisture of the films varied between 27.08 and 36.30%, being higher in the CHRE-0.3% film and lower in the CHRE-0.07% film (p ≤ 0.05) (Fig. 1B). According to Catanzano et al. [30], the addition of plant extracts in chitosan films can result in increased moisture content because of the introduction of hydrophilic molecules to the polymeric matrix. Kumar et al. [14] also found an increase in the moisture content of chitosan films containing increased concentrations of pomegranate peel extract (from 11.23% in the control film to 15.28% in the film with the highest extract concentration), attributing this result to the interactions and changes in the hygroscopic nature of the chitosan matrix. However, Hanani et al. [9] did not obtain an increase in the moisture content of the gelatin films after the incorporation of pomegranate peel powder.
Solubility decreased with increasing extract concentration in the films, being lower in the CHRE-0.3% film and higher in the CH film without the extract (p < 0.05, Fig. 1C). The presence of simple sugars, such as glucose and fructose, in the pomegranate peel promotes the formation of covalent bonds, which can decrease the water solubility of films [31]. Also, the increase in interactions between the phenolic compounds of the extract with the polymer can result in a lower affinity of the polymeric matrix with water [13]. Bertolo et al. [13] and Hanani et al. [9] also found lower solubility of chitosan/gelatine films and fish gelatine films, respectively, with the addition of increased concentrations of pomegranate peel extract.
The WVP is an important parameter that measures the ease of the passage of water vapor through a film, influencing the shelf life of food [32]. The WVP increased with the addition of increased concentrations of extract in the films (Fig. 1D), being higher in the CHRE-0.3% film (5.13 × 10–7 g mm/s m2 Pa), differing statistically (p ≤ 0.05) from the others. Researchers report that the presence of phenolic compounds in pomegranate peel results in alternative pathways and cracks in the matrix chemical bonds, causing a increase in WVP [14, 33]. Probably, weak molecular interactions between the matrix and extract also facilitate water vapor permeability [12]. Zeng et al. [12] and Moghadam et al. [29] also found an increase in WVP in chitosan films incorporated with pomegranate peel powder. The authors attributed this result to the formation of agglomerated particles of pomegranate peel powder, resulting in a heterogeneous polymeric structure that provides greater space for water vapor to cross the film.
Scanning Electron Microscopy (SEM), Colour and Transparency Properties of Film
Visually, the films showed a uniform, homogeneous, shiny and brown colour (Fig. 2). According SEM the film without the addition of extract (CH) exhibited greater uniformity and a smoother surface without the presence of bubbles, with only small agglomerations of chitosan (Fig. 3A). However, the films added with extract (Fig. 3B–E) showed less homogeneity, with clusters of white particles on their surfaces and rough surface, probably due to the aggregation of insoluble particles to the polymeric matrix [34], which may be related to the hydrophilic nature of some compounds in the extract and the hydrophobic nature of chitosan [9, 13]. Zeng et al. [12] also reported that the addition of pomegranate peel powder in chitosan films caused changes in the structure of the films, such as the formation of white dots, which may be related to the presence of insoluble particles. Bertolo et al. [13] obtained chitosan and gelatine films incorporated with pomegranate peel extract with a less compact structure and with agglomerated extract points, related to the hydrophilic nature of polyphenolic compounds.
The L parameter, which corresponds to brightness, varied between 49.10 and 67.83 and did not differ among the films (p > 0.05, Table 1). The coordinates a* (redness) and b* (yellowing) were higher in films containing a higher concentration of extract (p ≤ 0.05), being attributed to the presence of phenolic compounds and anthocyanin pigments in the extract [14]. More et al. [33], Kumar et al. [14] and Hanani et al. [9] also found increases in a* and b* values in starch-casein, chitosan and fish gelatine films, respectively incorporated with pomegranate peel extract.
Regarding the transparency of the films, it will be greater with transmittance; this means greater capacity for visible light to pass through the film sample [35]. Despite the darker coloration in the films with extract, no significant difference was obtained between the transparency values (p > 0.05). Ideally, the films must have the ability to prevent the passage of light to the food, to prevent its oxidation [12]. This result meant that the incorporation of the extract did not change the visible light barrier property when compared to the film without extract.
Thermogravimetric Analysis (TGA)
All film samples showed three stages of mass loss (Fig. 4A) at different temperatures, a common behaviour in chitosan films [36]. The first stage of decomposition, between 28.6 and 100.1 °C, can be characterized by the loss of absorbed water molecules, volatile compounds and other compounds present in the film matrix [37,38,39]. The addition of 0.04 and 0.07% extract in the films (CHRE-0.04% and CHRE-0.07%) provided greater thermal stability since there was an increase in the maximum degradation temperature (Tmax), to 100 and 120 °C, respectively, when compared to the CH film (93.7 °C). This result may be due to the interaction between the aromatic groups and the polymeric chains, resulting in higher degradation temperatures and, consequently, greater stability [10]
However, there was a decrease in Tmax in films with higher concentrations of extract, CHRE-0.1% (78.5 °C) and CHRE-0.3% (86.6 °C), probably due to the formation of spaced structures in the film by the phenolic compounds from extract, facilitating the dispersion of water molecules and the decomposition of chitosan chains [39]. Another hypothesis may be the transfer of polyphenolic compounds to sensitive-heat radicals, when exposed to heating temperatures, resulting in thermal degradation of the film at lower temperatures [40].
The second stage of weight loss of the films occurred at temperatures between 78.6 and 223.9 °C, probably due to the degradation of the glycerol present in the formulations and the pyrolytic precipitation of the chitosan chain [34, 40]. At this stage, a reduction in Tmax was observed in the films with extract when compared to the CH film, also probably due to the presence of phenolic compounds in the extract that can alter the crystalline structure of chitosan [38].
The last decomposition step was observed at 202.1 to 342.2 °C, caused by the breaking of glycosidic bonds and the acetylation of chitosan induced by high temperatures, in addition to the decomposition of the extract components [41,42,43]. At this stage, the Tmax increased as the extract concentration was increased in the films. This result may be due to the greater crystallinity of the polymeric structure caused by the increase in the extract concentration in the film, requiring higher temperatures to decompose the entire crystalline structure [41]. More et al. [33], Cui et al. [11] and Costa et al. [10] obtained increased thermal stability in starch-casein using from 10 to 100% of pomegranate peel extract, in zein film containing 10% of nanoparticles of chitosan with pomegranate extract and PVA/starch/acrylic acid films containing 1.25% of pomegranate extract, respectively. In the film of this work, thermal stability was achieved with lower concentrations of extract (0.04 and 0.07%) than the ones used by these researchers.
Differential Scanning Calorimetry Analysis (DSC)
In the DSC curves, the first endothermic peak occurred in the range of 109 to 128 °C, which can be attributed to the energy required for the loss of water contained in the films [44] (Fig. 4B). The glass transition temperature (Tg) of the CH film was lower when compared to the films with extract, indicating that the addition of the extract increased the thermal resistance of the films. The second peak occurred between 254 (film without extract) and 264 °C (CHRE-0.3%). This meant that the addition of increased concentrations of extract increased the degradation temperature, that is, the thermal stability of the films agreed with the results obtained in the TGA.
In the third peak, the films with extract, except CHRE-0.1, showed a higher Tg value (between 257 and 259 °C) than the CH film (254 °C). Films with lower extract concentrations (CHRE-0.04, CHRE-0.07) showed greater thermal stability. Probably, with the increase in extract concentration, there was a disruption of the dense and uniform structure of the films, resulting in weaker interactions with chitosan and reducing thermal stability [42]. Kumar et al. [14], Soltanzadeh et al. [16] and Cui et al. [11] also found an increase in Tg values in chitosan, gelatine and Zein films, respectively containing pomegranate peel extract.
Fourier Transform Infrared Spectroscopy (FT-IR)
Films incorporated with and without pomegranate peel extract did not show differences in the number of absorption bands (Fig. 4C); however, differences in the intensity of the bands were observed as the extract concentration increased. These alterations may be due to hydrogen bonds and electrostatic interactions between chitosan and the compounds present in the extract [45]. Also, the variation in the intensity of the peaks may have been caused by changes in the functional groups of the films with the incorporation of increased extract concentrations [12, 35].
All films showed a broad band at 3267 cm−1 due to the elongation vibration of hydroxyl groups associated with chitosan [46, 47]. The 2927 cm−1 and 2880 cm−1 bands correspond to the elongation of–CH groups [48]. The stretching of the band around 2927 cm−1 may be related to the C–H stretching of methyl, methoxyl and methylene groups in the phenolic acids present in the extract [13]. The vibration in bands 1638 cm−1 and 1559 cm−1 indicates the C=O stretching of amide I and N–H of amide II, respectively, which are characteristics of chitosan [49, 50]. However, the increase in peak intensity in the 1559 cm−1 band for films with increased extract concentrations may be due to an increase in the number of aromatic rings [51].
The films showed a band at 1026 cm−1, which can be attributed to glycosidic bonds and the C–O–C group of the polysaccharide chain [50]. The bands presented in 1411 cm−1 indicate –CH2 symmetric bending [52]. Changes in the intensity of the 1382 cm−1 band were observed in films containing pomegranate peel extract when compared to the CH film, and this behaviour may be due to the presence of carboxyl groups of phenolic compounds [49].
Antioxidant Activity (AA) of Films
The antioxidant activity of the films by the DPPH, ABTS (Fig. 5A) and FRAP (Fig. 5B) methods increased considerably with the addition of the extract. The CHRE-0.3 film showed greater AA, statistically differing from the others (p ≤ 0.05), being % inhibition of 51.9 and 59.3 by DPPH and ABTS, and 2.2 µmol Trolox/mL by FRAP method. This meant increases of antioxidant activity about 10.0, 5.2 and 36.5 times, respectively, relative to AA of the film without extract (% inhibition of 5.2 and 11.4 by DPPH and ABTS, respectively, and 0.06 µmol Trolox/mL by FRAP). This result is probably due to the presence of antioxidant compounds in the extract, such as ellagic acid, punicalagin, quercetin, punicalin, luteolin, kaempferol, glycosides, pedunculagin and hydrolysable tannins [14]. Zeng et al. [12], Soltanzadeh et al. [16] and Kumar et al. [14] also obtained an increase in the AA of chitosan films and films of gelatine and watercress seed gum when increased concentrations of pomegranate peel extract were used, demonstrating its potential to enhance the antioxidant effect of the films.
Antibacterial Activity of Films
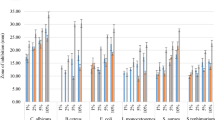
The antibacterial potential of the films was evaluated against B. subtilis, B. cereus, E. faecalis, E. coli, P. aeruginosa, S. marcescens, S. Enteritidis, S. Typhimurium and S. aureus strains (Fig. 5C).
The CH film inhibited all bacteria tested, except for S. marcensces, with the greatest inhibition for B. subtilis (65.4%) and S. Typhimurium (63.8%). The films CHRE-0.04% and CHRE-0.07% were more effective at inhibiting E. coli (30% and 51.6% inhibition, respectively), differing statistically from the others (p < 0.05). The CHRE-0.3% film was more effective in inhibiting P. aeruginosa (21.3%) and CH films (37.9%), CHRE-0.07% (37.4%) and CHRE-0.3% (33.9%) to inhibit E. faecalis, did not differ statistically between them (p < 0.05). The antimicrobial property of pomegranate peel extracts is related to the presence of hydrolysable polyphenols that act on the cell membrane of microorganisms [11]. In addition to chitosan, compounds present in pomegranate peel, such as ellagic acid, punicalagin and tannins, which act on sulfhydryl groups of proteins, resulting in protein precipitation and cell lysis, may be responsible for the antimicrobial activity of the films [33, 53]. For most bacteria, extract films showed less inhibition than CH film. A hypothesis for this result may be that hydroxyl groups in the extract form crosslinked structures with the protonated amino groups of chitosan, resulting in lower interaction between the polymer and the bacterial cells [15]. Hanani et al. [9], for example, obtained no zone of inhibition with fish gelatin films added with 1% pomegranate peel powder against S. aureus, L. monocytogenes and E. coli.
Conclusions
In this work, chitosan films incorporated with increased concentrations of pomegranate peel extract showed increased antioxidant activity, demonstrating potential to protect foods from oxidative damage. On the other hand, the films with extract showed higher susceptible for the preservation of non-photosensitive foods, since that the transparency was not changed by adding the extracts. The films with lowest extract concentrations (CHRE-0.04 and CHRE-0.07) were more thermally stable and the CHRE-0.07 was the highlight as greater inhibition of E. coli, a common foodborne bacterium. Also the films with lower concentrations of extract showed lower WVP, being more suitable for food preservation. In fact the films containing pomegranate peel extract showed potential of application however, studies are needed to evaluate their performance in food preservation.
References
Peng Y, Wu P, Schartup AT, Zhang Y (2021) Plastic waste release caused by COVID-19 and its fate in the global ocean. PNAS 118:1–6. https://doi.org/10.1073/pnas.2111530118
Tyagi P, Salem KS, Hubbe MA, Pal L (2021) Advances in barrier coatings and film technologies for achieving sustainable packaging of food products—a review. Trends Food Sci Technol 115:461–485. https://doi.org/10.1016/j.tifs.2021.06.036
Goel V, Luthra P, Kapur GS (2021) Biodegradable/Bio-plastics: myths and realities. J Polym Environ 29:3079–3104. https://doi.org/10.1007/s10924-021-02099-1
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res 63:20–31
Salari M, Khiabani MS, Mokarram RR, Ghanbarzadeh B, Kafil HS (2020) Use of gamma irradiation technology for modification of bacterial cellulose nanocrystals/chitosan nanocomposite film. Carbohydr Polym 253:117144. https://doi.org/10.1016/j.carbpol.2020.117144
Yildirim S, Röcker B, Pettersen MK, Nilsen-Nygaard J, Ayhan Z, Rutkaite R (2018) Active packaging applications for food. Compr Rev Food Sci Food Saf 217:165–199. https://doi.org/10.1111/1541-4337.12322
Salim A, Deiana P, Fancello F, Molinu MG, Santona M, Zara S (2023) Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: varietal screening through a multivariate approach. J Bioresour Bioprod 8:146–161. https://doi.org/10.1016/j.jobab.2023.01.006
Shahkoomahally S, Shin D, Habibi F, Kim J, Sarkhosh A (2023) Profiling phenolic compounds in juice and peel of fourteen pomegranate (Punica granatum L.) varieties grown in Florida, USA. Food Chem Adv 2:100225. https://doi.org/10.1016/j.focha.2023.100225
Hanani ZAN, Yee FC, Nor-Khaizura MAR (2019) Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll 89:253–259. https://doi.org/10.1016/j.foodhyd.2018.10.007
Costa NN, Faria Lopes L, Ferreira DF, Prado EML, Severi JA, Resende JA (2020) Polymeric films containing pomegranate peel extract based on PVA/starch/PAA blends for use as wound dressing: in vitro analysis and physicochemical evaluation. Mater Sci Eng C 109:110643. https://doi.org/10.1016/j.msec.2020.110643
Cui H, Surendhiran D, Li C, Lin L (2020) Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag Shelf Life 24:100511. https://doi.org/10.1016/j.fpsl.2020.100511
Zeng J, Ren X, Zhu S, Gao Y (2021) Fabrication and characterization of an economical active packaging film based on chitosan incorporated with pomegranate peel. Int J Biol Macromol 192:1160–1168. https://doi.org/10.1016/j.ijbiomac.2021.10.064
Bertolo M, Dias LD, Oliveira Filho J, Alves F, Marangon C, Martins V (2022) Central composite design optimization of active and physical properties of food packaging films based on chitosan/gelatin/pomegranate peel extract. SSRN Electron J 34:100986. https://doi.org/10.1016/j.fpsl.2022.100986
Kumar N, Pratibha, Petkoska AT, Khojah E, Sami R, Al-Mushhin AAM (2021) Chitosan edible films enhanced with pomegranate peel extract. Materials (Basel) 14:1–18. https://doi.org/10.3390/ma14123305
Yuan G, Lv H, Yang B, Chen X, Sun H (2015) Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 20:11034–11045
Soltanzadeh M, Peighambardoust SH, Ghanbarzadeh B, Amjadi S, Mohammadi M, Lorenzo JM (2021) Active gelatin/cress seed gum-based films reinforced with chitosan nanoparticles encapsulating pomegranate peel extract: preparation and characterization. Food Hydrocoll 129:107620. https://doi.org/10.1016/j.foodhyd.2022.107620
Catti J, Fidelis F, Marchi LB, Scapim MRS, Yamashita F, Roberto A (2022) Development of biodegradable films containing pomegranate peel extract and potassium sorbate. Food Sci Technol Int 160:113302. https://doi.org/10.1016/j.lwt.2022.113302
Scaramussa SAL, Soares LA, Santana LCLA (2022) Extracts from jatobá (Hymenaea courbaril L.) peel and seeds: antioxidant and antimicrobial activities and synergistic effect of extract combinations. Food Sci Technol Int. https://doi.org/10.1177/10820132221136589
Scaramussa SAL, Soares LA, Santana LCLA (2021) Filmes ativos biodegradáveis a base de quitosana incorporados com extrato de jatobá e método de produção (BR Patent No 1020210264349). Instituto Nacional da Propriedade Industrial. https://www.gov.br/inpi/pt-br
AOAC (2012) Official methods of analysis of AOAC International, vols. I and II, 19th ed., ed Latimer GW, Jr. AOAC International, Gaithersburg, p 930.15
Sothornvit R, Hong SI, An DJ, Rhim JW (2010) Effect of clay content on the physical and antimicrobial properties of whey protein isolate/organo-clay composite films. Lwt 43:279–284. https://doi.org/10.1016/j.lwt.2009.08.010
Sueiro AC, Faria-Tischer PCS, Lonni AASG, Mali S (2016) Filmes biodegradáveis de amido de mandioca, pululana e celulose bacteriana. Quim Nova 39:1059–1064. https://doi.org/10.5935/0100-4042.20160118
Campagner MR, Da Silva Moris VA, Pitombo LM, Do Carmo JB, De Paiva JMF (2014) Polymeric films based on starch and lignosulfonates: preparation, properties and evaluation of biodegradation. Polimeros 24:740–751. https://doi.org/10.1590/0104-1428.1700
Byun Y, Kim YT, Whiteside S (2010) Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J Food Eng 100:239–244. https://doi.org/10.1016/j.jfoodeng.2010.04.005
Martins JT, Cerqueira MA, Vicente AA (2012) Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll 27:220–227. https://doi.org/10.1016/j.foodhyd.2011.06.011
Moo-Huchin VM, Moo-Huchin MI, Estrada-León RJ, Cuervas-Glory L, Ortiz-Vázquez E, Betancur-Ancona D, Sauri-Duch E (2015) Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem 166:17–22. https://doi.org/10.1016/j.foodchem.2014.05.127
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Nadira PP, Mujeeb VMA, Rahman PM, Muraleedharan K (2022) Effects of cashew leaf extract on physicochemical, antioxidant, and antimicrobial properties of N, O-Carboxymethyl chitosan films. Carbohydr Polym Technol Appl 3:100191. https://doi.org/10.1016/j.carpta.2022.100191
Moghadam M, Salami M, Mohammadian M, Khodadadi M, Emam-Djomeh Z (2019) Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll 104:105735. https://doi.org/10.1016/j.foodhyd.2020.105735
Catanzano O, Gomez d’Ayala G, D’Agostino A, Di Lorenzo F, Schiraldi C, Malinconico M (2021) PEG-crosslinked-chitosan hydrogel films for in situ delivery of Opuntia ficus-indica extract. Carbohydr Polym 264:1–9. https://doi.org/10.1016/j.carbpol.2021.117987
Bhat R, Karim AA (2014) Towards producing novel fish gelatin films by combination treatments of ultraviolet radiation and sugars (ribose and lactose) as cross-linking agents. J Food Sci Technol 51:1326–1333. https://doi.org/10.1007/s13197-012-0652-9
Kanatt SR, Rao MS, Chawla SP, Sharma A (2012) Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll 29:290–297. https://doi.org/10.1016/j.foodhyd.2012.03.005
More PR, Pegu K, Arya SS (2022) Development and characterization of taro starch-casein composite bioactive films functionalized by micellar pomegranate peel extract (MPPE). Int J Biol Macromol 220:1060–1071. https://doi.org/10.1016/j.ijbiomac.2022.08.147
Riaz A, Lei S, Akhtar HMS, Wan P, Chen D, Jabbar S (2018) Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol 114:547–555. https://doi.org/10.1016/j.ijbiomac.2018.03.126
Fan X, Zhang B, Zhang X, Ma Z, Feng X (2023) Incorporating Portulaca oleracea extract endows the chitosan-starch film with antioxidant capacity for chilled meat preservation. Food Chem 18:100662. https://doi.org/10.1016/j.fochx.2023.100662
Bi F, Zhang X, Bai R, Liu Y, Liu J, Liu J (2019) Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int J Biol Macromol 134:11–19. https://doi.org/10.1016/j.ijbiomac.2019.05.042
D’souza OJ, Gasti T, Hiremani VD, Pinto JP, Contractor SS, Shettar AK (2023) Basella alba stem extract integrated poly (vinyl alcohol)/chitosan composite films: a promising bio-material for wound healing. Int J Biol Macromol 225:673–686. https://doi.org/10.1016/j.ijbiomac.2022.11.130
Kahya N, Kestir SM, Öztürk S, Yolaç A, Torlak E, Kalaycıoğlu Z (2022) Antioxidant and antimicrobial chitosan films enriched with aqueous sage and rosemary extracts as food coating materials: characterization of the films and detection of rosmarinic acid release. Int J Biol Macromol 217:470–480. https://doi.org/10.1016/j.ijbiomac.2022.07.073
Mittal A, Singh A, Benjakul S, Prodpran T, Nilsuwan K, Huda N (2021) Composite films based on chitosan and epigallocatechin gallate grafted chitosan: characterization, antioxidant and antimicrobial activities. Food Hydrocoll 11:106384. https://doi.org/10.1016/j.foodhyd.2020.106384
Nguyen TT, Thi Dao UT, Thi Bui QP, Bach GL, Ha Thuc CN, Ha Thuc H (2020) Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog Org Coat 140:105487. https://doi.org/10.1016/j.porgcoat.2019.105487
De Carli C, Aylanc V, Mouffok KM, Santamaria-Echart A, Barreiro F, Tomás A (2022) Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int J Biol Macromol 213:486–497. https://doi.org/10.1016/j.ijbiomac.2022.05.155
Ren G, He Y, Lv J, Zhu Y, Xue Z, Zhan Y (2023) Highly biologically active and pH-sensitive collagen hydrolysate-chitosan film loaded with red cabbage extracts realizing dynamic visualization and preservation of shrimp freshness. Int J Biol Macromol 233:123414. https://doi.org/10.1016/j.ijbiomac.2023.123414
Zhao P, Wang J, Yan X, Cai Z, Fu L, Gu Q (2022) Functional chitosan/zein films with Rosa roxburghii Tratt leaves extracts prepared by natural deep eutectic solvents. Food Packag Shelf Life 34:101001. https://doi.org/10.1016/j.fpsl.2022.101001
Koc B, Akyuz L, Cakmak YS, Sargin I, Salaberria AM, Labidi J (2020) Production and characterization of chitosan-fungal extract films. Food Biosci 35:100545. https://doi.org/10.1016/j.fbio.2020.100545
El Mouzahim M, Eddarai EM, Eladaoui S, Guenbour A, Bellaouchou A, Zarrouk A (2023) Food packaging composite film based on chitosan, natural kaolinite clay, and Ficus carica leaves extract for fresh-cut apple slices preservation. Int J Biol Macromol 233:123430. https://doi.org/10.1016/j.ijbiomac.2023.123430R
Liu F, Zhang X, Xiao X, Duan Q, Bai H, Cao Y (2023) Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbohydr Polym 312:120755. https://doi.org/10.1016/j.carbpol.2023.120755
Salim MH, Kassab Z, Abdellaoui Y, García-Cruz A, Soumare A, Ablouh E (2022) Exploration of multifunctional properties of garlic skin derived cellulose nanocrystals and extracts incorporated chitosan biocomposite films for active packaging application. Int J Biol Macromol 210:639–653. https://doi.org/10.1016/j.ijbiomac.2022.04.220
Ma J, Ye G, Jia S, Ma H, Jia D, He J (2022) Preparation of chitosan/peony (Paeonia suffruticosa Andr.) leaf extract composite film and its application in sustainable active food packaging. Int J Biol Macromol 222:2200–2211. https://doi.org/10.1016/j.ijbiomac.2022.10.012
Almeida AR, Brisola Maciel MVO, Machado MH, Sganzerla WG, Teixeira GL, da Rosa CG (2022) Production of chitosan and poly (vinyl alcohol) films functionalized with hop extract (Humulus lupulu L. var. Cascade) for food packaging application. Food Packag Shelf Life 32:100833. https://doi.org/10.1016/j.fpsl.2022.100833
Iaccheri E, Siracusa V, Ragni L, Aguiar Saldanha Pinheiro AC, Romani S, Rocculi P (2023) Studying physical state of films based on casava starch and/or chitosan by dielectric and thermal properties and effects of pitanga leaf hydroethanolic extract. J Food Eng 339:111280. https://doi.org/10.1016/j.jfoodeng.2022.111280
Liu X, Xu Y, Liao W, Guo C, Gan M, Wang Q (2023) Preparation and characterization of chitosan/bacterial cellulose composite biodegradable films combined with curcumin and its application on preservation of strawberries. Food Packag Shelf Life 35:101006. https://doi.org/10.1016/j.fpsl.2022.101006
Rodrigues MÁV, Marangon CA, Martins VCA, Plepis AMG (2021) Chitosan/gelatin films with jatobá resin: control of properties by vegetal resin inclusion and degree of acetylation modification. Int J Biol Macromol 182:1737–1745. https://doi.org/10.1016/j.ijbiomac.2021.05.160
Cai M, Zhang G, Li C, Chen X, Cui H, Lin L (2021) Pleurotus eryngii polysaccharide nanofiber containing pomegranate peel polyphenol/chitosan nanoparticles for control of E. coli O157:H7. Int J Biol Macromol 192:939–949. https://doi.org/10.1016/j.ijbiomac.2021.10.069
Acknowledgements
The authors thank the Coordination for the Improvement of Higher Education Personnel-CAPES-for the first author's PhD grant. This research utilized the equipment of the Multiuser Nanotechnology Center (CMNano- UFS) located at Federal University of Sergipe, a national multiuser research centre supported by FINEP. We thank the CMNano- UFS team for technical support during the experiments performed under proposal #073/2022. The authors thank the Laboratory for the Study of Colloidal Systems (NUESC) of the Institute of Technology and Research (https://itp.org.br/) for providing the equipment and technical support for thermogravimetric analysis and differential exploration calorimetry analysis, and the Flavor Analysis Laboratory (LAF) of the Federal University of Sergipe for technical support for Fourier transform infrared spectroscopy (FTIR).
Author information
Authors and Affiliations
Contributions
Larissa Almeida Soares performed the experiments. Luciana Cristina Lins de Aquino Santana acted in the supervision and scientific guidance. All authors wrote the main manuscript text, prepared all figures and reviewed the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida Soares, L., de Aquino Santana, L.C.L. Physicochemical Characterization, Antioxidant and Antimicrobial Potential of Biodegradable Chitosan-Based Films Containing Pomegranate (Punica granatum L.) Peel Extract. J Polym Environ 32, 1729–1740 (2024). https://doi.org/10.1007/s10924-023-03063-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-03063-x