Abstract
Curcumin at different contents (0.5% to 2.5% w/w of soy protein isolate (SPI)) were incorporated in SPI films plasticized with glycerol. Before casting into film, desired amount of curcumin was added in small amount (5 mL) of alkaline water and subjected to sonication followed by adding to neat SPI suspension. Curcumin incorporated SPI suspensions were further characterized in terms of molecular mass by using sodium dodecyl—sulfate polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE profile of curcumin loaded SPI films shows less intense bands as compared to control films, indicating the crosslinking between SPI and curcumin. The fabricated curcumin incorporated SPI films were structurally and morphologically characterized by Fourier transform infrared (FTIR) and scanning electron microscope (SEM), respectively. Mechanical properties of curcumin incorporated SPI films were determined and the results showed that tensile strength and elongation at break increased from 4.57 MPa to 7.03 MPa and 129% to 244% for 2% (w/w) curcumin incorporated SPI films. Thermal behaviour, water uptake, total leachable material and transmittance studies of curcumin incorporated SPI films were carried out. Antibacterial activity of curcumin incorporated SPI films against E. coli and L. monocytogenes were also studied. The film doesn’t show any zone of inhibition against both the bacteria, but it reduces the growth of bacteria and at 2.5% (w/w) content of curcumin, E. coli doesn’t grow on the curcumin incorporated SPI films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum based plastic materials need more than hundreds of years to decompose because of their microbial resistance leading to white pollution around the globe. Among several types of plastic materials, single-use plastics are major threat to the environment due to rampant use ultimately leading to accumulation in our ecosystem [1,2,3,4]. Non-biodegradable nature of plastic materials and especially single-use plastic material have compelled the researcher all over the world to focus on cost effective alternative material obtained from renewable resources which is inherently biodegradable in nature [5]. Biopolymers such as starch, cellulose and its derivatives [6], pectin [7], chitosan [8], lipids [9], proteins [10,11,12,13] have emerged as suitable alternatives to synthetic plastic. Among all these biopolymers, soy protein isolate (SPI) falling in the category of protein based biopolymers has emerged as a potential material in term of packaging films and sheets with better barrier properties to oxygen and its water vapor permeability as compared to packaging films obtained from lipids and polysaccharides [14,15,16]. Mediocre mechanical strength, high water sensitivity of SPI films with almost no antibacterial properties restrict its acceptability as packaging films and sheets [16,17,18]. Certain kind of additives such as organic acids [19], phenolic acids [20, 21], and polyphenols [22, 23] have been used to enhance the mechanical as well as water barrier properties and also to impart antibacterial properties. Sivarooban et al., fabricated SPI film with grape seed extract to enhance the mechanical properties of SPI film [22]. Similarly, Ciannamea et al., prepared red grape seed extract incorporated SPI film to impart antioxidant properties in film as well as to enhance the water resistance in film due to protein–polyphenol interaction [24].
Curcumin (bis-α, β-unsaturated β-diketone) is a low molecular weight natural polyphenolic compound [25]. It is widely known for its medicinal use in pharma industry for its anti-inflammatory, anticancer, antitumor, antioxidant, antibacterial activities and wound healing properties [25,26,27,28]. Curcumin has been incorporated in several biopolymers such as polylactic acid (PLA) [29], collagen [30], cellulose [31], gelatin [32], cellulose acetate [33] and hydrogel-silver nanoparticles [34] for the preparation of composite membranes of antibacterial nature as well as wound dressing materials. Liu et al., has incorporated curcumin in chitosan (a hydrophobic natural polymer) to prepare blend film that show increase in tensile strength from 6.62 MPa to 11.84 MPa and also the increase in zone of inhibition against S. aureus and R. solani [35]. Curcumin has been incorporated in SPI (hydrophilic natural polymer) to form SPI-curcumin complex at pH 2, 3 and pH 7 [36,37,38]. At pH 2, cold-set mixed protein-polysaccharide self-supported gels with high stability were obtained from 15% (w/v) SPI, 0.1% (w/v) xanthan gum and 5 mM of CaCl2 [36]. Recently, the emulsifying properties and emulsion oxidative stability of SPI after complexation with curcumin at pH 3.0 and 7.0 have been investigated so as to encapsulate water-insoluble bioactive compounds in functional food [37, 38]. Limited studies have been done for curcumin incorporated biopolymeric films [30,31,32, 35, 39] and almost no study has been reported for curcumin incorporated SPI films.
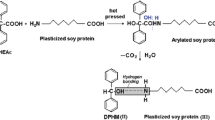
In this research paper, we have fabricated SPI film with curcumin at pH 9 to 9.5 by taking the concept of formation of protein phenol conjugates by alkaline method and ultrasonic heat treatment to curcumin for improved interaction [40,41,42]. Prior to formation of SPI incorporated SPI films, molecular mass profiles and structural changes of curcumin incorporated SPI suspension were carried out by sodium dodecyl—sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis and UV–Visible spectroscopy. The as-prepared films were characterized morphologically and structurally by scanning electron microscope (SEM) and Fourier transform infrared (FTIR) spectroscopy respectively. Mechanical, thermal, water uptake, antibacterial, and transmittance studies of curcumin reinforced SPI biopolymeric films were also carried out.
Materials and Methods
Materials
Soy protein isolate was procured from Zhenghou Ruikang Enterprise Co., Ltd. (Zhengzhou, China) having a purity of 90.27% (on dry basis). Curcumin and Luria Bertani (LB) powder was obtained from HiMedia. Glycerol as a plasticizer was acquired from Fisher Scientific and sodium hydroxide pellets was purchased from Titan Biotech Ltd. E. Coli /BL 21strain was procured from Gbiosciences. L. monocytogenes (MTCC 839) was purchased from IMTECH Chandigarh, India.
Sample Preparation
Samples were prepared in film form using solution casting method. SPI film (7% w/v) was fabricated by preparing a SPI suspension. In the first step, 1.05 g of glycerol (30% w/w of SPI) was added in 50 mL of water and the pH and temperature were maintained in the range of 9.5 to 10 with the help of 0.1 N NaOH and 60 °C, respectively. In the second step, 3.5 g of SPI (7% w/v) was gradually added to the plasticized water solution under stirring condition and the mixture was stirred for 1 h at 60 °C to get SPI suspension. The pH of resulting SPI suspension was again adjusted to 9.5–10 with the help of 0.1 N NaOH. After 1 h, the SPI suspension was kept in vacuum desiccator to remove air bubbles. Resulting suspension was then poured on silicon coated glass plate with a dimension of 15 cm × 10 cm and placed at 60 °C for 24 h without disturbance. The resulting SPI film fabricated on glass plate was maintained at 75% RH for 4–5 h so that of dried SPI film could be peeled off from the glass plate very easily.
The above mentioned protocol was also followed for preparation of curcumin incorporated SPI film with slight modification. It has been reported that curcumin tend to agglomerate in water as it is hydrophobic and it only gets dissolved in water at high pH [40, 42], so curcumin was dissolved initially in water at high pH. For this, 5 mL distilled water was taken in falcon and its pH was adjusted between 9.5 and10 with the help of 0.1 N NaOH. Then 0.35 g of curcumin (0.5% w/w of SPI) was added in 5 mL alkaline water and subjected to sonication. Afterward that 5 mL sonicated curcumin solution was added to neat 45 mL SPI suspension (obtained after 30 min stirring) to adjust the volume of 0.5% curcumin incorporated SPI suspension to 50 mL. The remaining 30 min stirring was done for 0.5% curcumin incorporated SPI suspension to complete total of 1 h stirring. Following same method as discussed in previous paragraph, 0.5% curcumin incorporated SPI film, designated as S-0.5CU, was prepared and peeled off. Hence, the SPI films prepared at all contents of curcumin were designated as S-0CU, S-0.5CU, S-1.0CU, S-1.5CU, S-2.0CU, S-2.5CU, here the numeric values denote the percentage of curcumin with respect to SPI.
Molecular Mass Profile by SDS-PAGE
Protein profile of prepared suspension in presence of different contents of curcumin was done using SDS-PAGE electrophoresis. For this study, sample suspension of each concentration of curcumin incorporated SPI suspension as well as neat SPI suspension was prepared by following procedure. Sample was mixed in a sample buffer (1 M Tris–HCl (pH 6.8) containing 10% (w/v) SDS, glycerol, β-mercaptoethanol, and 1% (w/v) bromophenol blue). β-mercaptoethanol cleaves disulphide bonds present in SPI and together with SDS it ensures unfolding of SPI thus transforming the secondary structure of protein to primary structure. The prepared samples were heated at 95 °C for 15 min and centrifuged at 12,000 rpm for 5 min at 4 °C. 5 µL of each concentration of curcumin incorporated SPI and neat SPI suspension was loaded onto each respective well of the polyacrylamide gel made of 5% stacking gel and 12% separating gel. The electrophoresis was carried out in a Hoefer electrophoresis unit (model miniVE, Hoefer, Holliston, MA, USA) using a current of 20 mA followed by staining with Coomassie blue R-250 in 40% (v/v) methanol and 10% (v/v) acetic acid and destaining with 45% (v/v) methanol and 10% (v/v) acetic acid. Wide-range molecular weight protein markers (Biorad Precision plus protein dual colour marker) were used to estimate the molecular weight of the proteins in presence of different content of curcumin.
UV–Visible Spectroscopy
The binding of curcumin with SPI was quantified by UV–Visible spectrophotometer from Motras Scientific. The absorbance of curcumin and SPI was measured by preparing stock solution of neat curcumin (4 µg/mL) and neat SPI i.e., S-0CU (980 µg/mL) at 426 nm [37]. Curcumin’s absorbance was measured at a constant SPI concentration (980 µg/mL) in the presence of different curcumin concentration 0.5% (4.9 µg/mL), 1.0% (9.8 µg/mL) and 2.0% (19.6 µg/mL). In this case, free curcumin samples without SPI were used as control, and absorbance was similarly recorded. The full wavelength UV–Vis absorption spectra of neat curcumin and curcumin incorporated SPI suspension were also carried out to show the interaction between curcumin and SPI.
Antimicrobial Studies
Curcumin incorporated SPI films were subjected to E. coli (Gram negative bacteria) and L. monocytogenes (Gram positive bacteria) for their antibacterial property if any. For this experiment, firstly the bacteria (both E. coli and L. monocytogenes) were revived overnight in 10 mL of LB broth in a shaker incubator at 37 °C. After that 10 μL of revived culture was taken (OD of 0.4 equivalent to 3.2 × 104 bacteria) and spreaded on plates, respectively for both the bacteria. Three plates for each concentration of S-0CU, S-0.5CU, S-1.0CU, S-1.5CU, S-2.0CU, S-2.5CU was prepared for both bacteria and the films (1 cm × 1 cm) were placed on each plates. Afterward the plates were incubated in static condition overnight at 37 °C to observe for bacterial growth after incubation.
Minimal inhibitory constant (MIC) of the bacteria was checked using Kirby-Bauer test. For this study, petri plates were spreaded with E. coli and L. monocytogenes and labelled for the different concentration of curcumin solution (87 mg curcumin in 50 mL water). Plates were punched to make wells at each labelled concentrations and the respective concentration of curcumin solution was added to the wells ranging from 10 to 60 μL (at an interval of concentration of 10 μL), where 1 μL solution contains 1.74 μg of curcumin. Afterwards the plates were incubated in static condition overnight and observed.
Characterizations
FTIR – Neat and curcumin incorporated SPI sample films were subjected to FTIR spectrophotometer from Perkin-Elmer, USA to record spectra. Sample films were scanned from a wavelength of 4000 to 400 cm–1 with a resolution of 4 cm–1 by infrared spectroscopy. 32 scans were taken at room temperature and the average of these scans was used to report the spectra.
Tensile strength – ASTM D 882 was followed to determine the tensile properties of sample films. Young’s modulus, tensile strength and elongation at break of neat and curcumin incorporated SPI films were measured by Universal Tensile Testing machine (Zwick, Germany) at IIT Patna. Sample dimension was 8 cm × 1 cm with a thickness of ~ 0.2 mm and cross head speed of 30 mm/min. Total of 5 tests were performed for every samples and the average value of five tests was reported as the tensile properties of sample films.
Thermogravimetric analysis – Thermal stability of the sample was determined using thermogravimetric analysis (TGA) instrument, TA Instruments, SDTQ600 (New Castle, DE, USA). The sample (10 ± 2 mg) was placed in an alumina pan and heated in the temperature range from room temperature to 700 °C under nitrogen atmosphere (at a gas flow rate of 100 mL/min) at a heating rate of 20 °C/min. The temperature at which maximum degradation takes place is denoted by Tmax. Char yield is obtained from the weight (%) vs. temperature curve.
Transmittance – This experiment was carried out using UV-2550 spectrophotometer (Shimadzu, Japan). For this experiment, sample films having a dimension of 4 cm × 1 cm was prepared and exposed to a wavelength ranging from 200 to 700 nm. The generated data was used as transmittance data.
SEM study – Cross-section morphology of neat and curcumin incorporated SPI films were carried out by SEM (EVO-SEM 15/18 (Carl Zeiss Microscopy, Ltd)) at an accelerating voltage of 20 kV. The samples were coated with gold prior to subjecting it to morphology experiment.
Water uptake – ASTM D570-81 was followed for water uptake studies. Sample films of neat and curcumin incorporated SPI films were prepared in a dimension of 1 cm × 1 cm (in triplets) and preconditioned at 60 °C for 24 h in the incubator followed by 1 h of cooling in desiccator maintained at 0% RH by using silica gel. Afterwards initial weight (W0) was taken, and film strip was transferred in well labelled bottles filled with distilled water and left undisturbed for 24 h. After the designated period, strips was carefully taken out and kept on tissue paper to remove water from strips surface and the strips were weighed again (final weight W1). The percentage water uptake of each strip was then calculated using below mentioned formula and the average water uptake percentage of all three strips of each concentration was reported as final water uptake value of the film.
Total leachable material – Total leachable material was tested to find out any leaching of curcumin from SPI film. For this study test tubes were taken, designated for each sample and weighed (M0). 1 cm × 1 cm of sample from each sample films were cut in triplicates, preconditioned and placed in the designated test tubes. 1 mL of water was added in the tube and left for 24 h. Afterward the film was taken out from the tube and the residual water of the test tube was dried followed by weighing (M1). Total leachable material was obtained using below mentioned formula.
Results and Discussion
Molecular Mass Profile by SDS-PAGE
Figure 1 depicts the molecular mass profile of neat and curcumin added SPI films. SPI is an impure protein of different molecular masses which contains major components of 7S and 11S. A band at 22 KDa represents the basic subunit while a band at 35–39 KDa represents the acidic subunit of glycinin (11S). Three distinct bands were observed at 50–52 KDa, 79 KDa and 85–89 KDa that represented the β, α and α’ subunits of beta-conglycinin (7S) fraction. From this gel image, it is evident that upon incorporation of curcumin, the intensity of the bands decreased as compared to the bands of neat SPI films. This may indicate towards the protein–polyphenol crosslinking, as curcumin is rich in polyphenol. Insaward et al., also reported that incorporation of polyphenol induces the protein polyphenol crosslinking which results in decreased band intensity [43].
SDS–PAGE analysis for 3 μL of marker in lane 1, and 5 μL of neat and curcumin added samples in increasing concentration in respective lanes. Lane 2, lane 3, lane 4, lane 5, lane 6 and lane 7 represent S-0CU, S-0.5CU; S-1.0CU; S-1.5CU; S-2.0CU; and S-2.5CU, respectively. Numbers on left are the molecular masses of marker proteins in kDa
UV–Visible Spectroscopy
Table 1 and Fig. 2 show the result of absorbance study of neat curcumin and curcumin with SPI at different concentrations. Upon addition of curcumin in SPI till S-2.0CU, the absorbance increases significantly from 0.037 to 0.028 and it is higher than that of neat curcumin at almost same concentration even for S-2.5CU. The increase in absorbance indicates towards the complexation of curcumin with SPI. Chen et al., in 2015 also suggested that SPI forms complexes with curcumin, through hydrophobic interactions resulting in improved solubility and stability of curcumin [41]. It has been reported that the solubility of free curcumin in water is 11 μg/mL [37, 41] while in our study we have added upto 24.5 µg/mL which suggested towards the increased solubility of curcumin in SPI, however it is less than the highest load capacity of curcumin (at a protein concentration of 3.5%, w/v) [41].
Visual Inspection and Transmittance of Curcumin-SPI film
Figure 3a shows the visual appearance of as prepared curcumin loaded SPI films. The photo clearly depicts that the colour of as-prepared film changes after incorporation of curcumin. The neat films without curcumin is pale yellow in appearance and the colour of curcumin incorporated films changed from brown to brown red and finally to intense dark brown with increasing content of curcumin SPI. Similar changes in colour were reported for SPI and other protein incorporated with caffeic acid, ferulic acid, gallic acid and catechin [43, 44]. It was suggested that the colour of the protein film changes due to phenolic-protein interaction and type and contents of phenolic compounds [42].
Figure 3b shows the transmittance curves for curcumin incorporated SPI samples in the visible range from 200 to 800 nm. As the contents of curcumin increases, the transmittance decreases and also the barrier properties of the curcumin incorporated SPI film to UV light are observed. The transmittance for S-0CU is reported as 97.83% and it decreases to 88.46% for S-2.0CU.
Mechanical Properties
Figure 4 depicts the mechanical properties of curcumin loaded SPI film. Data indicates that the tensile strength and elongation at break for curcumin incorporated SPI films are higher than that of neat SPI film. Similar trend was observed for curcumin incorporated chitosan blend film [35]. Tensile strength and elongation at break increases from 4.57 MPa to 7.03 MPa and 129% to 244% for S-2.0CU when compared to neat film. While Young’s modulus decreased from 43.30 MPa to 28.07 MPa for S-1.5CU but for S-2.0CU it remained almost same. A possible reason behind increased mechanical strength of curcumin incorporated SPI films can be the presence of polyphenol in curcumin which facilitate protein-phenol interaction as reported by Sivarooban et al., for grape seed extract incorporated SPI film [22].
Thermogravimetric Analysis
Figure 5 shows the thermogravimetric analysis (TGA) and differential thermogravimetry (DTG) curves of the curcumin incorporated SPI samples in the nitrogen environment. The char yield for curcumin incorporated SPI films during the whole thermo-degradation period was higher than that for neat SPI except for S-2.0CU thus indicates improvement of thermo-stability of the curcumin incorporated SPI films (Fig. 5a). In Fig. 5b, thermal decomposition of curcumin incorporated SPI films is divided into three stages. The first stage (Tmax1) is from room temperature to 125 °C and it is attributed to the evaporation of water from the samples. The weight loss in the second stage (Tmax2) in the temperature range of 175–288 °C is mainly related to the evaporation of glycerol that has been added as a plasticizer as well as decomposition of substituent groups present in SPI-curcumin complex. The values of Tmax2 increased from 259 °C for S-0.5CU to 261 °C for S-2.5 CU thus indicating that the evaporation of plasticizer occurs at higher temperature at higher content of curcumin in SPI. Weight loss (Tmax3) from at 308–525 °C is attributed to the degradation of protein backbone of SPI-curcumin complex. The values of Tmax3 also increased from 325 °C for S-0.5CU to 327 °C for S-2.5CU and that may be due to increasing amount of two benzene rings of curcumin at higher content of curcumin in SPI. The TGA and DTG results indicated that the addition of curcumin in SPI increased the thermal stability of curcumin incorporated SPI films.
FTIR Studies
Figure 6a shows the FTIR spectra of curcumin loaded SPI films. In neat SPI film, amide A broad band can be observed between 3200 and 3300 cm−1 which is generated due to N–H stretching. The curcumin incorporated SPI films shows a slight shift from 3284 to 3264 cm−1 indicating an interaction between SPI and curcumin. The peak at 1630 cm−1 indicates the amide I band of protein attributed to C = O stretching. Amide II bands can be observed at 1539 cm−1 and that peak is generated due to interaction between -CN stretching and -NH bending of SPI [19]. The peak at 1261 cm−1 also represents the interaction between -CN stretching and -NH bending and is attributed to amide III band. In curcumin, there is presence of aromatic rings and ketonic groups and that is the reason it is difficult to detect any new peaks because both these functional groups are already present in SPI also.
Curcumin incorporated SPI samples were monitored at 1700–1475 cm−1 regions that can give some information regarding the changes in the secondary structure of SPI (Fig. 6b). In this range, two typical peaks at 1630 cm−1 and 1539 cm−1 are associated with amides I and II, respectively. The peaks at 1630 cm−1 refer to β-sheet of SPI [45, 46]. The changes in amide I bond are more significant as compared to amide II bands. Till 1.5% of curcumin in SPI there is increase in amide 1 band and at above that content till 2.5% curcumin there is decrease in amide 1 band as compared to neat SPI. It is well-known that the secondary structure of SPI is stabilized by hydrogen bonds between functional groups of amino acids, including C = O and N–H. The rearrangement in the hydrogen-bonding network of the protein molecule cause changes in amide vibrational modes [47, 48]. The interactions of curcumin with amino acid residues of SPI can reorganize the hydrogen-bonding network and, finally, the protein structure that is evident from the change in amide 1 band of curcumin incorporated SPI film [49].
Water Uptake and Total Leachable Material
The magnitude of water uptake values does have effect on quality of the biofilms if it is used for packaging application. Water uptake values of SPI film before and after adding curcumin are shown in Fig. 7a. Neat SPI film showed water uptake of 171% which was similar to the several results cited in the literatures [14, 19]. Neat SPI film is hydrophilic in nature and the values of water uptake increased further after addition of curcumin that shows more hydrophilic nature of curcumin incorporated SPI film [35]. Tapal and Tiku in 2012 suggested that SPI enhances the solubility of curcumin, due to hydrophobic interaction between curcumin and SPI, which may expose the hydrophilic group of SPI leading to higher water uptake [37].
It has been reported that curcumin gets incorporated in SPI and forms complexes [41]. The formation of SPI-curcumin complex restricts the leaching of material and hence the leaching of curcumin incorporated SPI films is less and that is around 0.25% for S-2.0CU that showed highest tensile strength (Fig. 7b) [41].
Morphological Studies
Figure 8 shows the cross-sectional SEM images of neat and curcumin incorporated SPI films. The SPI film exhibits a relatively less coarse surface. In S-0.5CU the obvious change of cross sections can be observed in form of etched cross sectional morphology. The etched cross sectional morphology further progressed with the increase in the contents of curcumin. The original cross sectional structure of SPI is destroyed at high contents of curcumin in SPI matrix [50].
Antibacterial Properties
Figure 9 indicates the action of neat and curcumin incorporated SPI films against E. coli and L. monocytogenes. It has been reported that SPI does not exhibit antibacterial efficacy and similar result is found here [14]. Neat SPI i.e., S-0CU is completely covered by both the tested bacteria after 24 h of incubation. The incorporation of curcumin reduces the growth of bacteria on curcumin incorporated SPI film and clearly no visible growth of E. coli on S-2.5CU film was observed. Reductions in growth of bacteria on SPI after incorporation of curcumin in SPI films indicate the predominance of antibacterial effect of neat curcumin.
Minimum Inhibitory Concentration (MIC) Study
Figure 10 depicts the spot assay test for MIC study. The contents of curcumin for spot assay was maintained almost similar to that have been used in preparation of curcumin incorporated SPI films. However, zone of inhibition for both the bacteria upon exposure to curcumin were not observed despite of the antibacterial nature of curcumin. This may be due to the less concentration of curcumin in solution.
Conclusion
Curcumin incorporated SPI films with improved properties were successfully fabricated. Crosslinking between curcumin and SPI was evident by decreased intensity of bands upon addition of curcumin in SPI as evident from SDS-PAGE profile. FTIR study shows the changes in amide I and amide II bands, in curcumin incorporated SPI films that can be due to curcumin induced hydrogen bonds rearrangements in SPI matrix. Curcumin incorporation improved the thermal and mechanical properties of SPI films as compared to neat film. Tensile strength and elongation at break increase from 4.57 MPa to 7.03 MPa and 129% to 244% for S-2.0CU when compared to neat film. This may be attributed to the presence of polyphenols in curcumin, which is known to interact with protein. Curcumin incorporation increased the water sensitivity of SPI films that can be due to the fact that SPI induced improved solubility of curcumin through hydrophobic interactions. The incorporation of curcumin reduces the growth of E. coli and L. monocytogenes on curcumin incorporated SPI film and E. coli doesn’t grow on S-2.5CU film indicating towards the proven antibacterial properties of curcumin.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Madera-Santana TJ, Freile-Pelegrín Y, Azamar-Barrios JA (2014) Int J Biol Macromol 69:76–184
Moreno O, Atarés L, Chiralt A (2015) Carbohydr Polym 133:353–364
Shankar S, Rhim JW (2015) Carbohydr Polym 130:353–363
Song Y, Zheng Q (2014) Polym Rev 54:514–571
Rocha MD, De-Souza MM, Prentice C (2018) In: Grumezescu AM, Holban AM (eds) Food packaging and preservation, 1st edn. Elsevier
Halal SLME, Colussi R, Deon VG, Pinto VZ, Villanova FA, Carreño NLV, Dias ARG, Zavareze EDR (2015) Carbohydr Polym 133:644–653
Espitia PJP, Soares NDFF, dos Reis Coimbra JS, de Andrade NJ, Cruz RS, Medeiros EAA (2012) Food Bioprocess Tech 5:1447–1464
Arancibia MY, Alemán A, López-Caballero ME, Gómez-Guillén MC, Montero P (2015) Food Hydrocoll 91:99
Tzia C, Tasios L, Spiliotaki T, Chranioti C, Giannou V (2015) In: Varzakas T, Tzia C (eds) Handbook of food processing: food preservation, 1st edn. CRC Press
Zink J, Wyrobnik T, Prinz T, Schmid M (2016) Int J Mol Sci 17:1376
Tian HF, Guo GP, Fu XW, Yao YY, Yuan L, Xiang AM (2018) Int J Biol Macromol 120:475–490
Tian HF, Guo GP, Xiang AM, Zhong WH (2018) Polym Test 67:197–204
Li K, Li CM, Tian HF, Yuan L, Xiang AM, Wang CY, Li JL, Rajulu AV (2020) Macromol Mat Eng 305(8):2000239. https://doi.org/10.1002/mame.202000239
Rani S, Kumar R (2019) J Polym Environ 27:1613–1628
Koshy RR, Mary SK, Thomas S, Pothan LA (2015) Food Hydrocoll 50:174–192
Echeverría I, Eisenberg P, Mauri AN (2014) J Memb Sci 449:15–26
Zhang S, Xia C, Dong Y, Yan Y, Li J, Shi SQ, Cai L (2016) Ind Crops Prod 80:207–213
Xu F, Dong Y, Zhang W, Zhang S, Li L, Li J (2015) Ind Crops Prod 67:373–380
Rani S, Singh AK, Paswan RR, Kumar KD, Kumar R (2020) J Polym Environ 28:1841–1850
Friesen K, Chang C, Nickerson M (2015) Food Chem 172:18–23
Ou S, Wang Y, Tang S, Huang C, Jackson MG (2005) J Food Eng 70:205–210
Sivarooban T, Hettiarachchy NS, Johnson MG (2008) Food Res Int 41:781–785
Wang Z, Kang H, Zhang W, Zhang S, Li J (2017) Appl Surf Sci 401:271–282
Ciannamea EM, Stefani PM, Ruseckaite RA (2016) Food Sci Technol C 74:353–362. https://doi.org/10.1016/j.lwt.2016.07.073
Nong HV, Hung LX, Thang PN, Chinh VD, Vu LV, Dung PT, Trung TV, Nga PT (2016) Springerplus 5:1–9
Ak T, Gülçin I (2008) Chem Biol Interact 174:27–37
Fereydouni N, Darroudi M, Movaffagh J, Shahroodi A, Butler AE, Ganjali S, Sahebkar A (2019) J Cell Physiol 234:5537–5554
Sharma RA, Gescher AJ, Steward WP (2005) Eur J Cancer 41:1955–1968
Chen Y, Lin J, Fei Y, Wang H, Gao W (2010) Fibers Polym 8:1128–1131
Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R (2004) Biomaterials 25:1911–1917
Luo N, Varaprasad K, Reddy GVS, Rajulu AV, Zhang J (2012) RSC Adv 2:8483–8488
Musso YS, Salgado PR, Mauri AN (2017) Food Hydrocoll 66:8–15
Suwantong O, Opanasopit P, Ruktanonchai U, Supaphol P (2007) Polymer 48:7546–7557
Varaprasad K, Mohan YM, Vimala K, Raju KM (2011) J Appl Polym Sci 121:784–796
Liu Y, Cai Y, Jiang X, Wu J, Le X (2016) Food Hydrocoll 52:564–572
Brito-Oliveira TC, Bispo M, Moraes ICF, Campanella OH, Pinho SC (2017) Food Res Int 102:759–767
Tapal A, Tiku PK (2012) Food Chem 130:960–965
Chen S, Zhang N, Tang CH (2016) Food Hydrocoll 61:102–112
Roy S, Rhim JW (2020) Food Hydrocoll 98:105302
Rohn S, Rawel HM, Kroll J (2004) J Agric Food Chem 52:4725–4729
Chen FP, Li BS, Tang CH (2015) Food Res Int 75:157–165
Liu J, Yong H, Yao X, Hu H, Yun D, Xiao L (2019) RSC Adv 9:35825–35840
Insaward A, Duangmal K, Mahawanich T (2015) J Agric Food Chem 63:9421–9426
Prodpran T, Benjakul S, Phatcharat S (2012) Int J Biol Macromol 51:774–782
Yang Y, Wang Z, Wang R, Sui X, Qi B, Han F, Li Y, Jiang L (2016). Biomed Res Int. https://doi.org/10.1155/2016/5498639
Wang Z, Li Y, Jiang L, Qi B, Zhou L (2014). J Chem. https://doi.org/10.1155/2014/475389
Malik MA, Sharma HK, Saini CS (2016) J Food Sci Technol 53:3455–3464
Kanakis CD, Hasni I, Bourassa P, Tarantilis PA, Polissiou MG, Tajmir-Riahi HA (2011) Food Chem 127:1046–1055
Eczyk LS, Swieca M, Kapusta I, Gawlik-Dziki U (2019) Molecules 24:1–24
Li Y, Chen H, Dong YM, Li K, Li L, Li JZ (2016) Ind Crop Prod 82:133–140
Author information
Authors and Affiliations
Contributions
Statements are not mandatory.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work cited in this paper.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Only subscription based.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, S., Rani, P., Aggarwal, M. et al. Preparation and Characterization of Curcumin Incorporated Soy Protein Isolate Biopolymeric Films. J Polym Environ 30, 4877–4886 (2022). https://doi.org/10.1007/s10924-022-02566-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02566-3