Abstract
Zinc oxide (ZnO) is an inorganic compound widely used in everyday applications. ZnO is currently listed as a generally recognized as safe (GRAS) material by the Food and Drug Administration and is used as food additive. The advent of nanotechnology has led the development of materials with new properties for use as antimicrobial agents. Thus, ZnO in nanoscale has shown antimicrobial properties and potential applications in food preservation. ZnO nanoparticles have been incorporated in polymeric matrices in order to provide antimicrobial activity to the packaging material and improve packaging properties. This review presents the main synthesis methods of ZnO nanoparticles, principal characteristics and mechanisms of antimicrobial action as well as the effect of their incorporation in polymeric matrices. Safety issues such as exposure routes and migration studies are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne diseases are a global public health issue. The CDC has estimated 47.8 million foodborne illnesses, 127,839 hospitalizations and 3,037 deaths for 2011 in the U.S. alone, (CDC, 2011), which would result in medical expenses and productivity losses, and affect global health, trade and the economy.
As a result, the demand for new technologies to control foodborne pathogens has increased significantly in recent years. As such, food packaging plays an important role in providing safety and maintaining quality of food. Food packaging with new functions is known as active packaging, developed as a result of consumer demand for safety and more natural products with a longer shelf life, better cost–benefits and convenience (Ahvenainen, 2003).
According to regulations 1935/2004/EC and 450/2009/EC of the European Union, active packaging is defined as active materials in contact with food, with the ability to change the composition of the food or the atmosphere around it (Restuccia et al., 2010).
Antimicrobial packaging is a type of active packaging which interacts with the product or the headspace inside to reduce, inhibit or retard the growth of microorganisms that may be present on food surfaces (Soares et al., 2009).
In this way, the incorporation of antimicrobials into packaging materials allows the gradual diffusion of target bactericidal or bacteriostatic compounds into a food matrix, which eliminates the need for additional high concentrations of antimicrobials directly on the food product. In addition, researchers have considered antimicrobial packaging as an additional hurdle to food contamination after nonthermal processes; hence, they can play an important role in reducing the risk of pathogen contamination and extending the shelf life of food.
Organic compounds such as essential oils (Tripathi & Dubey, 2004), organic acids (Schirmer et al., 2009), enzymes like lysozyme (Appendini & Hotchkiss, 1997) and bacteriocins (Gálvez et al., 2007; Han, 2005) have been widely studied for their antimicrobial properties and tested for their potential application in polymeric matrices as antimicrobial packaging. However, organic compounds present some disadvantages. These include sensitivity to intense processing conditions that are present in many industrial processes (such as high temperatures and pressures) and the development of microorganism resistance.
The advent of nanotechnology, which involves the manufacture and use of materials with size of up to about 100 nm in one or more dimensions (Roco, 1999), has brought great opportunities for the development of materials with new properties for use as antimicrobial agents. Thus, the interest in inorganic compounds in nanosize has been steadily increasing over the last decade.
Inorganic compounds in nanosize present strong antibacterial activity at low concentrations due to their high surface area to volume ratio and unique chemical and physical properties (Rai et al., 2009). They are also more stable in extreme conditions such as high temperature and pressures (Sawai, 2003), and some are considered nontoxic and even contain mineral elements essential to the human body (Roselli et al., 2003). Most antibacterial inorganic materials are metallic nanoparticles and metal oxide nanoparticles such as silver, copper, titanium oxide and zinc oxide (ZnO) (Bradley et al., 2011; Chaudhry et al., 2008; Cioffi et al., 2005).
Research on ZnO as an antimicrobial agent started in the early 1950s. However, the real move towards the use of ZnO as an antimicrobial was in 1995, when Sawai and his colleagues found that MgO, CaO and ZnO powders had antimicrobial activities against some bacteria strains (Sawai, 2003; Sawai et al., 1997; Sawai et al., 1998). Currently, ZnO is one of the five zinc compounds that are listed as a generally recognized as safe (GRAS) material by the U.S. Food and Drug Administration (21CFR182.8991) (FDA, 2011).
The present review aims to describe and discuss research works that address the principal synthesis methods of ZnO nanoparticles, their antimicrobial activity as well as their mechanism of action and applications on polymeric matrices intended as food packaging applications. Also, safety aspects regarding the use of ZnO nanoparticles in food contact materials such as packaging are discussed.
Synthesis of ZnO Nanoparticles
Commercial production of ZnO nanoparticles is currently achieved by two main methods: mechanochemical processing (MCP) and physical vapor synthesis (PVS) (Casey, 2006). However, in other works, chemical reactions with different precursors and synthesis methods such as precipitation, thermal decomposition and hydrothermal synthesis have also been used.
Mechanochemical Processing (MCP)
MCP is a novel method for the production of nanosized materials, in which separated nanoparticles can be prepared. The method has been widely applied to the synthesis of a large variety of nanoparticles including ZnS, CdS, ZnO, SiO2 and CeO2 (Aghababazadeh et al., 2006).
MCP is a method of nanoparticle synthesis which combines a physical size reduction process in a conventional ball mill with chemical reactions that are mechanically activated at the nanoscale during grinding.
The application of MCP is a simple technique for the preparation of crystalline ZnO nanoparticles. In this process, the precursors zinc chloride (ZnCl2) and sodium carbonate (Na2CO3) are simultaneously milled in a ball mill to produce zinc carbonate (ZnCO3) and sodium chloride (NaCl) through ball–powder collisions and a chemical exchange reaction (Fig. 1). The ball mill acts as a low temperature chemical reactor where the reaction process results from local heat and pressure at contact surfaces at the nanoscale.
Moreover, the NaCl is used as an inert diluent added to the precursors. The product is considered a nanocomposite, with NaCl acting as the matrix phase (Casey, 2006). The mixture of the starting materials has the following chemical reaction equation:
The nanostructured product mix is then heat treated (170–380 °C) to thermally decompose ZnCO3 to ZnO, washed to separate the NaCl from ZnO and dried.
Nanoparticles produced by this method present an average particle size ranging from 20 to 30 nm (Aghababazadeh et al., 2006). The size of the obtained ZnO nanoparticles depends on the milling time and the heat treatment temperature. Experimental results have shown that increasing the milling time can effectively reduce the size of ZnO (Ao et al., 2006). Thus, there is an optimal grinding time in order to obtain ZnO nanoparticles with the smallest average size. According to Shen et al. (2006), optimal conditions are achieved by increasing the grinding time from 5 to 40 min, which reduces the size of ZnO nanoparticles from about 40 to 24 nm. However, with a higher grinding time, about 70 min, the size of ZnO nanoparticles slowly increases to 27 nm.
On the other hand, increasing the temperature of the heat treatment causes an increase in the size of the ZnO nanoparticles. The crystal size of ZnO nanoparticles increases slowly from about 18 nm at 400 °C to 21 nm at 600 °C, but increases rapidly above 600 °C, reaching 36 nm at 800 °C (Ao et al., 2006).
MCP is suitable for large-scale ZnO nanoparticle production due to its simplicity and low cost. Moreover, this process is attractive from an environmental point of view since the reactions involved do not include organic solvents (Lu et al., 2008). Moreover, drawbacks of the process such as particle agglomeration during milling are minimized by the presence of the salt matrix, which is then removed prior to calcination by a simple washing procedure.
Physical Vapor Synthesis (PVS)
In the PVS process, plasma arc energy is applied to a solid precursor in order to generate vapor at high temperature (Fig. 2). The plasma arc provides the energy needed to induce reactions that lead to supersaturation and particle nucleation when the precursor is injected into the plasma. This generally decomposes them fully into atoms, which can then react or condense to form particles when cooled by mixing with cool gas or by expansion through a nozzle (Swihart, 2003).
In this method, a reactant gas is added to the vapor, which is then cooled at a controlled rate and condensed to form nanoparticles. The nanoparticles produced by the PVS process consist of discrete, fully dense particles of defined crystallinity. This method typically produces particles with average sizes ranging from 8 to 75 nm (Casey, 2006).
Other Methods
There are several synthesis methods for preparing ZnO nanoparticles such as precipitation, thermal decomposition and hydrothermal synthesis, among others. Some characteristics of these methods such as their precursor and solvent as well as size and shape of the obtained nanoparticle are presented in Table 1.
In general, the preparation of nanoparticles is a complicated process, and different variables may affect the properties of the final product. Some important variables have distinct effects on the properties of the final product, while others may have only minor effects.
In the case of ZnO nanoparticle synthesis, variables such as nanoparticle size should be controlled, in order to obtain a uniform size distribution. In this way, theoretical considerations suggest that smaller particles with higher surface area have greater toxicity.
Moreover, nanoparticle morphology is another feature to be considered, since some works suggest that it influences nanoparticle toxicity when ZnO size and surface are controlled. The well-defined morphologies include nanowires, nanorods, nanospheres, nanoflowers, nanocubes, nanorings and nanoflakes, among others. Depending on the shape of the nanoparticles, the toxicity could be higher or lower, since interaction forces of lengthwise-oriented nanoparticles increase proportionally to their lengths. Interaction forces (Van der Waals forces) of nanoparticles with a cylindrical shape would be larger than those with spherical shape (Hsiao & Huang, 2011).
However, the control of all variables in the synthesis of ZnO is difficult to achieve, and different methods of synthesis will result in different sizes and shapes of nanoparticles. This depends mainly on factors such as the type of precursor and the solvent used as well as chemical and physical conditions such as pH and temperature of the components in the reaction.
Structure and Antimicrobial Properties
The food industry uses ZnO as a source of zinc, which is an essential micronutrient and serves important and critical roles in growth, development and well-being in humans and animals (Shi et al., 2008). In the food industry, ZnO has been used in food supplements, breakfast cereals and animal feedstuffs.
ZnO can present three crystal structures: Wurtzite, zinc blende and rocksalt (Fig. 3). At ambient conditions, the thermodynamically stable phase is the Wurtzite structure, in which every zinc atom is tetrahedrally coordinated with four oxygen atoms (Kulkarni et al., 2011).
ZnO crystal structures: cubic rocksalt (a), cubic zinc blende (b) and hexagonal Wurtzite (c). The shaded gray and black spheres represent zinc and oxygen atoms. Reprinted with permission from Özgür et al. (2005). Copyright [2005], American Institute of Physics
In each form, ZnO is a semiconductor material with direct wide band gap of ∼ 3.3 eV (Schmidt-Mende & MacManus-Driscoll, 2007) and has been extensively studied because of its versatility and broad applicability to many fields (Kulkarni et al., 2011). As such, ZnO is not a newly discovered material (Özgür et al., 2005), with reports of its physical characterization going back to 1935.
Recently, researchers have tested the in vitro antibacterial activity of ZnO, using pure nanoparticles or nanoparticle suspensions, also known as nanofluids.
Antimicrobial assessment in vitro has been performed using different methods (Table 2). The broth dilution method, followed by colony count, is one of the most used methods. It consists of determining the number of viable bacteria by plating, in suitable agar medium, serial dilutions of target microorganisms incubated in culture broths containing ZnO nanoparticles at different concentrations and proper time/temperature conditions. The antimicrobial activity of ZnO nanoparticles has been tested against some Gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus, which have presented sensitivity to these nanoparticles (Adams et al., 2006; Gordon et al., 2011; Reddy et al., 2007). Also, ZnO has shown antimicrobial activity against Gram-negative bacteria such as Pseudomonas aeruginosa, Campylobacter jejuni and Escherichia coli (Brayner et al., 2006; Ohira et al., 2008; Premanathan et al., 2011; Sawai, 2003; Xie et al., 2011).
E. coli has shown higher susceptibility to ZnO nanoparticles compared to S. aureus (Applerot et al., 2009a; Yamamoto, 2001). According to Applerot et al. (2009a), the higher resistance of S. aureus to ZnO nanoparticles can be explained by the differences between these two bacteria related to the intracellular antioxidant content such as carotenoid pigments in the interior of S. aureus, which promote a greater oxidant resistance as well as the presence of potent detoxification agents such as antioxidant enzymes, particularly catalase.
However, the increased sensitivity of S. aureus to ZnO nanoparticles has also been reported (Adams et al., 2006; Premanathan et al., 2011; Reddy et al., 2007; Sawai, 2003). Accordingly, Sawai (2003) has suggested a strong affinity between ZnO nanoparticles and the bacteria cells of S. aureus as the cause of higher activity against this microorganism.
There are two conditions for synergy between ZnO and S. aureus: the affinity of ZnO to the membrane of S. aureus and this microorganism's sensitivity to stress caused by H2O2 (Ohira et al. (2008). Previous work has indicated that ZnO nanoparticles generate H2O2.
According to Russell (2003), Gram-negative bacteria have shown less sensitivity to reactive oxygen species (ROS) when compared with Gram-positive bacteria. One of the main reasons for this higher resistance is the structural differences in the bacterial membrane (Fig. 4).
Gram-positive bacteria have a membrane, which surrounds the cell, and a cell wall primarily made up of peptidoglycan layer as well as teichoic and lipoteichoic acids. The cell wall of Gram-negative bacteria is more complex due to the presence of an outer membrane, which is composed mainly of lipopolysaccharide (LPS), in addition to a thin peptidoglycan layer (Epand & Epand, 2009; Jiang et al., 2004). Thus, the outer membrane of Gram-negative bacteria acts as a permeability barrier, so that the absorption of ROS into the cell is reduced (Russell, 2003).
Another explanation for the increased resistance to ZnO activity observed in E. coli compared with S. aureus is due to differences in the polarity of the cell membrane, since the membrane of S. aureus has less negative charge than E. coli (Sonohara et al., 1995). According to Gordon et al. (2011), this would allow a greater penetration level of negatively charged free radicals such as hydroxyl radicals, superoxide and peroxide ions causing damage and cell death in S. aureus at concentrations below that required to cause the same effect in E. coli.
Although several mechanisms have been proposed to explain the differences in the antimicrobial activity of ZnO against Gram-positive and Gram-negative bacteria, there are still some uncertainties; therefore, more studies should be done in order to clarify the sensitivity of these microorganisms to ZnO nanoparticles.
Furthermore, several factors can affect the antimicrobial activity of ZnO nanoparticles, among them the size of nanoparticles and, thus, the surface area as well as its activity in synergy with other antimicrobial agents.
The functional activity of nanoparticles is strongly influenced by their size. In this way, the antimicrobial activity of ZnO on E. coli and S. aureus has been improved with a diminution of particle size (Jones et al., 2008; Zhang et al., 2007). This is explained due to an increase in the surface area/volume ratio, which results in the increased reactivity of ZnO surface in nanometer size, since H2O2 generation depends strongly on ZnO surface area (Ohira et al., 2008). Thus, a larger surface area will result in more ROS on the surface of ZnO and, therefore, in a greater antibacterial activity of smaller nanoparticles (Padmavathy & Vijayaraghavan, 2008; Yamamoto, 2001).
In addition, theoretical considerations suggest that smaller particles, i.e., those with higher specific surface areas, should have more toxic effects on bacteria and fungi. However, according to Adams et al. (2006), the reasons for this toxicity could be due to other factors such as light intensity, surface chemistry, particle morphology and the concentration of the microorganism. Thus, more studies with rigorous control of variables and external factors should be conducted to clarify the effect of particle size on the activity of ZnO.
The antimicrobial activity of ZnO in synergy with other antimicrobial agents has also been an issue of interest. Chitosan capped ZnO nanorod showed higher antibacterial activity against E. coli compared to chitosan and also compared to uncapped ZnO individually, suggesting a synergistic effect (Bhadra et al., 2011). The researchers also observed that the antimicrobial effect of chitosan-capped ZnO was higher than the widely used antibiotic, amoxicillin, against this microorganism. According to Bhadra et al. (2011), chitosan-capped ZnO nanorods became attached to the outermost cell membrane of bacteria through the –NH2 group of chitosan, which enhanced cell membrane permeability and resulted in cell cytoplasm leaking out of the entire cell, leading to its destruction. Moreover, Gordon et al. (2011) combined ZnO with iron oxide to produce magnetic nanoparticles with antibacterial activity. The combined nanoparticles were tested against S. aureus and E. coli, and it was found that the antimicrobial activity was dependent on the weight ratio [Zn]/[Fe], i.e., the higher the ratio, the higher the antibacterial activity.
In addition to antibacterial activities, fungal pathogens of postharvest fruit have also been used to test the activity of ZnO, since concerns about the developed resistance of these microorganisms to many conventional fungicides such as benzimidazoles and dicarboximides have recently increased. According to He et al. (2011), ZnO nanoparticles showed significant antifungal activity against Botrytis cinerea and Penicillium expansum in concentrations higher than 3 mmol•L−1, with P. expansum being more sensitive than B. cinerea to the activity of these nanoparticles. Differences in the antifungal effects of ZnO can be produced by different growth morphologies of these fungi, since unlike B. cinerea, P. expansum tends to grow more densely on the surface of the culture medium. Thus, P. expansum has more exposure to ZnO nanoparticles in comparison with B. cinerea. Another possible reason for this difference is the innate tolerance of each fungus to ZnO nanoparticles.
Mechanism of Action
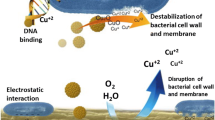
The exact mechanism of action of ZnO nanoparticles is still unknown. However, the antimicrobial activity of these nanoparticles is attributed to several mechanisms (Fig. 5) including the release of antimicrobial ions (Kasemets et al., 2009), interaction of nanoparticles with microorganisms, subsequently damaging the integrity of bacterial cell (Zhang et al., 2008) and the formation of ROS by the effect of light radiation (Jalal et al., 2010).
The release of Zn2+ antimicrobial ions has been suggested as a reasonable hypothesis about the toxicity of ZnO against S. cerevisiae (Kasemets et al., 2009). According to this author, the toxicity of ZnO nanoparticles could result from the solubility of Zn2+ ions in the medium containing the microorganisms. However, the solubility of metal oxides such as ZnO and Al2O3, is a function of their concentration and time (Wang et al., 2009). Thus, low concentrations of solubilized Zn2+ can trigger a relatively high tolerance by the microorganism. In the case of yeast, labile Zn2+ rapidly accumulates in dynamic vesicular compartments (vacuoles and zincosomes), which are an important cellular defense system to buffer both zinc excess and deficiency (Devirgiliis et al., 2004).
In addition, there are differences in the metabolic processes of Zn2+ ions, which depend on characteristics intrinsic to each microorganism. This could be one of the possible reasons for the observed differences in toxicity thresholds of ZnO nanoparticles in various microorganisms. In this way, Reddy et al. (2007) studied the toxicity of ZnO nanoparticles on E. coli and S. aureus. The results showed complete inhibition of E. coli growth at concentrations ≥3.4 mM, while growth of S. aureus was completely inhibited at concentrations ≥1 mM. Moreover, Reddy et al. (2007) observed that cells of E. coli treated with 1 mM of ZnO showed a consistent increase in the number of colony forming units (CFU) compared to control, due to the preference of this microorganism for low concentrations of Zn2+ in the growth medium. Conversely, S. aureus showed an efflux mechanism of Zn2+ during exposure to ZnO nanoparticles in millimolar range, indicating that the ion concentration resulted in undesirable and potentially toxic conditions to this microorganism. Thus, when studying the effect of ZnO against E. coli at low concentrations, rather than exercising antimicrobial activities, ZnO nanoparticles may actually increase bacterial growth.
Similar results have been reported by Padmavathy and Vijayaraghavan (2008), indicating that the ZnO nanoparticle suspensions in lower concentrations (0.01–1 mM) seem to have less antimicrobial activity against E. coli, and the presence of soluble Zn2+ ions may act as nutrients for this microorganism. Thus, while metals and metallic oxides are known to be toxic at relatively high concentrations, ZnO has shown no toxicity at low concentrations, since the zinc element is an essential cofactor in a variety of cellular processes.
On the other hand, interactions of ZnO with the bacterial cell membranes and the generation of damage on bacterial surface have been suggested as responsible for the antimicrobial activity of this metal oxide. In this way, Zhang et al. (2008) indicated that part of the antibacterial activity of ZnO results from the direct contact of nanoparticles with bacterial membrane and from the production of ROS close to the bacterial membrane.
Zhang et al. (2008) studied the effect of ZnO on E. coli cells, and as a result pointed out that the interaction between ZnO nanoparticles and E. coli cells is caused by electrostatic forces. According to Stoimenov et al. (2002), the global charge of bacterial cells at biological pH values is negative, due to the excess of carboxylic groups, which are dissociated and provide a negative charge to the cell surface. Conversely, ZnO nanoparticles have a positive charge, with a zeta potential of +24 mV (Zhang et al., 2008). As a result, opposite charges between the bacteria and nanoparticles generate electrostatic forces, leading to a strong bind between the nanoparticles and the bacteria surface and, consequently, producing cell membrane damage.
Furthermore, Brayner et al. (2006) showed that after the interaction between ZnO and E. coli, there is a disruption of the cell wall, causing the internalization of nanoparticles in bacterial cells. E. coli cells presented considerable damage, with disorganized cell walls, altered morphology and intracellular content leakage, as observed by these authors.
Zhang et al. (2007) obtained similar results, which clearly showed that the presence of ZnO nanoparticles caused the collapse of the bacteria membrane, leading to damage in E. coli surface. These researchers pointed out that this damage may be partly due to direct interaction between ZnO nanoparticles and the bacteria surface.
Similarly, interactions between ZnO nanoparticles and C. jejuni induced morphological changes, leakage of intracellular content and substantial increase in gene expression of oxidative stress in this microorganism (Xie et al., 2011). Thus, according to these studies, the inactivation of bacteria by ZnO involves mainly direct interaction between ZnO nanoparticles and the surface of cells, affecting the permeability of the membrane, allowing the internalization of nanoparticles and inducing oxidative stress in bacterial cells, resulting in the inhibition of cell growth.
However, unlike the previous mechanisms, several researchers have indicated the occurrence of ROS as the main mechanism responsible for the antimicrobial activity of ZnO nanoparticles (Gordon et al., 2011; Jalal et al., 2010; Padmavathy & Vijayaraghavan, 2008; Sawai et al., 1998; Yamamoto, 2001; Zhang et al., 2008). The generation of ROS such as hydroxyl radical (•OH), hydrogen peroxide (H2O2) and superoxide (O •−2 ), is the result of ZnO nanoparticle activation by visible light and UV.
Since ZnO is a semiconductor material, the incident radiation with photon energy higher than the value of its band gap (~3.3 eV) causes the movement of electrons from the valence band (vb) to the conduction band (cb) of the nanoparticle. The result of this process is the formation of a positive area, known as an electron hole (h +) in the valence band and a free electron (e−) in the conduction band (Seven et al., 2004).
The electron hole (h +) reacts with H2O molecules (from the suspension of ZnO) separating them into •OH and H+. In addition, O2 molecules dissolved in the medium are transformed into superoxide anion radicals (O2˙−), which in turn react with H+ to generate (HO •2 ). Subsequently, this species collides with electrons producing hydrogen peroxide anions (HO −2 ). Thus, the hydrogen peroxide anion reacts with hydrogen ions to produce H2O2 molecules (Gordon et al., 2011; Padmavathy & Vijayaraghavan, 2008). The following are the chemical equations that describe the generation of ROS in surface of ZnO nanoparticles:
Since the hydroxyl radicals and superoxide are negatively charged particles, they cannot penetrate the cell membrane and must remain in direct contact with the outer surface of the bacteria; however, H2O2 can penetrate the cell (Jalal et al., 2010; Padmavathy & Vijayaraghavan, 2008). The nanofluid concentration is comparable with the amount of H2O2, since the amount of H2O2 generated from the surface of ZnO should increase proportionally with increasing concentrations of nanoparticles (Jalal et al. 2010).
In addition to the aforementioned mechanism, Padmavathy and Vijayaraghavan (2008) suggest that the antibacterial effect of ZnO can also be the result of mechanical damage to the cell membrane caused by the abrasive surface of nanoparticles, since ZnO nanoparticles have been considered to be abrasive due to surface defects such as edges and corners (Stoimenov et al., 2002). However, additional related studies are needed to support this theory.
Moreover, the mechanism of action based on the generation of ROS on the ZnO nanoparticle surface seems contradictory, since some studies have shown the antimicrobial activity of ZnO nanoparticles even in dark conditions (Adams et al., 2006; Hirota et al., 2010; Zhang et al., 2007).
Adams et al. (2006) indicated that although the presence of light was a significant factor in the inhibition of bacterial growth, antimicrobial activity also occurred in the dark. Similar results have been presented by Hirota et al. (2010), indicating the sustainable antibacterial activity of ZnO nanoparticles (~30 nm) in the absence of light against E. coli on nutrient agar. Hirota et al. (2010) suggested that the antibacterial activity of ZnO in the dark could be produced by the generation of superoxide anions. These results indicated that there are probably additional mechanisms yet to be determined for the production of ROS in the absence of light and, therefore, more studies are needed to elucidate them.
On the other hand, the mechanism of action of ZnO on fungi has not been clearly determined. For example, a study of ZnO activity against the fungi P. expansum and B. cinerea showed inhibition of conidial development by distortion of conidiophores of P. expansum, while the fungal hyphae of B. cinerea were deformed (He et al., 2011). Deformations in the hyphal cells structure may be due to excessive accumulation of nucleic acids and carbohydrates, since the ZnO nanoparticles can affect cell function and produce increased levels of nucleic acids. According to Alvarez-Peral et al. (2002), an increase in nucleic acid can be considered as a stress response of fungal hyphae, and similarly, the increase in carbohydrates may be due to the mechanism of self-protection from ZnO nanoparticles.
Hence, the results of this study suggest that the ZnO has a mechanism of action in fungi different from those reported previously for bacteria. Therefore, further studies are needed to clarify the mechanism of action of ZnO in this type of microorganisms.
ZnO Applications on Food Packaging
Novel and efficient polymeric materials for food packaging developed with nanotechnology can provide solutions to food industry challenges related to product safety and materials performance as well as economic and environmental advantages (Silvestre et al., 2011). According to Chaudhry et al. (2008), food packaging materials developed with nanotechnology are the largest category of current nanotechnology applications for the food sector. In food preservation, the use of nanotechnology can extend and improve packaging functions, which traditionally have been containment, protection, preservation, marketing and communication, leading to a new kind of active food packaging.
In polymer science, composites are made of a polymeric matrix known as continuous phase and a discontinuous phase known as filler (Ajayan et al., 2003; Arora & Padua, 2010). Fibers, platelets and particles have been widely used as fillers, in order to improve the mechanical properties and heat resistance of polymers. Recent advances have allowed the application of nanotechnology in the development of new kinds of materials. Consequently, nanocomposites are hybrid materials where the filler incorporated in the polymeric matrix has at least one dimension (1D) in the nanometer scale.
The development of nanocomposites represents an alternative to conventional technologies used to improve the properties of polymers, since nanocomposites have improved barrier and mechanical properties as well as heat resistance when compared with the original polymers or conventional composites (Sorrentino et al., 2007). According to Thostenson et al. (2005), when nanocomposites are used as food packaging materials, they are able to withstand the thermal stress of food processing as well as the mechanical stress produced during transportation and storage.
A classic example of nanocomposites is the incorporation of the nanoclay montmorillonite (MMT) to improve thermal and mechanical properties of polymeric matrices such as nylon (Cho & Paul, 2001). In addition to MMT, other nanoparticles such as kaolinite and carbon nanotubes have been used to develop nanocomposites (Arora & Padua, 2010). However, in recent years, researchers have shown interest in the study of noble metals and metal oxides such as silver (Ag), titanium dioxide (TiO2), ZnO and copper oxides I and II (Cu2O, CuO) due to their stability at high temperatures and antimicrobial activity (Simoncic & Tomsic, 2010).
Silver and ZnO nanoparticles have some similarities such as their inorganic nature, a variety of synthesis methods and toxicity to the environment, among others. However, differences in bioavailability, applications and regulations offer some advantages to ZnO nanoparticles incorporated in polymeric matrices when compared to silver nanoparticles including providing solutions for safer and more affordable antimicrobial food packaging (Table 3).
The main applications of ZnO nanoparticles for food packaging materials include providing antimicrobial activity, since the presence of ZnO nanoparticles in the polymeric matrix allows the packaging to interact with the food and have a dynamic role in their preservation. In addition, ZnO nanoparticles allow for the improvement of packaging properties such as mechanical strength, barrier properties and stability.
ZnO Nanocomposites as Antimicrobial Food Packaging
ZnO nanoparticles have been incorporated in different materials including glass, low density polyethylene (LDPE), polypropylene (PP), polyurethane (PU), paper and chitosan using different incorporation methods (Table 4). The agar diffusion test and direct contact of the material with microorganisms contained in culture broth, followed by colony counting, has been used to test the antimicrobial activity of ZnO nanocomposite materials (Applerot et al., 2009b; Jin et al., 2009; Vicentini et al., 2010).
In relation to the active property of developed ZnO nanocomposites, the microorganisms used to perform the antimicrobial assessment include Gram-negative bacteria such as E. coli as well as Gram-positive bacteria such as B. subtilis, S. aureus and Lactobacillus plantarum (Applerot et al., 2009b; Emamifar et al., 2010; Jin & Gurtler, 2011).
For example, paper coated with ZnO nanoparticles (20 nm) has shown antimicrobial activity against E. coli (Ghule et al., 2006). Samples irradiated with UV light (365 nm, 1 mW cm−2) showed higher antibacterial activity with increasing exposure time. Therefore, while the UV light is considered as a contributing factor to the increased antibacterial activity of ZnO nanoparticles, exposure time and interaction time of bacteria with nanoparticles of ZnO are also important parameters (Ghule et al., 2006). On the other hand, the paper coated with ZnO nanoparticles also showed antibacterial activity even in the absence of light.
Moreover, PU films incorporated with ZnO (27 nm) have shown antimicrobial activity against E. coli and B. subtilis, with E. coli being more sensitive to the developed nanocomposite material (Li et al., 2009). The authors suggest that this may be the result of a strong affinity of the nanoparticles with E. coli cells and consider that the antibacterial activity of ZnO is due to the generation of H2O2 in nanoparticle surface.
Similar results were obtained by Applerot et al. (2009b), showing that glass coated with ZnO nanoparticles (300 nm) had antimicrobial activity against E. coli with an 89% reduction and, to a lesser extent, against S. aureus, with a reduction of 15%. The researchers suggest that the antibacterial activity of the nanocomposite material developed is influenced by the release of ROS generated at the surface of nanoparticles. In addition, Applerot et al. (2009b) indicated that difference in antibacterial activity between the two microorganisms tested may be explained by differences in their susceptibility to ROS.
However, there is no consensus related to the susceptibility of Gram-positive and Gram-negative bacteria to ZnO nanoparticles, since previous studies have also shown higher resistance of Gram-negative bacteria such as E. coli to the antimicrobial activity of ZnO nanoparticles in vitro. Moreover, chitosan and polyvinyl alcohol (PVA) films incorporated with ZnO nanoparticles (25–30 nm) showed antibacterial activity against S. aureus (Vicentini et al., 2010).
ZnO nanorods (30 nm in diameter and 500 nm in length) deposited onto glass surface showed antifungal activity against Candida albicans (Eskandari et al., 2011). In addition, researchers observed that the nanocomposite material developed was stable after 2 months of storage while maintaining its antifungal activity. Eskandari et al. (2011) suggested the formation of ROS, especially H2O2, on the surface of ZnO as responsible for its antifungal activity. However, recent studies have suggested that ZnO has a mechanism of action in fungi different from those reported previously for bacteria (He et al., 2011).
On the other hand, the antimicrobial activity of ZnO nanocomposites developed has been widely studied in vitro; however, few studies have shown the antimicrobial activity of these materials in contact with food. Orange juice has been used as a food matrix to test the antimicrobial activity of LDPE films incorporated with silver (Ag) and ZnO nanoparticles (Emamifar et al., 2010). According to the researchers, the application of active packaging containing ZnO nanoparticles prolonged the shelf life of fresh orange juice up to 28 days without causing negative effects on sensory parameters, while the developed active packaging containing silver nanoparticles had negative effects on the sensory quality of fresh juice, although this packaging had more activity in increasing orange juice shelf life.
Moreover, LDPE nanocomposite films containing Ag and ZnO were tested in orange juice inoculated with 8.5 log CFU ml−1 of L. plantarum (Emamifar et al., 2011). The results showed that the rate of microbial growth was significantly reduced by the use of the nanocomposite developed. However, the nanocomposite film containing silver nanoparticles showed a higher antimicrobial effect (Emamifar et al., 2011).
Modification of Structural Characteristics of Antimicrobial Food Packaging
Modification of structural characteristics of antimicrobial food packaging incorporated with ZnO nanoparticles can be determined by two main processes: structural analysis and property measurements (Table 5). Structural analysis is carried out using a variety of microscopic and spectroscopic techniques, while property characterization is more diverse and depends on the individual application (Koo, 2006). The techniques related to the structural analysis presented in Table 5 have been used to characterize packaging materials incorporated with ZnO nanoparticles as well as these nanoparticles before their incorporation in polymeric matrices.
The X-ray diffraction (XRD) technique uses the scattered intensity of an X-ray beam on the sample, revealing information about the crystallographic structure, chemical composition and physical properties of the material studied. This technique is nondestructive and does not require elaborate sample preparation, which partially explains its wide usage in materials characterization. XRD is one of the most commonly used techniques to characterize the nanodispersion degree of MMT in polymeric matrices intended for food packaging applications (Koo, 2006).
The crystal structure of ZnO nanorods coating a glass surface has been investigated by means of XRD (CuKα radiation and 1.54178 Å wavelength). The results showed the XRD pattern for ZnO nanorods with only one peak, which is indexed as (002) of the Wurtzite structure of ZnO. This indicated an orientation in the c-axis direction of the nanorod arrays over a large glass area. Also, no characteristic peaks were observed for impurities, thus, the samples studied presented a pure phase (Eskandari et al., 2011).
A similar result was obtained from previous work, in which a characterization of pure ZnO nanocrystals as well as ZnO sonochemically deposited on a glass slide after performing the XRD technique. A very intense and sharp peak at around 34° assigned to the (002) plane of Wurtzite ZnO in the spectra was observed. The large intensity of the peak and the weakness of the other diffractions indicated a very highly c-axis growth orientation on the glass surface. Also, the spectra of pure ZnO nanocrystals revealed the regular XRD pattern for hexagonal ZnO (indexed to the International Crystallographic data table, JCPDS card No. 79–0207), while no diffraction lines associated with impurities were detected (Applerot et al., 2009b).
Additionally, Applerot et al. (2009b) studied optical properties of ZnO nanocomposite glass by means of ultraviolet–visible spectroscopy, and according to them, the spectral transmittance of the pure glass had the highest transmittance. Meanwhile, an increase in the ZnO content caused a decrease in the transmittance, as observed in the UV, as well as in the visible region. This is due to a large surface area of absorbing ZnO nanoparticles, i.e., an increased surface area of nanoparticles and their uniform distribution on the glass surface lead to an increased UV absorption efficiency.
Although some structural features can be revealed by XRD, direct imaging of individual nanoparticles and their interaction with packaging materials is only possible using microscopy techniques such as scanning electron microscopy (SEM), transmission electron microscope (TEM) or atomic force microscopy (AFM).
As a result, researchers use more than one type of structural analysis to characterize the developed nanocomposites. For example, Applerot et al. (2009b) used AFM and SEM to study the surface morphology of the deposited ZnO on glass. Using SEM, they observed that the ZnO nanoparticles deposited on the glass were of spherical shape. Also, the ZnO coating on the glass changed as a function of reaction time. Thus, for a 2-h deposition process, a higher percentage of coating was observed, along with the growth of multilayers on glass surface, forming a pyramid-like structure. Additionally, AFM revealed the spherical shape of the deposited ZnO nanoparticles and the roughness profiles, which confirmed a multilayer growth and the formation of a pyramid-like structure on the glass surface after the 2-h deposition process.
Similarly, Lepot et al. (2011) used two techniques, SEM and TEM, to evaluate the dispersion quality as well as the morphology of the ZnO nanoparticles incorporated in biaxially oriented polypropylene (BOPP) films. Lepot et al. (2011) observed two ZnO morphologies, spherical nanoparticles and nanorods in 1D, and noted that spherical ZnO nanoparticles have a higher tendency to create agglomerates because of their higher surface area compared with 1D ZnO nanorods with the same diameter.
Moreover, Fourier transform infrared spectroscopy (FTIR) has been used to check the interaction of chitosan-capped ZnO nanoparticles with bacteria (Bhadra et al., 2011) as well as to study the photostability of polyethylene ZnO nanocomposites after UV exposure (Yang et al., 2010). The FTIR technique is based on vibrational spectroscopy, which allows the identification and determination of functional groups present in the polymeric sample.
In addition, to characterize the performance of polymer nanocomposites intended for food packaging applications, some mechanical properties such as Young's modulus (MPa), tensile strength (MPa) and elongation at break (%) have been studied. Barrier properties such as oxygen permeability have also been determined, and the thermal capacity has been studied by means of thermoanalytical techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Table 5).
According to Li et al. (2009), the study of developed PU-based coatings and films reinforced by ZnO nanoparticles (~27 nm) showed that the mechanical properties of films presented a significant improvement. Specifically, Young's modulus and tensile strength of PU films first increased and then decreased with the increase of ZnO content, with 2.0 wt.% being the optimal ZnO content for these properties. However, the elongation at rupture was inversely related to Young's modulus and tensile strength, indicating that ZnO nanoparticles can enhance the strength but not the flexibility of the nanocomposite films.
Furthermore, mechanical characterization of BOPP nanocomposites incorporated with ZnO (50–70 nm) showed that Young's modulus of the nanocomposites gradually increases by increasing the ZnO content, indicating an increase in the rigidity of the polymeric matrix related to the presence of the nanofillers (Lepot et al., 2011). Additionally, the developed films incorporated with ZnO nanoparticles presented improved barrier properties (oxygen permeability). According to Lepot et al. (2011), the improvement in the barrier property is explained by a decrease in the area available for diffusion, which resulted from two effects: impermeable nanoparticles replacing permeable spaces of the polymer and the biaxial orientation of the polymer chains.
Thermogravimetry allows knowing the thermal resistance of packaging materials. This technique studies the weight variation of packaging material (loss or gain) as a function of temperature and/or time, while the sample is subjected to a controlled temperature programming. Thermogravimetry is widely used, since it enables the determination of the temperature range in which the sample acquires chemical, fixed, defined and constant composition, and also the temperature in which packaging material start to decompose. The thermal stability of chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles was investigated by TGA studies, with a heating rate of 10 °C min−1 over the temperature range 0–300 °C in a nitrogen atmosphere (Vicentini et al., 2010). As a result, the developed films showed enhanced thermal stability in comparison to the control film. Vicentini et al. (2010) pointed out that the increase in thermal stability is in agreement with the XRD analysis, which showed a decrease in the interatomic distances, and consequently, more energy is required to decompose the films. According to the authors, the developed films are thermally stable up to 150 °C, which enables the application of a thermal process.
On the other hand, DSC is another widely used thermoanalytical technique. DSC consists of the direct measurement of heat changes, represented by enthalpy (∆H) that occurs in the sample during a controlled increase or decrease in temperature, making possible the study of packaging materials in their native state. In order to investigate the effect of the ZnO nanoparticles on the melting and the crystallization behavior of BOPP, DSC experiments were performed to determine the melting point temperatures and crystallinity (Lepot et al., 2011). The results showed a decrease in the melting temperatures after the addition of ZnO nanoparticles, explained by a decrease in lamellar thickness of the polymer crystals in comparison with pure BOPP. Moreover, after the addition of ZnO nanoparticles, crystallinity showed a decreasing trend with increasing weight percent of ZnO (Lepot et al., 2011).
Safety Issues
Food safety and quality as well as the potential impact on consumers are key issues related to food packaging developed with nanotechnology. Recently, interest has grown in safety issues regarding the use of nanoparticles in food packaging. Researchers are especially concerned with the possibility of nanoparticles migrating from the packaging material into the food and whether this migration would have a negative impact on the safety or quality of the packaged product (Bradley et al., 2011). According to Chaudhry et al. (2008), nanoparticles have much larger surface area to volume ratios, thus they may exhibit substantially different physicochemical and biological properties compared to conventionally sized particles.
There are three key factors of nanoparticle toxicity test strategies: physicochemical characterization, in vitro assays and in vivo studies (Oberdörster et al., 2005). However, there is currently a strong demand for low cost in vitro assays without reducing the efficiency and reliability of the risk assessment, since in vivo experiments are expensive, slow and ethically questionable.
Here, we analyze the main safety issues of ZnO nanoparticles: the current knowledge of ZnO nanoparticle toxicity towards carcinogenic and eukaryotic (healthy) cells as well as consumer exposure to ZnO nanoparticles by their migration from food packaging materials.
Toxicity of ZnO Nanoparticles Towards Carcinogenic Cells
Cancer is a malignant neoplasm that refers to more than 100 different diseases affecting various tissues and different types of cells. All forms of cancer are characterized by abnormal cell growth that results from a lack of the mechanism that controls normal cell division. This mechanism is programmed cell death (PCD), also known as apoptosis. The lack of apoptosis results from a multistep process involving genetic mutations induced by inheritance or environmental changes (Hütter & Sinha, 2001). Despite major advances in cancer therapy, there is considerable interest in the development of antitumor agents with a novel mode of action, since carcinogenic cells have shown the ability to develop resistance mechanisms to current chemotherapy (Hoskin & Ramamoorthy, 2008).
In vitro tests have shown that ZnO nanoparticles have antitumor activity against human colon carcinoma (LoVo) cells (De Berardis et al., 2010). The treatment with ZnO nanoparticles (11.5 μg ml−1) for 24 h resulted in a significant decrease of LoVo cell viability and apoptosis induction via ROS production. Moreover, higher doses of ZnO nanoparticles induced about 98% of cytotoxicity after 24 h of treatment. Also, the ZnO nanoparticle toxicity towards human osteoblast cancer cells suggested a selective toxicity of ZnO to cancer cells (Nair et al., 2009). Similarly, ZnO nanoparticles have exhibited a preferential ability to kill human myeloblastic leukemia cells (HL60) compared to healthy eukaryotic cells (normal peripheral blood mononuclear cells) (Premanathan et al., 2011).
Recent reports have demonstrated that some anticancer drugs could be assembled with biocompatible nanomaterials, which could effectively sustain drug delivery for the target carcinogenic cells and reduce the relevant toxicity towards normal cells and tissues. In this way, the combination of cancer therapy with ZnO nanoparticles is of interest for cancer research, since single modality treatment is not always effective.
A new strategy of using ZnO nanorods as carriers of the anticancer drug daunorubicin (DNR) was investigated (Zhang et al., 2011). The authors observed that the combination of ZnO nanorods with DNR induced a remarkable improvement in antitumor activity against human hepatocarcinoma cells. According to Zhang et al. (2011), ZnO nanorods increased the intracellular concentration of DNR and enhanced its potential antitumor efficiency, indicating that ZnO nanorods could act as efficient drug delivery carriers, importing DNR into target cancer cells.
The cytotoxic effect of DNR against leukemia cancer cells was investigated both in the presence and absence of ZnO nanoparticles (Guo et al., 2008). The results indicated that the combination of different-sized ZnO nanoparticles and DNR under UV irradiation could have a synergistic cytotoxic effect towards leukemia cancer cells, indicating the great potential of ZnO nanoparticles in relevant clinical and biomedical applications.
In addition to the use of antitumor drugs for cancer therapy, radiation therapy is commonly applied to carcinogenic cells due to its ability to control cell growth. The principle of cancer radiotherapy is that high energy radiation destroys malignant cells in a treated volume. However, using radiation to treat cancer requires high accuracy in delivering ionizing radiation to minimize toxicity to healthy surrounding tissues. To accomplish this, the use of ZnO nanoparticles as radiosensitizers has been proposed, which could enhance the effects of single or fractionated radiation therapy (Juzenas et al., 2008). Although more studies are needed to confirm these promising results, the key findings of these works support the view that ZnO nanoparticles induce toxicity in carcinogenic cells, which suggests they have potential as a novel alternative to cancer chemotherapy and radiation therapy.
Toxicity of ZnO Nanoparticles on Eukaryotic Cells
The application of nanotechnology in the food industry and food packaging and other areas such as the medical field, requires an evaluation of its health risks. Nanomaterials are considered to have more adverse effects on organisms than microscale materials because of their smaller sizes and corresponding larger specific surface areas. Some studies have shown cytotoxic effects of ZnO nanoparticles on different cell types such as human bronchial epithelium cells (Heng et al., 2010), human lung epithelium cells (Hsiao & Huang, 2011; Huang et al., 2010) and human kidney cells (Pujalté et al., 2011).
Mechanistic studies of ZnO cytotoxicity suggest that its activity against healthy cells is due to elevated oxidative stress and oxidative DNA damage. Although these toxicological studies were done by direct addition of ZnO nanoparticles to target cells, the effects of ZnO on human cells have not been confirmed. Moreover, these studies are disputed by others who suggest ZnO nanoparticles do not penetrate normal cells or compromise human or animal skin (Nohynek et al. (2010).
Consumer Exposure to ZnO by Migration from Packaging Material
An important factor to be considered in toxicity tests is the diversity of the exposure routes, which includes inhalation, dermal uptake, ingestion and injection. These routes of exposure can present unique toxicological outcomes that vary with the physicochemical properties of the nanoparticles in question (Oberdörster et al., 2005). However, in the case of nanocomposite packaging materials intended for food preservation, the main route of exposure to be evaluated is by ingestion (Fig. 6).
Exposure route and possible absorption pathways of ZnO nanoparticles incorporated in packaging material. Adapted with permission from Oberdörster et al. (2005). Copyright [2005], BioMed Central
On the other hand, for the final consumers of food packaged in nanocomposite materials, the first concern is to verify the migration of nanoparticles from the packaging into the food. If this migration happens, the next step to be studied is the effect of the ingestion of these nanoparticles inside the body from the mouth to the gastrointestinal tract, through in vitro as well as in vivo exposure tests (Silvestre et al., 2011).
In this context, Emamifar et al. (2010) studied the migration of silver (1.5% and 5%) and ZnO nanoparticles (0.25% and 1%) in LDPE and observed that the amount of silver ions (0.15 ± 0.002 μg L−1) migrating to orange juice after 112 days was below the allowable concentration (10 ppm). The zinc ions showed an increase in their migration (0.68 ± 0.002 μg L−1) when compared to silver, but its low concentration is in the range of acceptable foods to consumers, since ZnO is classified as a GRAS compound for food applications (Emamifar et al., 2011).
However, although these results are promising, there is a great need for further studies. These must include toxicological studies based on data obtained from migration tests to understand how nanoparticles as well as released ions may act within the body, their biotransformation and elimination routes, and their absorption by various organs.
Future Trends
Food packaging incorporated with ZnO nanoparticles has potential application in food preservation. So far, ZnO nanoparticles have been applied in petroleum-derived polymers such as LDPE and PP in order to develop antimicrobial materials. However, the uses of this nanoparticle in biodegradable polymeric matrices, although recent, promise to expand, since the incorporation of ZnO would allow for improvements in material performance, enhancing mechanical, thermal and barrier properties.
Moreover, additional research is needed to understand toxicological effects of ZnO nanoparticles, in order to determine the impact on consumers. In addition, there is a challenge to explore the use of food packaging developed with nanotechnology in synergy with other emerging technologies such as high hydrostatic pressures, electric fields, ultrasound, etc.
Conclusions
ZnO is a compound with many applications in everyday life. In addition, ZnO in nanosize is a promising antimicrobial agent due to its activity against a wide range of microorganisms and high resistance to severe processing conditions. ZnO nanoparticles have antimicrobial properties against foodborne pathogens. Moreover, these nanoparticles have maintained their antimicrobial activity even when incorporated in polymeric matrices, which indicates their potential for food preservation through their use as antimicrobial food packaging. Although the exact mechanism of action of ZnO nanoparticles has not been determined yet, their antimicrobial activity has been attributed to three main mechanisms: the release of antimicrobial ions, interaction of nanoparticles with microorganisms, subsequently damaging the integrity of bacterial cell and the formation of ROS by the effect of light radiation.
In addition to its antimicrobial properties, ZnO has presented modifications in the structure and properties of packaging materials such as mechanical and thermal resistance. This type of characterization is relevant in the design of a food packaging, since each characteristic influences the physical integrity of the developed packaging and, therefore, ensures the protection of the packaged food.
Lastly, antimicrobial food packaging developed with nanotechnology represents an impact on consumers. Few studies have focused on the migration of ZnO nanoparticles to food. However, the toxicological impact of ZnO nanoparticles must be evaluated to determine the positive or negative effects on food safety.
References
Adams, L. K., Lyon, D. Y., & Alvarez, P. J. J. (2006). Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Research, 40(19), 3527–3532.
Aghababazadeh, R., Mazinani, B., Mirhabibi, A., & Tamizifar, M. (2006). ZnO nanoparticles synthesised by mechanochemical processing. Journal of Physics Conference Series, 26(1), 312.
Ahvenainen, R. (Ed.). (2003). Novel food packaging techniques. Cambridge, UK: Woodhead Publishing Limited.
Ajayan, P. M., Schadler, L. S., & Braun, P. V. (Eds.). (2003). Nanocomposite science and technology. Weinheim: Wiley-VCH.
Alvarez-Peral, F. J., Zaragoza, O., Pedreno, Y., & Argüelles, J.-C. (2002). Protective role of trehalose during severe oxidative stress caused by hydrogen peroxide and the adaptive oxidative stress response in Candida albicans. Microbiology, 148(8), 2599–2606.
Ao, W., Li, J., Yang, H., Zeng, X., & Ma, X. (2006). Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technology, 168(3), 148–151.
Appendini, P., & Hotchkiss, J. H. (1997). Immobilization of lysozyme on food contact polymers as potential antimicrobial films. Packaging Technology and Science, 10(5), 271–279.
Applerot, G., Lipovsky, A., Dror, R., Perkas, N., Nitzan, Y., Lubart, R., & Gedanken, A. (2009). Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Advanced Functional Materials, 19(6), 842–852.
Applerot, G., Perkas, N., Amirian, G., Girshevitz, O., & Gedanken, A. (2009). Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Applied Surface Science, 256(3), S3–S8.
Arora, A., & Padua, G. W. (2010). Review: nanocomposites in food packaging. Journal of Food Science, 75(1), R43–R49.
Bhadra, P., Mitra, M. K., Das, G. C., Dey, R., & Mukherjee, S. (2011). Interaction of chitosan capped ZnO nanorods with Escherichia coli. Materials Science and Engineering: C, 31(5), 929–937.
Bradley EL, Castle L & Chaudhry Q (2011) Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends in Food Science & Technology, In Press, Accepted Manuscript, doi: 10.1016/j.tifs.2011.01.002
Brayner, R., Ferrari-Iliou, R., Brivois, N., Djediat, S., Benedetti, M. F., & Fiévet, F. (2006). Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Letters, 6(4), 866–870.
Casey, P. (2006). Nanoparticle technologies and applications. In R. H. J. Hannink & A. J. Hill (Eds.), Nanostructure control of materials (pp. 1–27). Cambridge, UK: Woodhead Publishing Limited.
CDC (2011) 2011 Estimates of foodborne illness in the United States. Center for Disease Control and Prevention, Atlanta, USA. Available at: http://www.cdc.gov/Features/dsFoodborneEstimates/. Accessed 6 May 2011.
Chaudhry, Q., Scotter, M., Blackburn, J., Ross, B., Boxall, A., Castle, L., Aitken, R., & Watkins, R. (2008). Applications and implications of nanotechnologies for the food sector. Food Additives & Contaminants: Part A, 25(3), 241–258.
Cho, J. W., & Paul, D. R. (2001). Nylon 6 nanocomposites by melt compounding. Polymer, 42(3), 1083–1094.
Cioffi, N., Torsi, L., Ditaranto, N., Tantillo, G., Ghibelli, L., Sabbatini, L., Bleve-Zacheo, T., D'Alessio, M., Zambonin, P. G., & Traversa, E. (2005). Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chemistry of Materials, 17(21), 5255–5262.
De Berardis, B., Civitelli, G., Condello, M., Lista, P., Pozzi, R., Arancia, G., & Meschini, S. (2010). Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicology and Applied Pharmacology, 246(3), 116–127.
Devirgiliis, C., Murgia, C., Danscher, G., & Perozzi, G. (2004). Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochemical and Biophysical Research Communications, 323(1), 58–64.
Emamifar, A., Kadivar, M., Shahedi, M., & Soleimanian-Zad, S. (2010). Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innovative Food Science & Emerging Technologies, 11(4), 742–748.
Emamifar, A., Kadivar, M., Shahedi, M., & Soleimanian-Zad, S. (2011). Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control, 22(3–4), 408–413.
Epand, R. M., & Epand, R. F. (2009). Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochimica et Biophysica Acta (BBA)—Biomembranes, 1788(1), 289–294.
Eskandari, M., Haghighi, N., Ahmadi, V., Haghighi, F., & Mohammadi, S. R. (2011). Growth and investigation of antifungal properties of ZnO nanorod arrays on the glass. Physica B: Condensed Matter, 406(1), 112–114.
FDA (2011) Part 182—substances generally recognized as safe. Food and drug administration, Washington DC, USA. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=786bafc6f6343634fbf79fcdca7061e1&rgn=div5&view=text&node=21:3.0.1.1.13&idno=21#21:3.0.1.1.13.9. Accessed 28 March 2011.
Gálvez, A., Abriouel, H., López, R. L., & Omar, N. B. (2007). Bacteriocin-based strategies for food biopreservation. International Journal of Food Microbiology, 120(1–2), 51–70.
Ghule, K., Ghule, A. V., Chen, B.-J., & Ling, Y.-C. (2006). Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chemistry, 8(12), 1034–1041.
Gordon, T., Perlstein, B., Houbara, O., Felner, I., Banin, E., & Margel, S. (2011). Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 374(1–3), 1–8.
Guo, D., Wu, C., Jiang, H., Li, Q., Wang, X., & Chen, B. (2008). Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. Journal of Photochemistry and Photobiology B: Biology, 93(3), 119–126.
Han, J. H. (2005). Antimicrobial packaging systems. In H. H. Jung (Ed.), Innovations in food packaging (pp. 80–107). London: Academic Press.
He, L., Liu, Y., Mustapha, A., & Lin, M. (2011). Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiological Research, 166(3), 207–215.
Heng, B. C., Zhao, X., Xiong, S., Woei Ng, K., Yin-Chiang Boey, F., & Say-Chye Loo, J. (2010). Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food and Chemical Toxicology, 48(6), 1762–1766.
Hirota, K., Sugimoto, M., Kato, M., Tsukagoshi, K., Tanigawa, T., & Sugimoto, H. (2010). Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceramics International, 36(2), 497–506.
Hoskin, D. W., & Ramamoorthy, A. (2008). Studies on anticancer activities of antimicrobial peptides. Biochimica et Biophysica Acta: Biomembranes, 1778(2), 357–375.
Hsiao, I. L., & Huang, Y.-J. (2011). Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Science of the Total Environment, 409(7), 1219–1228.
Huang, C.-C., Aronstam, R. S., Chen, D.-R., & Huang, Y.-W. (2010). Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicology In Vitro, 24(1), 45–55.
Hütter, G., & Sinha, P. (2001). Proteomics for studying cancer cells and the development of chemoresistance. Proteomics, 1(10), 1233–1248.
Jalal, R., Goharshadi, E. K., Abareshi, M., Moosavi, M., Yousefi, A., & Nancarrow, P. (2010). ZnO nanofluids: green synthesis, characterization, and antibacterial activity. Materials Chemistry and Physics, 121(1–2), 198–201.
Jiang, W., Saxena, A., Song, B., Ward, B. B., Beveridge, T. J., & Myneni, S. C. B. (2004). Elucidation of functional groups on Gram-positive and Gram-negative bacterial surfaces using infrared spectroscopy. Langmuir, 20(26), 11433–11442.
Jin, T., & Gurtler, J. B. (2011). Inactivation of Salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. Journal of Applied Microbiology, 110(3), 704–712.
Jin, T., Sun, D., Su, J. Y., Zhang, H., & Sue, H. J. (2009). Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. Journal of Food Science, 74(1), M46–M52.
Jones, N., Ray, B., Ranjit, K. T., & Manna, A. C. (2008). Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiology Letters, 279(1), 71–76.
Juzenas, P., Chen, W., Sun, Y.-P., Coelho, M. A. N., Generalov, R., Generalova, N., & Christensen, I. L. (2008). Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Advanced Drug Delivery Reviews, 60(15), 1600–1614.
Kasemets, K., Ivask, A., Dubourguier, H.-C., & Kahru, A. (2009). Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicology In Vitro, 23(6), 1116–1122.
Koo, J. (2006). Polymer nanocomposites: Processing, characterization, and application. New York, USA: McGraw-Hill.
Kulkarni, S. B., Patil, U. M., Salunkhe, R. R., Joshi, S. S., & Lokhande, C. D. (2011). Temperature impact on morphological evolution of ZnO and its consequent effect on physico-chemical properties. Journal of Alloys and Compounds, 509(8), 3486–3492.
Lepot, N., Van Bael, M.K., Van den Rul, H., D'Haen, J., Peeters, R., Franco, D., & Mullens, J. (2007). Synthesis of ZnO nanorods from aqueous solution. Materials Letters, 61(13), 2624–2627.
Lepot, N., Van Bael, M. K., Van den Rul, H., D'Haen, J., Peeters, R., Franco, D., & Mullens, J. (2011). Influence of incorporation of ZnO nanoparticles and biaxial orientation on mechanical and oxygen barrier properties of polypropylene films for food packaging applications. Journal of Applied Polymer Science, 120(3), 1616–1623.
Li, J. H., Hong, R. Y., Li, M. Y., Li, H. Z., Zheng, Y., & Ding, J. (2009). Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Progress in Organic Coatings, 64(4), 504–509.
Lu, J., Ng, K. M., & Yang, S. (2008). Efficient, one-step mechanochemical process for the synthesis of ZnO nanoparticles. Industrial and Engineering Chemistry Research, 47(4), 1095–1101.
Nair, S., Sasidharan, A., Divya Rani, V., Menon, D., Nair, S., Manzoor, K., & Raina, S. (2009). Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. Journal of Materials Science. Materials in Medicine, 20, 235–241.
Nohynek, G. J., Antignac, E., Re, T., & Toutain, H. (2010). Safety assessment of personal care products/cosmetics and their ingredients. Toxicology and Applied Pharmacology, 243(2), 239–259.
Oberdörster, G., Maynard, A., Donaldson, K., Castranova, V., Fitzpatrick, J., Ausman, K., Carter, J., Karn, B., Kreyling, W., Lai, D., Olin, S., Monteiro-Riviere, N., Warheit, D., & Yang, H. (2005). Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and Fibre Toxicology, 2(8), 1–35.
Ohira, T., Yamamoto, O., Iida, Y., & Nakagawa, Z. (2008). Antibacterial activity of ZnO powder with crystallographic orientation. Journal of Materials Science. Materials in Medicine, 19(3), 1407–1412.
Özgür, Ü., Alivov, Y. I., Liu, C., Teke, A., Reshchikov, M. A., Doğan, S., Avrutin, V., Cho, S.-J., & Morkoç, H. (2005). A comprehensive review of ZnO materials and devices. Journal of Applied Physics, 98(4), 041301.
Padmavathy, N., & Vijayaraghavan, R. (2008). Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Science and Technology of Advanced Materials, 9(3), 035004.
Prasad, V., Shaikh, A.J., Kathe, A.A., Bisoyi, D.K., Verma, A.K., & Vigneshwaran, N. (2010). Functional behaviour of paper coated with zinc oxide-soluble starch nanocomposites. Journal of Materials Processing Technology, 210(14), 1962–1967.
Premanathan, M., Karthikeyan, K., Jeyasubramanian, K., & Manivannan, G. (2011). Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine: Nanotechnology, Biology and Medicine, 7(2), 184–192.
Pujalté, I., Passagne, I., Brouillaud, B., Tréguer, M., Durand, E., Ohayon-Courtès, C., & L'azou, B. (2011). Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Particle and Fibre Toxicology, 8(10), 1–16.
Rai, M., Yadav, A., & Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83.
Reddy, K. M., Feris, K., Bell, J., Wingett, D. G., Hanley, C., & Punnoose, A. (2007). Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Applied Physics Letters, 90(21), 213902.
Restuccia, D., Spizzirri, U. G., Parisi, O. I., Cirillo, G., Curcio, M., Iemma, F., Puoci, F., Vinci, G., & Picci, N. (2010). New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control, 21(11), 1425–1435.
Roco, M. C. (1999). Towards a US national nanotechnology initiative. Journal of Nanoparticle Research, 1(4), 435–438.
Roselli, M., Finamore, A., Garaguso, I., Britti, M. S., & Mengheri, E. (2003). Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. Journal of Nutrition, 133(12), 4077–4082.
Russell, A. D. (2003). Similarities and differences in the responses of microorganisms to biocides. Journal of Antimicrobial Chemotherapy, 52(5), 750–763.
Sawai, J. (2003). Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. Journal of Microbiological Methods, 54(2), 177–182.
Sawai, J., Kojima, H., Ishizu, N., Itoh, M., Igarashi, H., Sawaki, T., & Shimizu, M. (1997). Bactericidal action of magnesium oxide powder. Journal of Inorganic Biochemistry, 67(1–4), 443–443.
Sawai, J., Shoji, S., Igarashi, H., Hashimoto, A., Kokugan, T., Shimizu, M., & Kojima, H. (1998). Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. Journal of Fermentation and Bioengineering, 86(5), 521–522.
Schirmer, B. C., Heiberg, R., Eie, T., Møretrø, T., Maugesten, T., Carlehøg, M., & Langsrud, S. (2009). A novel packaging method with a dissolving CO2 headspace combined with organic acids prolongs the shelf life of fresh salmon. International Journal of Food Microbiology, 133(1–2), 154–160.
Schmidt-Mende, L., & MacManus-Driscoll, J. L. (2007). ZnO—nanostructures, defects, and devices. Materials Today, 10(5), 40–48.
Seven, O., Dindar, B., Aydemir, S., Metin, D., Ozinel, M. A., & Icli, S. (2004). Solar photocatalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO2, ZnO and Sahara Desert dust. Journal of Photochemistry and Photobiology A: Chemistry, 165(1–3), 103–107.
Shen, L., Bao, N., Yanagisawa, K., Domen, K., Gupta, A., & Grimes, C. A. (2006). Direct synthesis of ZnO nanoparticles by a solution-free mechanochemical reaction. Nanotechnology, 17(20), 5117.
Shi, L., Zhou, J., & Gunasekaran, S. (2008). Low temperature fabrication of ZnO—whey protein isolate nanocomposite. Materials Letters, 62(28), 4383–4385.
Silvestre, C., Duraccio, D., & Cimmino, S. (2011). Food packaging based on polymer nanomaterials. Progress in Polymer Science, 36(12), 1766–1782.
Simoncic, B., & Tomsic, B. (2010). Structures of novel antimicrobial agents for textiles—a review. Textile Research Journal, 80(16), 1721–1737.
Soares, N. F. F., Silva, C. A. S., Santiago-Silva, P., Espitia, P. J. P., Gonçalves, M. P. J. C., Lopez, M. J. G., Miltz, J., Cerqueira, M. A., Vicente, A. A., Teixeira, J., Silva, W. A., & Botrel, D. A. (2009). Active and intelligent packaging for milk and milk products. In J. S. R. Coimbra & J. A. Teixeira (Eds.), Engineering aspects of milk and dairy products (pp. 155–174). New York, USA: CRC Press Taylor & Francis Group.
Sonohara, R., Muramatsu, N., Ohshima, H., & Kondo, T. (1995). Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophysical Chemistry, 55(3), 273–277.
Sorrentino, A., Gorrasi, G., & Vittoria, V. (2007). Potential perspectives of bio-nanocomposites for food packaging applications. Trends in Food Science & Technology, 18(2), 84–95.
Stoimenov, P. K., Klinger, R. L., Marchin, G. L., & Klabunde, K. J. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir, 18(17), 6679–6686.
Swihart, M. T. (2003). Vapor-phase synthesis of nanoparticles. Current Opinion in Colloid & Interface Science, 8(1), 127–133.
Thostenson, E. T., Li, C., & Chou, T.-W. (2005). Nanocomposites in context. Composites Science and Technology, 65(3–4), 491–516.
Tripathi, P., & Dubey, N. K. (2004). Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology, 32(3), 235–245.
Vicentini, D. S., Smania, A., Jr., & Laranjeira, M. C. M. (2010). Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Materials Science and Engineering: C, 30(4), 503–508.
Wang, H., Wick, R. L., & Xing, B. (2009). Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environmental Pollution, 157(4), 1171–1177.
Xie, Y., He, Y., Irwin, P. L., Jin, T., & Shi, X. (2011). Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Applied and Environmental Microbiology, 77(7), 2325–2331.
Yamamoto, O. (2001). Influence of particle size on the antibacterial activity of zinc oxide. International Journal of Inorganic Materials, 3(7), 643–646.
Yang, R., Christensen, P. A., Egerton, T. A., & White, J. R. (2010). Degradation products formed during UV exposure of polyethylene-ZnO nano-composites. Polymer Degradation and Stability, 95(9), 1533–1541.
Zak, A.K., Majid, W.H.A., Darroudi, M., & Yousefi, R. (2011). Synthesis and characterization of ZnO nanoparticles prepared in gelatin media. Materials Letters, 65(1), 70–73
Zhang, H., Chen, B., Jiang, H., Wang, C., Wang, H., & Wang, X. (2011). A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials, 32(7), 1906–1914.
Zhang, L., Ding, Y., Povey, M., & York, D. (2008). ZnO nanofluids—a potential antibacterial agent. Progress in Natural Science, 18(8), 939–944.
Zhang, L., Jiang, Y., Ding, Y., Povey, M., & York, D. (2007). Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). Journal of Nanoparticle Research, 9(3), 479–489.
Acknowledgments
The authors would like to thank to Mr. Nicholas J. Walker for providing language help and writing assistance. Financial support for this research was provided by a doctoral scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espitia, P.J.P., Soares, N.F.F., Coimbra, J.S.R. et al. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol 5, 1447–1464 (2012). https://doi.org/10.1007/s11947-012-0797-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0797-6