Abstract
Poly (3-hydroxybutyrate) (PHB) is a good alternative to petroleum-based plastics due to its biodegradable and biocompatible properties. In the present work, Cupriavidus necator ATCC 25207 was used for the first time to produce PHB using fructose, glucose, and acid or enzyme hydrolyzed molasses, a cheap carbon source. The bacterium produced highest PHB with 4.99 g/L, 3.94 g/L, and 15.28 g/L using fructose, glucose, and acid hydrolyzed molasses, respectively. Sulfuric acid and invertase treated molasses yielded 1288% and 529% more PHB than untreated molasses. In addition, acid treated molasses resulted in 151% more PHB but 34.04% less cell growth than enzyme hydrolyzed molasses. This novel finding could have applications in fermentations using molasses in growth or production media. The utilization of glucose by the bacterium was increased by 67.03% through spontaneous mutagenesis. The characterizations of the produced polymers were carried out using FTIR, NMR, XRD, DSC, TGA, and GPC. The molecular weights of PHB samples from acid hydrolyzed molasses and fructose are 3.84 \(\times \) 105 and 1.43 \(\times \) 106 g/mol, respectively. This study shows that C. necator ATCC 25207 has a potential to be used in PHB production using acid hydrolyzed molasses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic products have been widely used by humans making daily lives comfortable; however, these products don’t decompose in nature easily, causing environmental pollution. According to recent estimates, more than 250 million tons of non-degradable plastics, a large amount of which is discarded in the sea, have been produced annually [1]. During decomposition of plastic materials, hydrogen chloride and hydrogen cyanide are released from acetonitrile-derived plastics, creating a threat for the environment [2].
Biodegradable plastics such as polyhydroxyalkanoates (PHAs), on the other hand, would be a part of the solution. Production of biodegradable plastics instead of petrochemical derived plastics is thus becoming increasingly important and the studies on this subject have increased recently [3]. PHAs have been produced commercially by several companies in pilot or industrial scales [4].
PHAs, consisting of hydroxyalkanoate monomers, are polyesters which are non-toxic, insoluble in water, and have a high degree of polymerization. These polymers are divided into three different groups according to their monomeric units; short, medium, and long-chain-length PHA. Most of the short-chain-length PHAs like PHB are highly crystalline; however, many medium-chain-length PHAs form amorphous structures [5].
PHAs are synthesized by several microorganisms including Azotobacter, Bacillus, Alcaligenes, Pseudomonas, Rhizobium, and Rhodospirillum using such substrates as carbohydrates (fructose, glucose, xylose, arabinose, maltose, and sucrose), n-alkanes (hexane, octane, and dodecane), n-alkanoic acids (acetic acid, propionic acid, butyric acid, valeric acid, lauric acid, and oleic acid), n-alcohols (methanol, ethanol, octanol, and glycerol), and gases (methane and carbon dioxide). PHAs accumulate intracellularly at large amounts and are used as carbon and energy sources by the microorganism under limited carbon sources [6].
PHAs have a wide range of uses including packaging films, paper coatings, shampoo bottles, and such medical products as stents, vascular grafts, patches, and drug carriers [7].
They are degraded to carbon dioxide and water within 5–6 weeks in aerobic conditions; however, degradation of the polymer is faster in anerobic conditions producing methane and carbon dioxide [8].
PHB, a member of PHAs, was first isolated and characterized by Lemoigne in 1926 [9]. One of the microorganisms producing PHB at large amounts is Cupriavidus necator, known also as Ralstonia eutropha which exists in soil and fresh water and has versatile metabolic capabilities for adaptation in changing enviromental conditions [10, 11]. Cupriavidus necator H16 strain could utilize fructose, N-acetylglucosamine, and gluconate for bacterial growth and PHB production [12]. The C. necator strain has also been used to obtain PHA-PHV from cassava and pineapple resides [13, 14]. This strain does not have a glucose transport system and so it is unable to utilize glucose [15]. However, Franz et al. reported that a glucose positive phenotype was formed by spontaneous mutation when C. necator H16 was incubated in a medium containing glucose (20 g/L) for about 70 h. They showed that glucose positive phenotypes of C. necator H16 acquire the ability to consume glucose possibly due to mutations in the permease component of the N-acetylglucosamine phosphotransferase system allowing glucose uptake by facilitated diffusion [11]. This method is a simple and rapid way for adaptation of microorganism to glucose without any mutagenic process [11]. König et al. also observed that glucose positive phenotypes were formed when C. necator H16 was cultivated with fructose for several weeks [16]. New C. necator strains capable of utilizing glucose have been recently isolated and used for PHA productions [17, 18]. C. necator NCIMB 11,599 and C. necator DSM 545 strains, used in many PHB studies, are the glucose-utilizing recombinant mutant strains of C. necator DSM 529 and C. necator H16 strains, respectively [19,20,21,22,23].
The high cost of production is the major problem associated with the industrial production of PHB. The production cost of this polymer is still 8 to 10 times more than that of petroleum-based plastics [24]. 49% of the total cost of PHB production is raw materials, 60% of which are carbon sources [25]. The cost of PHB production could be reduced by developing new strains, improving fermentation methods, and using such cost effective carbon sources as molasses and whey [26].
Molasses, a by-product of sucrose production from sugarcane or sugar beet industry, are generated by global sugar industry 50 million tons annually [26]. It is an inexpensive carbon source containing sucrose, fructose, glucose, various B-vitamins (thiamine, riboflavin, niacin, pyridoxine, biotin, and folic acid), and minerals (potassium, phosphate, calcium, magnesium, copper, and iron) and has been used for production of some fine chemicals in industrial scales [27, 28].
In the present study, C. necator ATCC 25207 was explored for the first time to produce PHB using such economical carbon sources as olive oil mill wastewater, whey, molasses, and hydrolyzed molasses, giving rise to 0.01, 0.50, 0.17, and 2.36 g PHB in 1 L of culture, respectively. The best result was obtained using acid hydrolyzed molasses and thus further studies were carried out using acid hydrolyzed molasses, fructose, and glucose.
Material and Methods
Chemicals and Enzyme
Meat extract, peptone, and yeast extract were acquired from Fluka (Buchs SG, Switzerland). Glycerol and antifoam A were provided by Sigma-Aldrich (St. Louis, Missouri, USA). Sodium phosphate dibasic, sodium phosphate monobasic, magnesium sulfate heptahydrate, iron (III) chloride, calcium chloride dihydrate, copper sulfate pentahydrate, cobalt (II) chloride hexahydrate, zinc sulfate hexahydrate, nickel (II) chloride, fructose, glucose, sucrose, and thin layer chromatography (TLC) plates were purchased from Merck (Darmstadt, Germany). Beet molasses was provided by Pakmaya (Kocaeli, Turkey) and its composition is given in Table 1. Sucrose content in molasses was determined as explained in Sect. “Analytical Methods”. The moisture in molasses was determined according to Food Analysis Laboratory Manuel [29]. The other parameters of the molasses were provided by the supplier.
Invertase was purchased from Novo Nordisk (Denmark). The enzyme activity was 103.59 International Units (IU) per mg solid. IU is the amount of enzyme forming one µmol fructose from 0.16 M sucrose at 30 °C and pH 4.50 in 1 min.
Microorganism
Cupriavidus necator ATCC 25207 (formerly known as Wautersia eutropha NRRL B-2804 or C. necator DSM 30029) was obtained from American Type Culture Collection as a vacuum dried culture.
Growth and PHB Production Media
Bacterial growth and PHB productions were carried out in growth and production media, respectively. The media compositions are given in Table 2.
Inoculum Preparation
The microorganism was cultivated on growth medium and late exponential phase growth cultures (1 mL) were stored in glycerol [0.5 mL, 80.00% (w/w)] at – 80 °C in 2 mL cryovials as a stock culture. All the growth media contained glucose (5 g/L) in the inoculum preparation experiments which were carried out by consecutive cultivations of microorganism in growth media for 18 h at 30 °C using a shaker (Heidolph Unimax 1010, Germany) at 175 rpm. The stock culture (0.3 mL) was used in the preparation of pre-culture 1 by inoculating 5 mL of growth medium in 20 mL test tubes. The consecutive cultivations (pre-cultures 2–4) were performed by inoculating 5 mL of growth medium in 20 mL test tubes using 0.3 mL of pre-cultures. The final cultivation in a 250 mL shake flask containing 50 mL growth medium was carried out using 1 mL of pre-culture 4. Then, the final culture was centrifuged under aseptic conditions at 5800 rpm, 5 °C for 30 min (Centurion Scientific, K3 series) to obtain cell pellets which were washed twice with 0.90% (w/v) NaCl solution (10.00 mL/1.00 g pellet) and then isolated by centrifugation [11, 30]. These cell pellets were used as a inoculum.
Molasses Hydrolyses
The molasses, diluted fivefold (w/w) with distilled water, was centrifuged (5800 rpm, Centurion Scientific, K3 series, 20 °C, 20 min), and then filtered (Sartorious stedim 388 filter papers). The filtrate was used for acid and enzymatic hydrolyses.
In acid hydrolysis, the 1 L of filtrate (pH 3.00, 2 M H2SO4), kept at 25 °C for 8 h, was centrifuged, followed by heating at 120 °C for 15 min [22]. The hydrolyzed molasses solution, retained at 25 °C for 8 h, was centrifuged and filtered. It was then neutralized with 2 M NaOH solution and sterilized at 120 °C for 20 min.
In enzymatic hydrolysis, 40.00 mL of filtrate (pH 4.30, 2 M HCl) was reacted with free invertase (252 IU) at 30 °C for 30 min under continuous stirring at 300 rpm with a magnetic stirrer (Heidolph MR 3004 safety) [23]. Then, the filtrate was centrifuged and filtered. The pH of the filtrate was set to 7.00 with 2.00 M NaOH. Finally, the filtrate was sterilized at 120 °C for 20 min.
PHB Productions in Shake Flasks and Bioreactors
Batch and fed-batch modes were used for PHB productions in shake flasks and bioreactors, respectively. All experiments were carried out at least in triplicate and standard deviations were calculated using Excel.
Batch mode PHB productions were carried out in shake flasks (250.00 mL) in which production medium (100.00 mL), inoculated with the cell pellet (0.30 g containing 0.04 g dry cell), was incubated in a shake flask at 30 °C and 175 rpm using molasses (30–55 g/L) or hydrolyzed molasses (40.00–70.00 g/L) for 60 h or carbohydrates [fructose (20.00–60.00 g/L), and glucose (20.00–60.00 g/L)] for 68 h at pH 7.00 by addition of 2 M HCI or 2 M NaOH solutions. The consecutive PHB productions were carried out thrice in the production media (100 mL) containing 20 g/L of glucose or fructose, inoculated with a previous PHB production cell cultures (2 mL). The cell cultures were then centrifuged to obtain cell pellets which were washed with 0.90% (w/v) NaCl solution (10.00 mL/g pellet) and isolated by centrifugation (5 °C, 5800 rpm, 20 min). The purified cell pellets were lyophilized for 48 h (Scanvac cool safe), then PHB within the cell pellets was extracted and purified as explained in Sect. “Extraction and Purification of PHB”.
PHB productions in bioreactors were carried out in a 5 L bioreactor (Sartorious Biostat B-plus) equipped with an autoclavable jacketed culture vessel, a control system and a software (MFCS SCADA, Germany) for controlling dissolved oxygen, pH, temperature, the stirring speed, gas flow, and foam formation.
The pH of the culture was maintained at 7.00 by addition of 2.00 M HCl or 2.00 M NaOH solutions. Dissolved oxygen concentrations were maintained at 80% for microbial growth and 30% for PHB production using air flow (2.50 L/L/min) and cascade control of agitation speed. Foam formation was controlled by the addition of the silicone-based antifoam solution (10.00% v/v). Production medium (2.50 L) containing acid hydrolyzed molasses was inoculated with cell pellets (8.00 g containing 1.20 g dry cell) and incubated at 30 °C for 52 h for the production of PHB, in which molasses were used in various feeding rates and concentrations in three experiments called FB-M1, FB-M2, and FB-M3. The initial molasses concentrations in production media were 55.00 g/L for FB-M1/FB-M2 and 70.00 g/L for FB-M3. The fermentations took place in batch mode until the amounts of carbohydrates (a mixture of fructose and glucose) are about 11.00 g/L for FB-M1 and FB-M2, 14.57 g/L for FB-M3. It was then switched to fed-batch mode. In the FB-M1, the molasses solution was fed into the bioreactor at the following feeding rates; 51.20 mL/h in between 21 and 26th h, and 91.92 mL/h in between 39 and 41th h. In the FB-M2, the feeding rates were 29.00 mL/h in between 15–24th and 27–30th h, 17.12 mL/h in between 35 and 38th h, and 48.40 mL/h in between 45 and 46th h. The feeding rate was 58.12 mL/h in between 24 and 29th h for FB-M3. In 52 h of fermentation experiments taking place in FB-M1, FB-M2, and FB-M3, the cell cultures were centrifuged to get cell pellets, which were purified and dried by washing with 0.90% (w/v) NaCl solution (10.00 mL/g pellet), centrifugation (5800 rpm, 4 °C, 20 min), and lyophilization for 48 h.
Calculations
The overall performance of fermentations was characterized by the PHB yield, PHB and biomass yields per g sugar consumed and volumetric productivity at the end of the cultivation time (52 h) using Eqs. (1)–(5):
where RCW, [PHB], [Total biomass], [consumed sugar], YX/S, YP/S, qPHB, qRCW, and [RCW] are the residual cell weight, total PHB, total biomass, consumed sugar concentrations (g/L), biomass and product yields per gram sugar consumed, PHB productivity, residual cell productivity, and residual cell concentration (g/L), respectively.
Extraction and Purification of PHB
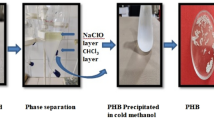
1.00 g dry cells in chloroform (25.00 mL) were disrupted using a homogenizer (IKA T25 digital Ultra Turrax) at 13,500 rpm and 25 °C for 10 min and filtered through the filter paper. The filtrate was concentrated by a rotary evaporator (Buchi R200 V805, Germany) to obtain a viscous solution which was dropped into 10 volume of 95.00% ethanol solution to precipitate PHB. The white precipitate was separated by centrifugation (5800 rpm, 4 °C, 20 min), washed twice with ethanol (95.00% v/v) and then dried under vacuum (MMM Vacucell vacuum drying oven) at 0.30 bar and 50 °C for 24 h [31]. The dried PHB in powder form was used for characterization.
Analytical Methods
The optical density was measured at 600 nm using a microplate reader (molecular devices spectramax plus 384) [32]. Residual cell weight was determined gravimetrically by the subtraction of the amount of PHB from the dry cell weight.
The residual ammonia nitrogen and phosphorus in the supernatant were determined using Kjeldahl method [33] and colorimetric assay [34], respectively.
The total amount of reducing sugar was determinated by dinitrosalicylic acid method (DNS) [35]. The quantitative determinations of fructose, glucose and sucrose were carried out using thin layer chromatography/densitometry (CAMAG TLC scanner 4 using software winCATS v.3, Switzerland). 2.00 µL sample was spotted on TLC plate with the CAMAG Linomat V automatic sample spotter as bands of 6 mm in length at a constant application rate 4.00 s/µL, keeping 6.00 mm space between each band with a x and y axis position of 10 and 15 mm, respectively. TLC was performed using three ascents of solvent mixture of acetonitrile/water [90.00: 10.00 (v/v)]. TLC plates were visualized by dipping the plates into 5% (v/v) H2SO4 in ethanol containing 0.5% (w/v) α-naphthol, followed by heating on a hot plate (CAMAG TLC plate heater III, Switzerland) at 120 °C for 10 min. The plates were cooled to room temperature and then scanned at a wavelength of 520 nm using a slit length of 6 mm and a slit width of 0.45 mm in the reflectance mode for visible absorption. Scanning speed and data resolution were 100 µm/step and 10 mm/s, respectively.
Characterization of PHB
Powdered PHB samples were characterized by FT-IR, NMR (1H and 13C), differential scanning calorimetry, gel permeation chromatography, thermogravimetric analysis, and XRD.
FT-IR-spectrum was measured between the 650 and 4000 cm−1 (Perkin Elmer FT-IR spectrometer). PHB samples in D-chloroform were used for the NMR (1H and 13C) spectra (Varian Inova 500 NMR spectrometer) [36].
The morphological state of PHB, melting temperature, and the enthalpy of fusion were measured using the differential scanning calorimeter (DSC822 Mettler Toledo) with cooling and heating under N2 flow of 20.00 mL/min. 1.26 mg sample was used for each thermal analysis in the range of − 25 °C to 190 °C at a heating rate of 10 °C/min. The first and second cooling runs were performed from 190 to − 25 °C at rates of 10 °C/min, holding for 5 min at − 25 °C prior to second heating [31].
The thermal stability of PHB granules was examined with the thermogravimetric analyzer using TGA/SDTA851 Mettler Toledo at a nitrogen flow rate of 20.00 mL/min and a scanning rate of 10 °C/min [36].
The molecular weights of PHB samples were determined at 35 °C by gel permeation chromatography (GPC) (model EcoSEC 8320, Tosoh EcoSEC ambient system) using two columns [TSKgel GMHXL (PN: 16,141)] and a refractive index detector, with chloroform as the elution solvent (flow rate 1.00 mL/min). PHB sample in chloroform (2.50 mg/mL) was injected (50.00 µL) into the column [31]. Monodisperse polystyrenes (Mw: 474–6,770,000 g/mol) in chloroform (2.50 mg/mL) were used as standards.
The XRD measurements were carried out using a Bruker D8 Advance diffractometer with Ni-filtered Cu Kα radiation (λ = 0.154 nm) in the range of 2θ = 10°–50° at 25 °C [36].
Results and Dıscussıon
Hydrolysis of Sucrose in Molasses
Sucrose in molasses was hydrolyzed by enzymatic and chemical methods. The TLC showed that all the sucrose was converted into fructose and glucose by invertase at 30 °C in 35 min (Fig. S1). Sulphuric acid treatment also resulted in complete hydrolysis (Fig. S2A).
Productions of PHB in Shake Flasks
Cupriavidus necator (H16) is unable to utilize glucose due to the absence of a glucose transport system [15]. Franz et al. reported that incubation of C. necator H16 in a medium containing glucose (20 g/L) rapidly induced a glucose positive phenotype [11]. In the present study, glucose utilization by C. necator ATCC 25207 was also increased inducing glucose positive phenotypes by incubation of the microorganism in a medium containing glucose (20 g/L): the three consecutive cultivation studies lasting for 68 h resulted in glucose consumptions of 8.90 g, 13.85 g, and 13.93 g in 1 L of culture, respectively. Under similar conditions, wild type C. necator ATCC 25207 consumed only 8.34 g glucose. These results show that glucose positive phenotypes were indeed formed (Fig. S3A), resulting in 67.03% increase in glucose consumption by the bacteria. In addition, the cultivations using these mutant bacteria produced more dry cell weight (DCW) (4.41–4.49 g/L) than the wild type bacteria (2.84 g/L) (Fig. S3A). It is clear that most of the mutagenesis was completed during the second consecutive cultivation. PHB productions and DCW were not changed in the consecutive cultivations using fructose (Fig. S3B).
PHB production by this microorganism increases as the concentration of carbohydrates (glucose or fructose) in culture media increases [37, 38]. We have also observed similar results in PHB productions by C. necator ATCC 25207 when the initial sugar concentrations (fructose and glucose) in the medium were in the range of 20–60 g/L, resulting in increases up to 1391 and 7780%, respectively (Tables S1 and S2). In all the cultivations, PHB productivities increased when concentration of carbohydrates (fructose or glucose) in the medium was more than 10.00 g/L (Tables S1 and S2); however, consumption of PHB began for cell growth when concentration of carbohydrates (fructose or glucose) in the medium decreased to 10.00 g/L (Fig. 1a and d, Tables S1, S2).
Batch mode PHB productions by C. necator ATCC 25207 in shake flasks incubated at 30 °C and 175 rpm for 68 h using production medium containing 20 g/L of glucose (a), 40 g/L of glucose (b), 60 g/L of glucose (c), 20 g/L of fructose (d), 40 g/L of fructose (e), 60 g/L of fructose (f).Glc glucose,Fru fructose
We found that RCW in addition to PHB was also increased with the increasing glucose concentrations up to 60 g/L in shake flask cultures (Fig. 1a–c, Table S2). However, cell growth and PHB production by Alcaligenes eutrophus NCIMB 11,599, a glucose utilizing mutant strain, were totally inhibited at 40.00 g/L of glucose in the medium. PHB production by A. eutrophus Glu 9 was weakly inhibited at 20.00–60.00 g/L of glucose [32].
The bacterial strain used in the present study prefers fructose over glucose for PHB production when carbohydrate concentration is up to 40 g/L where fructose produced 4.99 g/L PHB (17.75% yield) whereas glucose produced only 1.60 g/L PHB (8.24% yield) at the 68 h of fermentation at which consumption rate of glucose is slower than that of fructose and glucose is utilized mostly for microbial growth. 19.44 g/L of consumed glucose produced 5.43 g/L of RCW; however, 29.11 g/L of consumed fructose produced less RCW (3.39 g/L), resulting in biomass yields (YX/S) of 36.16% and 28.79% from glucose and fructose media, respectively (Tables S1 and S2). At higher concentration of carbohydrate (60 g/L), the amount of PHB produced by glucose (3.94 g/L) gets close to that produced by fructose (4.92 g/L). In addition, RCW in glucose medium (5.68 g/L) was still higher than that of fructose medium (4.00 g/L). Based on these findings, this microorganism could first be incubated in the medium with glucose (≥ 60 g/L) for microbial growth, followed by addition of fructose into fermentor for optimal PHB productions.
The maximum PHB obtained in our study was 3.94 g/L using 60.00 g/L of glucose in the medium (Table S3). Our finding is comparable to some of the results published: 3.30 g/L PHB was produced using C. necator DSMZ 545 in a medium containing 40.71 g/L glucose in 48 h. 4.60 g/L PHB was obtained in 72 h when C. necator ATCC 17,699 was incubated in a medium containing 30.00 g/L glucose [39]. In another study, batch cultivation with R. eutropha NCIMB 11,599, a recombinant strain, yielded 6.54 g/L PHB in 72 h using 20.00 g/L of glucose [21].
We have shown that initial fructose concentration up to 40.00 g/L is important for biomass and PHB productions by C. necator ATCC 25207. Increasing fructose concentrations resulted in better biomass and PHB yields: 0.33 g/L PHB and 4.98 g/L DCW were obtained using 20.00 g/L of fructose in the medium (Fig. 1d); however, the amount of PHB (4.99 g/L) and DCW (8.38 g/L) were increased by 1412% and 68%, respectively, at 40.00 g/L of fructose in the medium (Fig. 1e, Tables S1, S3). At 60.00 g/L of fructose, the PHB production (4.92 g/L) was almost the same as that of obtained from 40.00 g/L of fructose (4.99 g/L) in the medium (Fig. 1e and f). In addition, DCW was increased only by 6.00% in respect to DCW obtained using 40.00 g/L of fructose (Table S1). These results show that cell growth and PHB production are not largely affected by fructose when its concentration is about 60 g/L.
PHB yield and productivity obtained from shake flask experiments using fructose in the present work were higher than those produced by similar strains (Table S3) [7, 40]. 1.40 g/L PHB and 3.25 g/L DCW were obtained using C. necator NRRL B14690 at 40.00 g/L fructose in a fermentation lasted for 48 h [7]. Aramvash obtained 2.16 g/L PHB and 4.76 g/L DCW using C. necator ATCC 17,699 in a medium containing 20.00 g/L fructose in 48 h (Table S3) [40]. However, C. necator DSM 545 produced more PHB (5.80 g/L) and DCW (10.74 g/L) than those in our work at 39.00 g/L fructose in a batch mode fermentation in 72 h [40].
Economical production of PHB requires an inexpensive carbon source such as molasses, more than half of which is sucrose. Some C. necator strains grow in sucrose medium but their PHB productions are inefficient [7, 41]. We have also observed similar results for PHB production using molasses medium containing 16.08 g/L, 21.06 g/L, and 29.39 g/L of sucrose (Table 3). The best PHB production was only 0.17 g/L using molasses medium containing 29.39 g/L sucrose (Table 3).
Thus, sulphuric acid or invertase hydrolyzed molasses was used in PHB productions and found that acid hydrolyzed molasses gave rise to higher PHB productions while enzyme hydrolyzed molasses yielded higher cell weights. Various concentrations of acid hydrolyzed molasses (40.00–70.00 g/L) and enzyme hydrolyzed molasses (40.00 and 55.00 g/L) were used in cultivations and the results were given in Table 3. The best result for PHB production (2.36 g/L) together with RCW (3.10 g/L) was obtained using 55.00 g/L of acid hydrolyzed molasses, higher concentration of which (70.00 g/L) resulted in decreases in productions of PHB (1.24 g/L) and RCW (3.76 g/L). Enzyme hydrolyzed molasses at 40.00 g/L yielded PHB (1.07 g/L) and RCW (4.53 g/L); however, at higher concentration of enzyme hydrolyzed molasses (55.00 g/L) lower PHB (0.94 g/L) and more RCW (4.70 g/L) were obtained, showing that 40.00 g/L is better in terms of PHB production using enzyme hydrolyzed molasses. On the contrary, the most amount of PHB (2.36 g/L) was biosynthesized using 55.00 g/L acid hydrolyzed molasses.
These results show that the acid hydrolyzed molasses (55 g/L) medium resulted in 151% more PHB production but 34.04% less cell growth than enzymatic hydrolyzed molasses (55 g/L) medium. During acid hydrolysis of sucrose in molasses, a water insoluble material was formed, which was then removed by filtering (Fig. S2B). However, no precipitation was observed in enzymatic hydrolysis of molasses. It is likely that the soluble components of the water insoluble material, found in medium containing enzyme hydrolyzed molasses, are responsible for the slowing PHB production down by inhibiting enzyme(s) responsible for PHB biosynthesis (Fig. S2B). This assumption is supported by the fact that high concentration of enzyme hydrolyzed molasses (55.0 g/L) yields lower production of PHB (0.94 g/L) than that of 40.00 g/L enzyme hydrolyzed molasses (1.07 g/L). This novel finding could have applications in fermentations using molasses in growth or production media.
Sulphuric acid or enzyme hydrolyzed molasses media were also used as sole carbon sources by various researches for PHB productions by C. necator strains; however, PHB productions in these studies were lower than those obtained in the present study and the effects of acid and enzyme hydrolyzed molasses on PHB productions were not compared. Sen et al. produced 0.78 g/L PHB using C. necator and acid pretreated cane molasses medium [41]. Baei et al. produced 1.30 g/L of PHB using acid hydrolyzed sugar beet molasses medium [22]. Bozorg and co-workers obtained 1.63 g/L PHB using acid hydrolyzed cane molasses medium [42]. Poletto et al. used acid (HCI or H2SO4) or enzymatic (invertase or baker’s yeast) hydrolyzed molasses for the growth of C. necator DSM 545 and found that invertase hydrolyzed molasses was the best; however, they did not study the production of PHB during the cell growth. Thus, they used invertase hydrolyzed molasses together with sugarcane vinasse for the production of PHB (11.70 g/L). We also found that enzyme hydrolyzed molasses (55 g/L) yielded more cell growth (51.61%) than acid hydrolyzed molasses (55 g/L) which, on the contrary, resulted in 151% more PHB than enzyme hydrolyzed molasses.
Productions of PHB in Bioreactor
RCW and PHB obtained in shake flasks were low due to such insufficient conditions as dissolved oxygen, agitation, and pH. Thus, PHB productions were also carried out in a bioreactor under nitrogen limitation using acid hydrolyzed molasses as a carbon source. In batch cultivations in the bioreactor using 55 g/L of acid hydrolyzed molasses solution, the microorganism started growing exponentially after a lag phase in 4 h (Fig. 2a and b). PHB accumulation in the microorganisms began in 18 h where nitrogen concentrations were between 0.10 and 0.20 g/L (Fig. 2a and b) and 4.23 g/L PHB was obtained in 9.53 g/L DCW at 36 h of the fermentation (Fig. 2a and b, Table 4). The overall PHB yield (Yp/s) and productivity were 0.26 g PHB/(g sugar) and 0.18 g/(L h), respectively (Table 4). The amount of PHB produced in the cells started to decrease when the total sugar concentration in the medium was reduced to 10.00 g (fructose and glucose)/L, which happened in 40 h (Fig. 2a and b, Table 4).
Batch mode PHB productions by C. necator ATCC 25207 in bioreactors incubated at 30 °C, 2.50 L/L/min of air flow rate for 52 h using production medium containing acid hydrolyzed molasses. a The profiles of total sugar, DCW, PHB, and RCW; b the profiles of N, P, DCW, and PHB; c acid, base,and antifoam added to the bioreactor, pH of the medium; d oxygen, agitation, and temperature. S sugar concentration (fructose and glucose), Sc consumed sugar concentration, DCW dry cell weight, RCW residual cell weight, N nitrogen concentration, P phosphate concentration, pO2 dissolved oxygen
The pH of the culture both decreased and increased during fermentation due to formations of acids (hydrochloric and organic) and ammonium ions by protein catabolism, respectively; thus, it was maintained at 7.00 by addition of NaOH (2.00 M) or HCl solution (2.00 M) (Fig. 2c) [43].
The PHB productivity in the batch cultivations using bioreactor increased by 79% compared to shake flask studies due to the strict controlled such fermentation conditions as dissolved oxygen, agitation, and pH in the bioreactor studies (Fig. 2c and d).
The maximum amount of PHB (4.23 g/L) obtained from batch culture in bioreactor in the present work was lower than the following batch mode studies in bioreactors. Gahlawat obtained 6.24 g/L PHB using Azohydromonas australica DSM 1124 in sucrose medium [38]. Srivastava obtained 6.10 g/L PHB using C. necator NRRL 14,690 in fructose medium [44]. Tripathi produced 8.80 g/L PHB using C. necator NCIM 5085 and hydrolyzed cane molasses (Table 4) [45]. In order to increase PHB production in our work, a fed-batch mode production was designed so that the sugar concentration was kept over 10.00 g/L during the fermentation (52 h) where the best PHB yields were obtained.
Acid hydrolyzed molasses solution (197.24 g/L containing 72.56 g/L carbohydrate) with various feeding rates were used in PHB productions called FB-M1, FB-M2, and FB-M3. All the productions were started as a batch system with 2.50 L of production medium containing 0.44 g/L initial cell. Initial total sugar concentrations in FB-M1/FB-M2 and FB-M3 were 30.00 and 40.39 g/L, respectively at the beginning of the fermentations (Table 4). When the total sugar concentrations dropped to approximately 11.00 g/L, FB-M1 and FB-M2 were converted to fed-batch modes (Figs. S4A and 3a). However, in the FB-M3, batch mode was converted to the fed-batch mode in 24 h where the sugar concentration in the medium was 14.57 g/L (Fig. S5A).
Fed-batch mode PHB productions (FB-M2) by C. necator ATCC 25207 in the bioreactor incubated at 30 °C, 2.50 L/L/min of air flow rate for 52 h using production medium containing acid hydrolyzed molasses solution containing 72.56 g/L fructose and glucose. a The profiles of total sugar, PHB, DCW, and molasses; b the profiles of P, N, RCW, and PHB; c acid, base, and antifoam added to the bioreactor, pH of the medium; d oxygen, agitation, and temperature. Arrow points show the feeding times of acid hydrolyzed molasses. S sugar concentration (fructose and glucose), Sc consumed sugar concentration, DCW dry cell weight, RCW residual cell weight, N nitrogen concentration, P phosphate concentration, pO2 dissolved oxygen
In FB-M1 production, acid hydrolyzed molasses solution was fed into the bioreactor at rates of 51.20 mL/h in between 21 and 26th h and 91.92 mL/h in between 39 and 41th h (Fig. S4A). The lower feeding rate (51.20 mL/h) resulted in 0.29 g/(L h) PHB and 0.33 g/(L h) RCW productivities; however, the higher feeding rate (91.92 mL/h), gave rise to lower PHB (0.17 g/(L h)) and higher RCW productivities [0.63 g/(L h)] compared to the results of lower feeding rate (51.20 mL/h) possibly due to the accumulation of glucose in the medium which favors cell growth as shown in the flask studies (Figs. S4A, S4B, S6A, Table S4). PHB and biomass concentrations in FB-M1 production were 10.68 and 25.26 g/L, respectively (Fig. S4A, S4B, Table 4). The overall product yield (YP/S), biomass yield (YX/S), and PHB productivity were 0.28 g PHB/(g-sugar), 0.60 g DCW/(g-sugar), and 0.26 g/(L h) PHB, respectively (Table 4).
In the FB-M2 production, acid hydrolyzed molasses solution was fed into the bioreactor at three different feeding rates; 29.00 mL/h in between 15–24th h and 27–30th h, 17.12 mL/h in between 35 and 38th h, and 48.40 mL/h in between 45 and 46th h. The feeding rate of 29 mL/L resulted in 0.50 g/(L h) PHB and − 0.38 g/(L h) RCW productivities, showing that RCW decreases in this feeding rate. 17.12 mL/h feeding rate gave rise to 0.49 g/(L h) PHB and − 0.26 g/(L h) RCW productivities. At 48.40 mL/h feeding rate, productivities of PHB and RCW were 0.29 g/(L h) and 0.23 g/(L h), respectively (Fig. 3a and Table 4). Feeding rates affected the productivities of PHB and DCW: at feeding rates of 17.12 mL/h, PHB productivity was almost the same as that of feeding rate of 29.00 mL/h; however, there was no cell growth at this feeding rate. At higher feeding rate (48.40 mL/h), lower PHB productivity (0.29 g/L h) and higher RCW [0.23 g/(L h)] were obtained due to accumulation of glucose (Fig. 3a and b, Table 4). In FB-M2 production, the overall yield of DCW (YX/S, 0.44 g DCW/g sugar) decreased while the overall yield of PHB (YP/S, 0.50 g PHB/g sugar) increased compared to results of FB-M1 production (Table 4). 15.28 g/L PHB and 13.61 g/L RCW were obtained at the end of fermentation (Fig. 3a and b, Table 4). Quantitative determinations of PHB produced in this production were carried out using gravimetric and TGA analyses, both of which yielding similar results (Fig. S11 and Table 4).
In FB-M3 productions, the hydrolyzed molasses solution was fed into the bioreactor at only one feeding rate (58.12 mL/h), resulting in 5.84 g/L PHB, 12.34 g/L dry cell, 6.50 g/L residual biomass, and 0.25 g/(L h) PHB productivity. DCW and PHB productions were lower than those obtained in FB-M1 and FB-M2 production modes possibly due to the inhibitory effect of high initial molasses concentration on the microbial growth and PHB Production (Fig. S5A and Table 4) as observed in PHB productions by Azohydromonas lata MTCC 23311 [46]. The overall biomass (YX/S) and product yields (YP/S) were 0.30 g DCW/(g-sugar) and 0.27 g PHB/(g-sugar), respectively (Table 4).
In all the fed-batch productions in bioreactors similar to batch productions, the pH of the cultivations decreased at the growth phase due to acid formation. However, the pH increased at the PHB production phase due to protein catabolism and/or formation of extracellular polymeric substances (EPS) as observed by Wang et al. and Ribeiro et al. [30, 47] (Figs. 3c, S4C, S5C). In all of the fed-batch productions we carried out, excessive foam formations were observed at the PHB production phases (Figs. 3c, S4C, S5C). Bacterial growth and PHB productions were affected by ammonium and phosphate ions resulting in increased PHB productions and cessation of bacterial growth when the concentrations of ammonium and phosphate ions were between 0.10–0.20 g/L and 0.80–1.00 g/L, respectively, in all the productions (Figs. 2a, b, 3a, b, S4A, S4B, S5A, S5B). However, an increase in the amount of RCW was observed when the molasses solution was fed into the biorector since the ammonium and phosphate ions in molasses were available for bacterial growth (Figs. 3a, b, S4A, S4BB, S5A, S5B). We observed that fructose in the bioreactor was consumed faster by the microorganism than glucose (Fig. S6A and S6B, Table S4) and thus glucose accumulated in the medium inducing bacterial growth and reducing PHB production.
There is no work on PHB production by C. necator ATCC 25207 using sucrose or hydrolysed molasses. The best PHB production (15.28 g/L) was obtained from lab scale fed-batch production (FB-M2) in the present work by C. necator ATCC 25207 using acid-hydrolyzed beet molasses (Fig. 3, Table 4).
Based on findings of the present work, C. necator ATCC 25207 could have a potential to be used for PHB productions provided that the random mutagenesis of microorganism and optimization studies (glucose/fructose, nutrient composition, pH, dissolved oxygen, and feeding rate) are carried out.
Characterization of PHB
PHB samples produced using acid hydrolyzed molasses and fructose are called PHBm and PHBf, respectively. Structural and thermal properties of the polymers were determined by spectral measurements (FT-IR, H-NMR, and 13C-NMR), TGA, DSC, XRD, and GPC analyses.
FTIR spectra of the PHBm and PHBf are the same as shown in Fig. S7. The bands found at 1721 cm−1 correspond to the stretching of the C=O bond, whereas a series of intense bands located at 1000–1300 cm−1 correspond to the stretching of the C–O bond of the ester group. Also, bands of minor relevance, such as those found at 3438 cm−1, show the presence of terminal –OH group. The bands found at 2976 and 2934 cm−1 correspond to the stretching of the C–H bond. These are similar to the bands of PHB standard [13, 14].
1H NMR and 13C spectra of PHBm are shown in Figs. S8 and S9, respectively. The doublet at 1.21 ppm in 1H NMR spectrum is associated with methyl group, while multiplet peaks between 2.43 and 2.52 are ascribed to methylene group adjacent to asymmetric carbon atom. The multiplet at 5.20 ppm is related to -CH group. 13C NMR spectrum proved that the product synthesized is PHB. The peaks in 13C NMR spectrum were assignable to the methyl (19.76 ppm), methylene (40.79 ppm), methine (67.60 ppm), and carbonyl (169.12 ppm) carbon resonances of PHB. 1H NMR and 13C NMR spectrums of PHB obtained in our work are similar to those of PHB produced by Pradhan et al. [36].
The TG analyses of PHB samples are shown in Fig. 4a, b, and Table 5. The thermal degradation of PHB started with melting temperature range of 170–180 °C due to cleavage reaction of polymer ester groups by random chain scission as observed by Hablot et al. [48]. Degradation of PHBm and PHBf began at 262.00 °C and 261.47 °C, respectively. T%5 values were 274.16 for PHBm and 271.76 °C for PHBf. PHBm and PHBf decomposed completely at 302.80 °C and 300.77 °C, respectively. The standard PHB decomposes completely at 310 °C and its starting points of weight loss and T%5 are 230.00 and 274.00 °C, respectively [49]. These results show that the thermal stabilities of PHBm and PHBf are similar to those of standard PHB. Table 5 shows the temperatures of degradation steps from which the purities of PHBm and PHBf were determined as 98.86% and 98.88%, respectively.
Differential scanning calorimetry (DSC) was used to determine the melting temperature and enthalpy of PHB. Melting enthalpy, determined by calculating the area under the melting curve, was used to find the crystallinity of PHB (Xc) which determines the mechanical properties of the material [50]. The ratio of the PHB melting enthalpy to the melting enthalpy of 100% crystalline PHB (ΔHm100) (ΔHm/ΔHm100 × 100) gives the % crystallinity. The melting enthalpies of PHBm and PHBf were determined to be 89.15 and 83.62 J/g, respectively, while the melting enthalpy of the 100% crystalline PHB was taken as 146 J/g [51]. The crystallinity of PHBm and PHBf were calculated as 61.06% and 57.27%, respectively, and these values are within range of 50–70% previously found for PHB homopolymers [52]. The melting points of the PHBm and PHBf are 172.43 and 175.27 °C, respectively (Fig. 4c and d, Table 5). These values are similar to the melting point of standard PHB having a Mw of 2.75 × 105 g/mol [31] (Table 5).
The X-ray diffractograms of the PHB samples are shown in Fig. S10. Major peaks and their corresponding 2θ values are: (020) 13.53°, (110) 16.92°, (101) 21.00°, (111) 22.00°, (121) 25.00°, (040) 27.00°. The presence of intense peaks at 13.53°, 16.92°, and 22.00° in PHB samples are similar to peaks of standard PHB studied by S̆krbić and Divjaković, who determined that polymer in their work are arranged in the regular helicoidal conformation with two antiparallel chains in rhombic unit cell within the crystalline domain [53].
Molecular weights of the PHB samples produced were determined by gel permeation chromatography. Mws of PHB samples, depending on microorganism, carbon sources, and enviromental conditions, are in the range of 2.00 \(\times \) 105 and 3.00 \(\times \) 106 g/mol [54, 55]. In our study, the Mw and Mn of PHBf were 1.43 \(\times \) 106 g/mol and 3.66 \(\times \) 105 g/mol. These are higher than those of PHBm (Mw: 3.84 \(\times \) 105 g/mol, Mn: 1.01 \(\times \) 105 g/mol) (Fig. S12A, S12B, Table 6) due to the fact that fructose is preferred over glucose by C. necator ATCC 25207 for PHB productions, leading to higher Mw of polymers (Fig. S12A, S12B, Table 6). The Mn and Mw of polymers produced by Alcaligenes latus DSM 1124 in sucrose medium were 4.60 \(\times \) 104 and 1.31 \(\times \) 105 g/mol. The same microorganism in milk waste medium yielded polymers having higher Mn and Mw of 4.31 \(\times \) 105 and 5.64 \(\times \) 105 g/mol [56]. Mw and Mn of polymer synthesized by C. necator DSMZ 4058 in glycerol medium were 6.20 \(\times \) 105 g/mol, 1.68 \(\times \) 105 g/mol, respectively. However, Mw and Mn of polymer synthesized by the same microorganism grown in fructose medium changed to 1.16 \(\times \) 106 g/mol, 2.52 \(\times \) 105 g/mol, respectively [57].
Conclusıon
It was shown for the first time that C. necator ATCC 25207 could be used for PHB productions using fructose, glucose, acid and enzyme hydrolyzed molasses as a sole carbon source. This bacterium prefers fructose over glucose for PHB production and glucose is utilized mostly for microbial growth. Sulfuric acid and invertase treated molasses yielded 1288% and 529% more PHB than untreated molasses, respectively. It was found that acid treated molasses was 151% better in PHB production; however, enzyme hydrolyzed molasses was 51.61% better than acid hydrolyzed molasses in cell growth. This novel finding could have applications in fermentations using molasses in growth or production. Fructose and acid hydrolyzed molasses media yielded PHB samples with different molecular weights. 15.28 g/L PHB was obtained using acid hydrolyzed molasses and the yield of PHB production could be increased by media optimization and random mutagenesis of the microorganism.
References
Haider TP, Volker C, Kramm J, Landfester K, Wurm FR (2019) Plastics of the future? the impact of biodegradable polymers on the environment and on society. Angew Chem Int Ed Engl 58:50–62. https://doi.org/10.1002/anie.201805766
Atlas R, Bartha R (1998) Microbial ecology: fundamentals and applications, 4th edn. Benjamin Cummings Publishing Company Inc, Addison Wesley Longman Inc, San Francisco
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174. https://doi.org/10.1016/j.ijbiomac.2016.04.069
Alves MI, Macagnan KL, Rodrigues AA, de Assis DA, Torres MM, de Oliveira PD, Furlan L, Vendruscolo CT, Moreira AdS (2017) Poly (3-hydroxybutyrate)-P (3HB): review of production process technology. Ind Biotechnol 13:192–208
Koller M (2018) Chemical and biochemical engineering approaches in manufacturing polyhydroxyalkanoate (PHA) biopolyesters of tailored structure with focus on the diversity of building blocks. Chem Biochem Eng Q 32:413–438
Tan GYA, Chen CL, Li L, Ge L, Wang L, Razaad IMN, Li Y, Zhao L, Mo Y, Wang JY (2014) Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymers 6:706–754. https://doi.org/10.3390/polym6030706
Khanna S, Srivastava AK (2005) Statistical media optimization studies for growth and PHB production by Ralstonia eutropha. Process Biochem 40:2173–2182. https://doi.org/10.1016/j.procbio.2004.08.011
Siracusa V, Rocculi P, Romani S, Dalla Rosa M (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol 19:634–643
Lemoigne M (1926) Produits de deshydration et de Polymerisation de L’acide β= oxybutyrique. Bull Soc Chim Biol 8:770–782
Volodina E, Raberg M, Steinbuchel A (2016) Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit Rev Biotechnol 36:978–991. https://doi.org/10.3109/07388551.2015.1079698
Franz A, Rehner R, Kienle A, Grammel H (2012) Rapid selection of glucose-utilizing variants of the polyhydroxyalkanoate producer Ralstonia eutropha H16 by incubation with high substrate levels. Lett Appl Microbiol 54:45–51. https://doi.org/10.1111/j.1472-765X.2011.03171.x
Kersters K (1984) Genus alcaligenes castellani and chalmers 1919. Berg Man Syst Bacteriol 1:361–373
Vega-Castro O, Contreras-Calderon J, León E, Segura A, Arias M, Pérez L, Sobral PJ (2016) Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralsthonia eutropha. J Biotechnol 231:232–238
Vega-Castro O, León E, Arias M, Cesario MT, Ferreira F, da Fonseca MMR, Segura A, Valencia P, Simpson R, Nuñez H (2020) Characterization and production of a polyhydroxyalkanoate from cassava peel waste: manufacture of biopolymer microfibers by electrospinning. J Polym Environ. https://doi.org/10.1007/s10924-020-01861-1
Raberg M, Peplinski K, Heiss S, Ehrenreich A, Voigt B, Doring C, Bomeke M, Hecker M, Steinbuchel A (2011) Proteomic and transcriptomic elucidation of the mutant Ralstonia eutropha G+1 with regard to glucose utilization. Appl Environ Microbiol 77:2058–2070. https://doi.org/10.1128/AEM.02015-10
Konig C (1969) Constitutive glucose-6-phosphate dehydrogenase in mutants utilizing glucose, which are derived from cryptic wildtype strains. Arch Mikrobiol 67:51–57
Volova T, Kiselev E, Vinogradova O, Nikolaeva E, Chistyakov A, Sukovatiy A, Shishatskaya E (2014) A glucose-utilizing strain, Cupriavidus euthrophus B-10646: growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS ONE 9:e87551. https://doi.org/10.1371/journal.pone.0087551
Poomipuk N, Reungsang A, Plangklang P (2014) Poly-β-hydroxyalkanoates production from cassava starch hydrolysate by Cupriavidus sp. KKU38. Int J Biol Macromol 65:51–64. https://doi.org/10.1016/j.ijbiomac.2014.01.002
Kim BS, Lee SC, Lee SY, Chang HN, Chang YK, Woo SI (1994) Production of poly (3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng 43:892–898
Beaulieu M, Beaulieu Y, Melinard J, Pandian S, Goulet J (1995) Influence of ammonium salts and cane molasses on growth of Alcaligenes eutrophus and production of polyhydroxybutyrate. Appl Environ Microbiol 61:165–169
Song JY, Kim BS (2005) Characteristics of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) production by Ralstonia eutropha NCIMB 11599 and ATCC 17699. Biotechnol Bioprocess Eng 10:603. https://doi.org/10.1007/BF02932302
Baei SM, Najafpour G, Younesi H, Tabandeh F, Issazadeh H, Khodabandeh M (2011) Growth kinetic parameters and biosynthesis of polyhydroxybutyrate in Cupriavidus necator DSMZ 545 on selected substrates. Chem Ind Chem Eng Q/CICEQ 17:1–8
Dalsasso RR, Pavan FA, Bordignon SE, de Aragão GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem 85:12–18
Sharma SK, Mudhoo A (2011) A handbook of applied biopolymer technology: synthesis, degradation and applications. Royal Society of Chemistry, Cambridge
Nonato R, Mantelatto P, Rossell C (2001) Integrated production of biodegradable plastic, sugar and ethanol. Appl Microbiol Biotechnol 57:1–5
Favaro L, Basaglia M, Casella S (2019) Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: a review. Biofuels Bioprod Bior 13:208–227. https://doi.org/10.1002/bbb.1944
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110:621–632. https://doi.org/10.1016/j.jbiosc.2010.07.014
Koller M, Salerno A, Reiterer A, Malli H, Malli K, Kettl K-H, Narodoslawsky M, Schnitzer H, Chiellini E, Braunegg G (2012) Sugarcane as feedstock for biomediated polymer production. In: Goncalves JF, Correia KD (eds) Sugarcane: production, cultivation and uses. Nova Publishers, New York, pp 105–136
Nielsen SS (2010) Determination of moisture content. Food analysis laboratory manual. Springer, Boston, pp 17–27
Ribeiro PLL, Campos MI, Druzian JI (2017) Novel extracellular polymeric substances produced by Cupriavidus necator IPT 027 grown on glucose and crude glycerol originated from biodiesel. Polym Adv Technol 28:549–556
Chaijamrus S, Udpuay N (2008) Production and characterization of polyhydroxybutyrate from molasses and corn steep liquor produced by Bacillus megaterium ATCC 6748. Agric Eng Int 10:1–12
Kim H-Y, Park J-S, Shin H-D, Lee Y-H (1995) Isolation of glucose utilizing mutant of Alcaligenes eutrophus, its substrate selectivity, and accumulation of poly-β-hydroxybutyrate. J Microbiol 33:51–58
Horwitz W, Chichilo P, Reynolds H (1970) Official methods of analysis of the association of official analytical chemists. Association of Official Analytical Chemists, Washington, DC, USA
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Pradhan S, Dikshit PK, Moholkar VS (2018) Production, ultrasonic extraction, and characterization of poly (3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym Adv Technol 29:2392–2400
Serafim LS, Lemos PC, Oliveira R, Reis MA (2004) Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol Bioeng 87:145–160. https://doi.org/10.1002/bit.20085
Gahlawat G, Srivastava AK (2013) Development of a mathematical model for the growth associated Polyhydroxybutyrate fermentation by Azohydromonas australica and its use for the design of fed-batch cultivation strategies. Bioresour Technol 137:98–105. https://doi.org/10.1016/j.biortech.2013.03.023
Dahman Y, Ugwu CU (2014) Production of green biodegradable plastics of poly(3-hydroxybutyrate) from renewable resources of agricultural residues. Bioprocess Biosyst Eng 37:1561–1568. https://doi.org/10.1007/s00449-014-1128-2
Aramvash A, Shahabi ZA, Aghjeh SD, Ghafari M (2015) Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int J Environ Sci Technol 12:2307–2316. https://doi.org/10.1007/s13762-015-0768-3
Sen KY, Hussin MH, Baidurah S (2019) Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal Agric Biotechnol 17:51–59. https://doi.org/10.1016/j.bcab.2018.11.006
Bozorg A, Vossoughi M, Kazemi A, Alemzadeh I (2015) Optimal medium composition to enhance poly-β-hydroxybutyrate production by Ralstonia eutropha using cane molasses as sole carbon source. Appl Food Biotechnol 2:39–47. https://doi.org/10.22037/afb.v2i3.8883
Stanley A, Kumar HNP, Mutturi S, Vijayendra SVN (2018) Fed-batch strategies for production of PHA using a native isolate of Halomonas venusta KT832796 strain. Appl Biochem Biotechnol 184:935–952. https://doi.org/10.1007/s12010-017-2601-6
Patwardhan PR, Srivastava AK (2004) Model-based fed-batch cultivation of R-eutropha for enhanced biopolymer production. Biochem Eng J 20:21–28. https://doi.org/10.1016/j.bej.2004.04.001
Tripathi AD, Srivastava SK, Singh RP (2013) Statistical optimization of physical process variables for bio-plastic (PHB) production by Alcaligenes sp. Biomass Bioenergy 55:243–250. https://doi.org/10.1016/j.biombioe.2013.02.017
Zafar M, Kumar S, Kumar S, Dhiman AK (2012) Artificial intelligence based modeling and optimization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) production process by using Azohydromonas lata MTCC 2311 from cane molasses supplemented with volatile fatty acids: a genetic algorithm paradigm. Bioresour Technol 104:631–641. https://doi.org/10.1016/j.biortech.2011.10.024
Wang J, Yu HQ (2007) Biosynthesis of polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Appl Microbiol Biotechnol 75:871–878. https://doi.org/10.1007/s00253-007-0870-7
Hablot E, Bordes P, Pollet E, Averous L (2008) Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polym Degrad Stab 93:413–421. https://doi.org/10.1016/j.polymdegradstab.2007.11.018
Venkateswar Reddy M, Yajima Y, Mawatari Y, Hoshino T, Chang Y-C (2015) Degradation and conversion of toxic compounds into useful bioplastics by Cupriavidus sp. CY-1: relative expression of the PhaC gene under phenol and nitrogen stress. Green Chem 17:4560–4569. https://doi.org/10.1039/c5gc01156f
Kong Y, Hay JN (2002) The measurement of the crystallinity of polymers by DSC. Polymer 43:3873–3878. https://doi.org/10.1016/S0032-3861(02)00235-5
Barham P, Keller A, Otun E, Holmes P (1984) Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J Mater Sci 19:2781–2794
Laycock B, Halley P, Pratt S, Werker A, Lant P (2014) The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci 39:397–442. https://doi.org/10.1016/j.progpolymsci.2013.06.008
S̆krbić Z, Divjaković V (1996) Temperature influence on changes of parameters of the unit cell of biopolymer PHB. Polymer 37:505–507
Taidi B, Mansfield DA, Anderson AJ (1995) Turnover of Poly(3-hydroxybutyrate) (Phb) and its influence on the molecular-mass of the polymer accumulated by alcaligenes-eutrophus during batch culture. FEMS Microbiol Lett 129:201–205. https://doi.org/10.1111/j.1574-6968.1995.tb07580.x
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Progress Polym Sci 25:1503–1555. https://doi.org/10.1016/S0079-6700(00)00035-6
Wong PAL, Cheung MK, Lo WH, Chua H, Yu PHF (2004) Investigation of the effects of the types of food waste utilized as carbon source on the molecular weight distributions and thermal properties of polyhydroxybutyrate produced by two strains of microorganisms. E-Polymers. https://doi.org/10.1515/epoly.2004.4.1.324
Mothes G, Schnorpfeil C, Ackermann JU (2007) Production of PHB from crude glycerol. Eng Life Sci 7:475–479. https://doi.org/10.1002/elsc.200620210
Acknowledgements
This project was supported by TUBITAK (The Scientific and Technological Research Council of Turkey; Grant no: 109Y372) and Gebze Technical University (GTU) (Grant No: BAP-2017-A102-18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ertan, F., Keskinler, B. & Tanriseven, A. Exploration of Cupriavidus necator ATCC 25207 for the Production of Poly(3-hydroxybutyrate) Using Acid Treated Beet Molasses. J Polym Environ 29, 2111–2125 (2021). https://doi.org/10.1007/s10924-020-02020-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-02020-2