Abstract
With the development of biofilms by various microorganisms, bacterial species are more and more resistant to antibiotics. Thus, the quest for finding novel antibiofilm agents continues to fight against these pathogenic infections. Carbon nanoparticles and nanocomposites such as carbon nanotubes, fullerenes, graphene oxide nanoparticles, carbon quantum dots, etc.; shows effective antibiofilm activities against different biofilm-forming bacteria and fungi. This is due to their shape and size which plays a major role in the disintegration of the extracellular polymeric matrix of the bacterial biofilms causing their inhibition. Carbon nanotubes are found to reduce the use of antibiotics as well as reduce the resistance possessed by these biofilms forming bacterial and fungal species, increase their bioavailability, and increase their capabilities for on-site drug delivery. The present review provides insight into the antibiofilm efficiencies of different carbon nanoparticles and nanocomposites and their mechanism of action.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Excessive usage and unregulated prescription of antibiotics have resulted in the development of resistance in microorganisms leading to serious health problems in recent years [1]. This problem paves way for the researchers to search for new and alternative agents which can effectively inhibit microbial growth. Nanoparticles are nanometer-sized materials, that find wide applications in different optical devices, various semiconductors, different fuel cells, catalysts, biosensors, delivery of different types of drugs; etc. [2]. Nanoparticles as a new system for drug delivery is a novel application for the improvement of various physicochemical properties and therapeutic consequences of drugs. In the last few decades, the application of nanoparticles and nano-carriers in the field of drug delivery and pharmaceutical sciences as well as in the field of microbiology has become a very popular subject of research [3]. One of the main reasons for using nanotechnology is for overcoming antimicrobial resistance possessed by the majority of microorganisms. The majority of work on nanoparticles and nanotechnology is focused on developing antimicrobial, antibacterial, antifungal, and antibiofilm nano-agents like metal oxide nanoparticles, green synthesized nanoparticles; etc. [4]. Various metals have been explored in developing either metal or metal oxide nanoparticles such as silver nanoparticles, silver oxide nanoparticles, titanium oxide nanoparticles, zinc nanoparticles, zinc oxide nanoparticles, gold nanoparticles, calcium oxide nanoparticles, silicon dioxide nanoparticles, silica nanoparticles, copper nanoparticles, copper oxide nanoparticles, and magnesium oxide nanoparticles; which shows antibacterial and antibiofilm activities [5]. Moreover, through various research works, it has been known that carbon nanoparticles or carbon nanotubes possess extreme effectiveness against bacterial biofilms [6].
The surface area and size of carbon nanoparticles and nanocomposites are essential factors that decide their antibiofilm, antibacterial or antifungal abilities. Several research works have shown that the larger the surface area of the nanoparticles and the smaller the size of the nanoparticles, the more effective they can serve as antibiofilm, antibacterial, or antifungal agents [7]. Moreover, there are a lot more parameters on which the antibiofilm activities of the nanoparticles depend. For example, it depends on the composition of the nanoparticles and nanocomposites, their various intrinsic characteristics, the modifications taking place on their surfaces, and finally, the type of biofilm-forming bacterial species they are dealing with. Oxidative stress plays an important role in the antibiofilm activities of these carbon nanocomposites [8]. The oxidative stress causes them to penetrate the extracellular polymeric matrix of the bacterial biofilms and cause disruption of the biofilm. This is followed by the disruption of the cell walls of the individual bacterial cells by the nanocomposites which kill the bacterial cells and the bacterial biofilms [9]. Another reason for the disruption of biofilms might be due to the physical interaction between the carbon nano-materials and bacterial biofilms. Although a lot of research and literature is not available to support this fact [10].
With the increase in population and advancement in the health system in the 21st century the incidence of microbial infections and multi-drug resistance has become an alarming concern. In October 2020 the World Health Organisation has declared antimicrobial resistance as one of the top 10 global health threats to mankind. Twenty-five thousand deaths are reported per year in the European Union, more than twenty-three thousand deaths in the United States, and an astonishing count of fifty-eight thousand deaths per year in India due to antimicrobial resistance. In this era of antibiotic resistance, it is very important to find an alternative treatment strategy that can combat microbial infections and multi-drug resistance. This review cohesively highlights the structural chemistry and varied synthesis procedures of carbon-based nanomaterials and nanocomposites. It also throws light onto the antibiofilm activity of carbon-based nanomaterials like carbon nanotubes, fullerenes, graphene oxide, and carbon quantum dots. To the best of our knowledge, this review is the first of its kind that elaborately explains the antibiofilm activity of most carbon-based nanoparticles and nanocomposites.
2 Carbon Nanotubes
Carbon nanotubes are nano-dimensional cylindrical-shaped particles that are usually hollow. Since its invention in the year 1991, it has found application in different disciplines of science, especially in chemistry and biology [11].
2.1 Structural Chemistry
Single-walled carbon nanotubes are classified into three different types based on the nature of the rolling of the graphene layer. These are zigzag-patterned nanotubes, chiral nanotubes, and armchair-shaped nanotubes. There are various parameters that determine the characterization of single-walled carbon nanotubes. The pair of indices determine the chiral vector of the nanotube and also determines the various electrical and magnetic properties of nanotubes [12]. Among the pair of indices consisting of m and n, when m equals zero, the nanotubes take the zigzag form whereas when m equals n, the nanotubes take the shape of armchairs or become chiral shaped. The chiral vector is represented by C and it equals (na1 + ma2) where n and m are the indices and a1 and a2 are the graphite base cellular vectors. The cell vector is also responsible for the tube diameter which is represented as d. It finds out the direction in which the graphene sheet rolls. The calculation of the diameter of the carbon tube is done by the formula (d × ¼ × a ×m2 × mn × n2 × p × π). Here p is responsible for the lattice constant of the graphite sheet. If the measurement of (n-m) is a multiple of three, the formed nanotubes possess different metallic properties, or the nanotubes are considered good conducting materials [12]. If the (n-m) value is not a multiple of three, in that case, the nanotube is considered a semiconductor or material with semi-metallic characteristics. The armchair form of the nanotube is usually metallic in nature, but in other types, they can be either metallic or semi-metallic.
There are multiple parameters on which the structural characterization of nanotubes depends. The first one is the chiral vector which is determined by the formula, Ch = na1 + na2» (n, m). Where Ch is the chiral vector, n, and m are indices, and a1 and a2 are the graphite base cellular vectors [13]. The translational vector is another important parameter that is determined by the formula T = t1a1 + t2a2» (t1, t2), where T is the transitional vector. It is very important to know about the length of the chiral vector. This parameter is calculated by the formula, L = a √ (n2 + m2 + n × m), where L is the length of the chiral vector and a is the lattice constant [14]. The chiral angle determines the angle of rotation of the nanotube and also determines its structural orientation. This angle can be calculated by the formula cosθ = (2n + m)/ (2 × √ (n2 + m2 + n * m)), where θ is the angle of chirality. The total number of hexagons that are present in the nanotube can be measured in a unit cell by the formula N = (2 × (n2 + m2 + n × m)/dR), where N is the number of hexagons present in the unit cell of a nanotube [15]. The diameter of the nanotube and its rotation angles belonging to the symmetry vector can be determined by the mathematical formulations dt = L/π and ψ = 2π/N respectively. Here dt represents the diameter, L represents the length, ψ represents the rotational angle and 2π/N is measured in radians. The symmetry vector calculation is done by the R = pa1 + qa2» (p, q) formula, where R is the symmetry vector and p, q are the constraints determining the symmetry of the nanotubes. Lastly, the pitch of the symmetry vector is calculated by the formula τ = ((m ×(p–n) × q) × T)/N, where τ is the pitch [16]. Synthesis of multi-walled carbon nanotubes can be done by two different models. The first one is the Parchment model while the second one is the Russian Doll model. If the carbon nanotube encircles another nanotube and the outer nanotube possesses a larger diameter compared to the thinner nanotube, then that carbon nanotube is known as the Russian Doll model. On the other hand, if there is only a graphene sheet that is wrapped around itself a number of times just like a piece of paper that is rolled up, then that way of preparation of carbon nanotube is known as the Parchment model [17].
2.2 Mechanism of Synthesis
The availability of different technologies is seen in the production and development of carbon nanotubes of different sizes, shapes, and morphologies. Mostly, these technologies include processes of gas phases. However, three of these processes are widely used and very popular methods of carbon nanotube synthesis [18]. These are chemical vapor deposition (CVD) methods, and ablation technology with the help of laser and arc discharge technology associated with carbon.
2.2.1 CVD or Chemical Vapour Deposition Technology
Preparation processes involving increases in temperatures like arc discharge and laser ablation were first put to use for the purpose of the formation of carbon nanotubes. But nowadays, these technologies have been replaced by chemical vapor deposition technologies and synthesis by evaporation of carbon molecules; both of which undergo the involvement of lower temperatures. These methods also involve the usage of different metal particles for their catalytic roles. Some examples of these are cobalt, nickel, iron, magnesium, etc. [19]. Direct electricity is passed by the process of arcing inside the reaction chamber. Then, pressure is put on the chamber and the temperature is raised to approximately 400 0 F. In the arcing process, almost half of the total amount of carbon that underwent evaporation was collected by its solidification in the cathode [20]. This is known as Cigar-like structure formation or cylindrical hard deposition. Whereas, in the positive electrode, consumption or erosion occurs. This is followed by the deposition of carbon, which remains in the form of a hardened grey shell, on the periphery and undergoes condensation to form cathode soot on the cathode and chamber soot in the surrounding walls of the chamber. The cathode soot, chamber soot, and inner core lead to the formation of three types of structures which are polyhedral-shaped graphene sheets, single-walled carbon nanotubes, and multiple-walled carbon nanotubes. These carbons are soft and dark [21].
2.2.2 Laser-Ablation Technology
The technology involving the ablation of laser uses high-power vaporization of laser in a tube, which is made of quartz and blocks of pure graphite. The tube is heated from below with the help of a furnace at a temperature of more than 12000 C with the surrounding environment covered with argon [22]. The main reason for using a laser in this method is the vaporization of the graphite inside the quartz. Same to the process of formation of single-walled carbon nanotubes through the method involving arc discharge, the laser technology adds particles of metal as catalysts that target the graphite in the tube. Multiple research reports show that the diameter of the carbon nanotubes is dependent on the power of the laser used [23]. When the pulse power of the laser is increased, the diameter of the carbon nanotubes becomes very thin. However, it is seen that using ultrafast laser pulses which has the ability to move in sub-pico-seconds can synthesize even larger volume of carbon nanotubes. The majority of the nanotubes produced are single-walled [24].
2.2.3 Carbon Arc-Discharge Technology
The standard process for the synthesis of carbon nanotubes by CVD is nanotube synthesis by carbon arc discharge (Fig. 1). Multiple types of carbon arc discharges are present plasma-enhanced need CCVD or catalytic chemical vapor deposition, thermal CCVD or catalytic chemical vapor deposition, CVD assisted by oxygen, CVD assisted by water, microwave plasma chemical vapor deposition, radio-frequency involved chemical vapor deposition, hot filament involved chemical vapor deposition; etc. [25]. However, among all of these methods, CCVD or catalytic chemical vapor deposition is the most accepted and trusted method and is considered the most standard process for the synthesis of both single-walled and multi-walled carbon nanotubes [26].
2.3 Antibiofilm Activities
Single-walled carbon nanotubes are seen to possess antibiofilm activities against biofilm-forming bacteria Escherichia coli. The mode of action involves the rupture of the Extracellular polymeric matrix (EPM) of the biofilm, followed by cell membrane rupture of the individual bacterial cells leading to the death of the bacteria [27]. But many research works confirmed that the size of the synthesized carbon nanotube is perhaps the most important parameter in finding out the antibiofilm property [28]. Again, it is seen that multi-walled carbon nanotubes do not work better on biofilms of Escherichia coli compared to single-walled carbon nanotubes. It is seen that the toxicity level of single-walled carbon nanotubes is much more significant compared to multi-walled carbon nanotubes [29]. Direct contact between the single-walled carbon nanotubes and the cell membrane of biofilm-forming bacteria occurs which results in the disruption and loss in integrity of the cell membrane, affecting the metabolism process and also affecting the shape and morphology of the bacterial biofilm as well as bacterial cells [30]. Also, because single-walled carbon nanotubes possess a very small radius and hence a very small circumference compared to the radius and circumference of multi-walled carbon nanotubes, they can easily penetrate inside the EPM matrix of the biofilm and the cell membrane of the bacterial cells and exert its effect, which the later cannot do [31].
Many authors have found evidence that proves that the addition of various surface groups to single-walled carbon nanotubes and multiple-walled carbon nanotubes increases their antibiofilm activities against biofilms formed by round-shaped and rod-shaped biofilm-forming bacteria as well as biofilms formed by both Gram-positive and Gram-negative bacterial species [32]. The addition of surface groups the like hydroxyl group and carboxylic acid group to single-walled carbon nanotubes result in increased antibiofilm activities against biofilm-producing Gram-positive and Gram-negative bacterial species [33]. However, multi-walled carbon nanotubes along with the addition of these same surface groups do not show that much antibiofilm effectiveness against the same biofilm forming bacterial species [34]. Although, both types of nanotubes have the ability to damage and destroy the cell membrane of the bacteria which made their DNA content come out of the cell, causing their death; the extent of this destruction and damage will depend on the efficiency of the concerned nanotube [35].
Various experiments conducted on these nanotubes also show antibacterial and antibiofilm activities of the single-walled carbon nanotubes, when they are found in a dispersed state in various surfactant solutions such as sodium dodecyl benzenesulfonate, sodium holate, sodium dodecyl sulphate; etc. against an array of biofilm-forming bacterial species such as Escherichia coli, Enterococcus faecium, Salmonella enteric; etc. [36]. Single-walled carbon nanotubes possess antibiofilm activities mainly against Escherichia coli; however, it is seen that it possesses antibiofilm activities against biofilm-forming bacterial species like Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus sanguinis; etc. [37]. Single-walled carbon nanotubes in various surfactant solutions are seen to possess very low toxicity toward animal and human cells. So, it can be applied as a treatment procedure against biofilm infections that occurs in the human body [38]. Especially in human astrocytoma cells 1321N1, the application of these nanotubes can be done to solve and treat biomedical problems involving biofilm formation and proliferation specifically against antimicrobial-resistant microorganisms [39].
2.4 Mechanism of Antibiofilm Activities
One of the plausible mechanisms of action of carbon nanotubes against biofilm-forming microbial species will be direct contact between the carbon nanotubes and the surface of the biofilms specifically the extracellular polymeric matrix (Fig. 2) [40]. This direct contact will result in serious effects on the cell membrane integrity, morphology, and, various metabolic processes of the bacterial biofilms and individual cells [41]. More research should be carried out on the antibiofilm activities of carbon nanoparticles especially because of their large surface-to-volume ratio, high inner volume, and various other physical and chemical characteristics which possess uniqueness [42].
2.5 Applications of Carbon Nanotubes
Carbon nanotubes have a wide range of applications (Table 1). They are largely used for their properties like high field emission, high thermal conductivity, large capacity for energy storage, better conductive characteristics, conductive adhesiveness; etc. [43]. Carbon nanotubes are largely applied in molecular electronics and other structural applications. Various fabrics and fibers are made out of carbon nanotubes which have a lot of industrial applications. They are largely used in the biomedical industries, air, and water filtration systems, hydrogen storage; etc. Carbon nanotubes are also widely used for molecular sieves, heat transfer, and enzymatic and catalytic roles [44].
3 Fullerenes
Fullerenes are a football-shaped allotrope of carbon and are widely known for their antibiofilm activities. The biofilm-forming bacterial species against which it shows maximum effectiveness is Salmonella typhi, Streptococcus mutants, Escherichia coli; etc. [57].
3.1 Structural Chemistry
In the fullerenes, the structure plays a very important role as it determines its unique physical and chemical properties. All the carbon atoms in fullerenes are linked to each other forming a polyhedron. So, in order to do this, each carbon atom undergoes the formation of a double bond and one single bond. In C60-type fullerene, two types of bonds are present. First is the double bond which is located between two different hexagons and second is the single bond which is located between a pentagon and a hexagon [58]. These bonds cover the entire fullerene surface forming a network of double bonds in conjugation. The structures of fullerene which have a reduced number of hexagons shows higher SP3 bonding properties for example increased carbon sites of reaction as well as higher strains. However, in the case of the pentagons located adjacent to hexagons, reduced stability is shown along with relatively abundant fullerenes with different extracted pentagons [59]. In the latter case, the π bond delocalization in the structure is an obvious phenomenon.
3.2 Mechanism of Synthesis
Formation of fullerenes is mostly carried out by the application of carbon laser vaporization which is done by maintaining an atmosphere that is inert in nature. However, the amount of synthesized fullerene by this method is relatively low. Instead, if methods like graphite-mediated arc heating or poly-aromatic hydrocarbon irradiation by laser are done, the number of fullerenes synthesized will be relatively much higher [60].
3.2.1 Laser Vaporization of Carbon
Laser vaporization of carbon into fullerenes is a widely used method and it has gained a lot of popularity over the years. Here, the production of fullerene is achieved through a pulsed laser-mediated supersonic expansion nozzle which is particularly concentrated on the target which is graphite [61]. The entire process is done in an inert atmosphere preferably helium. At first, the vaporization of carbon happens by placing a graphite piece on a solid disc, which is rotated continuously throughout the process. This is done in presence of a high density of helium gas in the atmosphere which is achieved by focusing a pulsed laser on its target [62].
3.2.2 Electric Arc Heating of Graphite
The concept of this method of fullerene synthesis was invented by two scientists named Huttman and Kratchmer. This method is initiated by the generation of an electronic arc in between two rods of graphite which is also done while maintaining an atmosphere filled with inert gases (Fig. 3). The electronic arc leads to the formation of a condensed fluffy containing toluene extractible fullerenes, which is also known as soot. Then extraction of fullerenes from this condensed fluffy is done with help of removal of a small amount of solvent toluene with the help of a rotary evaporator [63]. The remaining solid mixture is in most cases pure fullerene C60 along with little amounts of higher fullerenes. Liquid chromatography is carried out to filter the C60 fullerene into its purest form.
3.2.3 Resistive Arc Heating of Graphite
In this process, at first, some carbon rods are evaporated with the help of resistive heating. In this case, the surrounding environment is filled with inert gas helium partially. With the help of resistive heating, the carbon rods are heated in such a way that they undergo an emission of a substance that looks like soot and which is made of fullerenes and is a faint grey-white plume in appearance [64]. Glass shields are placed covering the carbon rods and on them, these substances made of fullerenes are collected.
3.2.4 Laser Irradiation of Polycyclic Hydrocarbons
Formation of fullerenes directly is not usually done for the purpose of getting a new fullerene family homolog because it is not received in sufficient amounts because of some of the uncontrollable parameters involved in the method of evaporation of graphite [65]. Instead, the synthesis of fullerenes with the help of PAHs or Polycyclic Aromatic Hydrocarbons is a much better and preferred method. This process is based on PAHs or polycyclic aromatic hydrocarbons which already possess carbon frameworks that is required. These polycyclic aromatic hydrocarbon molecules are folded to synthesize fullerenes by the process of FVP or flash vacuum pyrolysis [66]. It has been reported that polycyclic aromatic hydrocarbons are composed of sixty different carbon atoms that undergo synthesis of C60 type of fullerene by the process of laser irradiation at a wavelength of 337 nm [67].
3.3 Antibiofilm Activities
The antibiofilm activity of fullerenes occurs mainly because of the metabolism of energy that takes place after internalizing the nanoparticles inside the extracellular polymeric matrix of the bacterial biofilms and eventually into each of the bacterial cells [68]. The antibiofilm activities may also rise from inhibition of the respiratory chains of the individual bacterial cells that lead to the death of the bacterial cells and eventually disruption of the biofilm [69]. The derivatives of fullerenes are mainly involved in this mode of action. Here, a very low concentration of fullerene derivatives affects the uptake limit of oxygen at first and then subsequently increases the level of oxygen uptake [70]. The increase occurs once hydrogen peroxide is produced and attached to the fullerene derivatives [71]. An alternative antibiofilm mode of action suggests that the extracellular polymeric matrix disruption of biofilm followed by disruption of the cell membrane of the individual cells could be a possible explanation [72]. The hydrophobic parts of fullerenes might undergo intercalation with the lipid membranes and also interact with the lipid membranes (Fig. 4). There are various classes of fullerenes and all of them show different levels of anti-biofilm activities. Fullerenes can be positively charged, neutral, and negatively charged [73].
3.4 Mechanism of Antibiofilm Activities
The cation-containing derivatives of fullerene possess maximum antibiofilm activity against biofilms of Shewanella oneidensis and Escherichia coli. However, the anion-containing derivatives of fullerene didn’t show that much antibiofilm effectiveness [74]. There have been different scientific opinions pertaining to this observation. One opinion suggests that the surface negative charge of the Gram-negative bacteria prevents interaction with the anionic fullerenes which results in no bond formation between fullerenes and cell membrane proteins [75]. This is why they cannot penetrate into the surface as they could not bind to the membrane. Another opinion suggests that the anionic fullerenes were extremely soluble in aqueous solutions [76]. Thus, in aqueous solutions, they cannot interact with the bacterial biofilms and hence cannot show their antibiofilm activities. Fullerenes can also be engineered such that their structural modifications can be done with the attachment of protonated amine side chains the and attachment of deprotonated carboxylic side chains. This is done by some linkers organically [77]. The results were found to be similar to the cationic and anionic fullerene derivatives. Fullerenes with protonated amine side chains showed effective results against Escherichia coli biofilms whereas fullerenes with deprotonated carboxylic side chains did not show any noticeable amount of antibiofilm activity against any biofilm-forming bacterial species [78].
There are a lot of applications of fullerenes. They can be used as photosensitizers in photodynamic therapy (PDT). This is mainly done by enhancing the solubility of the fullerenes with the artificial addition of multiple hydrophilic side chains [79]. Once these fullerenes are made water soluble, specific reducing agents of biological origin are mixed in the aqueous solution which results in the formation of superoxide [80]. However, this procedure can be cytotoxic; but the cytotoxicity is way more in the case of biofilm-forming bacterial cells compared to human cells or other animal cells. Scientists have also shown that fullerene derivatives such as fullero-pyrrolidinium when used for photo-irradiation, result in effective antibiofilm activity against bacterial as well as fungal biofilms [81].
It is seen that fullerene derivatives that are substitutes for cations can be irradiated with white light. This results in the enhancement of their antibiofilm activities against an array of microorganisms [82]. Whereas, when we talk about synthetic derivatives of fullerenes, they are always composed of a quaternary group or at least one basic amino group [83]. This makes them an antibiofilm agent against many pathogenic bacteria causing biofilm-related infections such as Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa; etc., and many pathogenic fungi causing infections related to fungal biofilms such as Candida albicans [84]. It was seen that the more the number of cationic groups existing quaternarily in these fullerene derivatives, the more their antibiofilm effectiveness. Such derivatives of fullerenes were most effective against biofilms of Staphylococcus aureus which was followed by Escherichia coli [85]. Candida albicans showed the most resistance against these fullerenes but ultimately the fullerenes could successfully penetrate through the extracellular polymeric matrix and cell wall of these fungal biofilms [86]. Many researchers have found that the sulfobutyl derivative of fullerene possesses effective antibacterial as well as antibiofilm activities against the majority of the bacterial species living in the environment [87]. The mode of action followed is photo-irradiation. These fullerenes can also be effective against burns and wounds, especially those in which light cannot penetrate inside the tissues [88].
3.5 Applications of Fullerenes
Fullerenes are widely used for their applications as essential antioxidants that often react at an extremely high rate in combination with free radicals (Table 2). This is one of the reasons for cell death or cell damage. They also have a wide range of applications in the healthcare industry in which the main purpose of their use is to enhance oxidative death of cells or cell damage. Thus, it has immense applications in the spoilage of food, deterioration of plastics, and corrosion of metals [89]. Fullerenes play an important role in regulating neuro-degeneracy and other neurological diseases such as Lou Gehrig’s disease and Alzheimer’s disease which are caused by radical damage. Fullerenes are also used in the development of many drugs for different therapies and diseases like photodynamic therapy, atherosclerosis, anti-viral therapy; etc. Because fullerenes possess the ability to neutralize more than twenty free radicals with just one molecule of fullerene, it is termed a “radical sponge” [90]. The antioxidant properties of fullerenes are almost one hundred times more efficient than that of other antioxidants like vitamin E. They also possess a very high solubility in oils. Because fullerenes are soluble in different oils like almond oil, they are widely used for screen tests to treat ocular toxicity in tissues after showing no indication of any side effects [91].
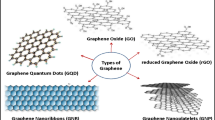
4 Graphene and Graphene Oxide (GO)
Graphene is basically a single layer of carbon atoms, placed very close to one another in a 2-D crystal structure. Graphene sheets in nano-dimensions possess the ability to disperse into the water very easily [102]. Once they disperse themselves in any aqueous solution, the development of side chains or functional groups in these graphene sheets takes place. These groups can be carboxyl groups, epoxy, or even hydroxyl groups.
4.1 Structural Chemistry
The morphology of graphene oxides was more and more researched and studied in detail in the last few years. Various technologies involving 13 C NMR, 1 H NMR, transmission electron microscopy with ultra-high resolution, dark field microscopy, XRD or X-ray diffraction; etc. were used to study the morphological properties of graphene [103]. Despite so many attempts and using so many technologies to search for the structure and morphology of graphene oxides, certain types are still not clearly explained and need further studies into variability in graphene oxide stoichiometry [104]. In simple terms, graphene oxides are a single-layered sheet of graphite that contains oxygen groups on its edges as well as basal planes and different side chains of carboxyl, hydroxyl, epoxy; etc. nature. This leads to the formation of a mixture of sp2 and sp3 carbon atoms which is in a hybrid state. Models and structures of graphene oxides have been predicted based on various mathematical, analytical, and theoretical simulation studies [105].
The first structure of graphene oxide was described by two scientists named Rudolf and Hofmann in 1939 and confirmed the presence of various randomly distributed epoxy groups throughout the single-layered graphite sheet [106]. In the year 1946, Ruess improved this existing model by adding hydroxyl groups along with the epoxy groups. This model also used two types of carbon atoms having sp2 and sp3 hybridization [107]. The two types of carbon atoms were arranged in an alternative pattern to make up the graphite sheet.
Boem and Scholz proposed a structure of graphene oxide that is relatively less organized in the year 1969. Their structure consisted of carbon-carbon double bonds and carbon-carbon single bonds which are repeated periodically in various layers of carbon [108]. These carbon layers are further associated with several carbonyl side chains and hydroxyl side chains. However, in this structure, ether oxygen was not involved. In the year 1994, Matsuo and Nakajima invented a model which possessed some resemblance with different intercalation compounds of graphite [109].
Four years later in 1998, scientists Klinowski and Lerf invented a model which was ideally an LK model, consisting of two sections. The first section consists of aromatic benzene rings which are not oxidized, whereas the second section is in association with aliphatic rings that are of six carbon chains each [110]. The dimensions of the two different sections depend on how much material has been oxidized. The main constituents of this model are epoxy groups, multiple double bonds, and lastly a number of different aromatic groups. Wrinkling inside the single layer is the result of a little bit of distortion in a tetrahedral structure of hydroxyl groups which is bonded with the atoms of carbon. The functional groups such as oxygen are bonded to the single-layered carbon above and below the various materials of the carbon layer. This generates two-layered oxygen atoms with different concentrations which are made mainly from hydroxyl groups and epoxide groups [111]. Both these groups are in close contact with one another. All aromatic groups, different functionalities of oxygen, oxidized rings; etc. are randomly seen throughout the single layer of carbon. The oxygen groups present here are also responsible for the acidity of graphene oxides. The oxygen groups which are located at the edges of the lattice-structured carbon and which are of carboxyl or hydroxyl origin are mainly involved in the determination of graphene oxide acidity [112]. These are precisely the reasons why the LK model is considered one of the most acceptable models for graphene oxides with mild oxidation.
4.2 Mechanism of Synthesis
Graphene oxides can be synthesized by multiple processes. Probably the easiest of all is the usage of various oxidizing agents while maintaining an acidic environment. Whereas there are different other methods that are widely used such as microbial synthesis of graphene oxide and electrochemical synthesis of graphene oxides.
4.2.1 Permanganate Method and its Modifications
This method is also known as Tour’s method and the synthesized product by this method was named Tour’s graphene oxides [113]. In this method, sulphuric acid and phosphoric acid are at first mixed in a ratio of 9:1 (Fig. 5). In a separate container, graphite, and potassium permanganate are mixed inside an ice bath in a ratio of 1:6. Now the two mixtures are mixed together which is followed by heating of the mixture up to 50 0 C and stirring the mixture on a magnetic stirrer for 12 h continuously and cooled [114]. The mixture is further cooled by placing it on ice, and 30% hydrogen peroxide solution is added for the removal of extra levels of potassium permanganate. In this method, phosphoric acid acts as an agent of etching as well as a dispersive agent. It stabilizes the process of oxidation making the synthesis of graphene oxide in a safe way. The yield of generated graphene oxide is extremely high and the graphene obtained are all of regular shapes and morphologies [115].
4.2.2 Jones Method and its Modification
Various new methods have been developed to carry out the oxidation of graphite for the preparation of graphene oxides. These methods include the usage of potassium chromate from a mixture of nitric acid or perchloric acid. Preparation of graphene oxides can also be done following the Jones conditions [116]. Again, potassium ferrate is used in a solution of sulfuric acid for the preparation of graphene oxides. Graphite undergoes oxidation inside the water at a temperature of 50 0 C in the presence of hydrogen peroxide. It can also undergo oxidation by metallic iron in the presence of benzoyl peroxide at a temperature of 110 0 C [117]. It should be kept in mind that the graphene oxides prepared by any of the chemical methods cause structural distortion and change in the morphology of the graphene oxides. This is mainly because of the extremely acidic environments in which the synthesis is carried out or also can be because of the impurities present in the mixture [118]. In these cases, the produced graphene oxides are not suitable for usage in the different electronic, sensor-based, and point-of-care diagnostic tools in which they are widely used. It should also be noted that even if the chemical methods of graphene oxide synthesis like the permanganate method or the chlorate method produce graphene oxides with unsatisfactory electrical properties, they are still preferred and widely used, and also more and more research is conducted to explore these processes and innovate them to find out a better alternative [119].
4.2.3 Tour’s Method and its Modification
In the year 2017, research works reported a lot of modifications to Tour’s method of graphene oxide synthesis. The most important modification was decreasing the time of reaction which was reduced from almost 12 h to 1 h. But surprisingly there were no striking differences in the synthesized graphene oxides. Further modifications to Tour’s method were carried out in 2018 in which graphite flakes were oxidized in the presence of permanganate in a graphite: permanganate ratio of 1:6 [120]. The prepared mixture was further mixed with another mixture which is prepared by mixing phosphoric acid and sulfuric acid in a ratio of 1:9. Now the newly mixed solution was heated at a temperature of 65 0 C for a time span of 12 h. All the chemicals were cooled at 5 0 C before they were used. Except for the various chemical methods used for the synthesis of graphene oxide, electrochemical methods are also promising methods for graphene oxide synthesis on an industrial scale [121]. They are even more eco-friendly and economical methods compared to chemical methods of synthesis. This is mainly because of the recycling of the electrolyte for a number of cycles and washing of the appliances for a minimum time. The quality of electrochemically synthesized graphene oxides is much better than the other types produced by the standard processes because of the usage of electrolytes, which is aqueous in nature. This is because once the electrolytes are aqueous, there is no need for oxidizing agents and the formation of impurities does not take place. Also because of the modern experimental setups used nowadays, the regulation of defects and the presence of impurities can be controlled easily [122].
4.3 Antibiofilm Activities
Much emphasis was laid on the antibiofilm effects of two particular nano-dimensional compounds of graphene oxide. These are graphene oxide-chlorophyllin and graphene oxide-chlorophyllin conjugated with metallic zinc [123]. These two types of graphene oxide nanoparticles were effective against a large number of biofilm-forming bacterial species like Escherichia coli, methicillin-resistant Staphylococcus aureus, Streptococcus sanguinis, Streptococcus mutants, pseudomonas aeruginosa; etc. [124]. Their mechanism of action was somewhat similar to the normal graphene oxide nanoparticles which involves cell membrane disruption of the biofilm-forming bacterial species. However, in the case of graphene oxide-chlorophyllin conjugated with metallic zinc, the metal toxicity due to the presence of metallic zinc and the modified surface chemistry because of the conjugation resulted in the enhancement of their antibiofilm activity [125]. Graphene oxide nanoparticles in conjugation with metallic zinc target the hydrogen bonds of the tetrapyrroles, which are usually colorless and generate hydroxyl radicals in the bacterial cellular components [126]. The leaching of zinc ions in the aqueous solution helps in this process. This mechanism also results in their antibiofilm activity.
Nanocomposites of carbon that are formed of core metallic nanoparticles and conjugated nanostructures of carbon are also been checked for their antibiofilm activities [127]. Silver nanoparticles conjugated with carbon nanotubes and silver nanoparticles conjugated with graphene oxide nanoparticles are the major examples in this category [128]. However, because of the excellent dispersed action of carbon nanotubes over graphene oxide nanoparticles, the former is seen to possess a much better antibiofilm activity than the latter against several biofilm-forming bacteria. Silver nanoparticles conjugated with carbon nanotubes show inhibitory effects against biofilms of pathogens that cause extremely harmful infections to the human body such as methicillin-resistant Staphylococcus aureus, Burkholderia cepacia, Klebsiella pneumoniae, Acinetobacter baumannii; etc. [129]. Among these Acinetobacter baumannii is known to possess extreme antibiotic and drug resistance. Bio-defensive bacterial species like Yersinia pestis can also be effectively inhibited with the help of these conjugated nanoparticles [130].
4.4 Mechanism of Antibiofilm Activities
The development of extreme stress throughout the nano-membranous structure of graphene sheets results in their antibiofilm efficacies (Fig. 6). Escherichia coli biofilms are a major target for both graphene oxide and graphene nanoparticles [131]. The antibiofilm property of graphene and graphene oxide nanoparticles is also because of the extremely sharp nano-edges of these nanoparticles. Once the bacterial biofilms come in contact with these edges, their extracellular polymeric matrix gets damaged [132]. The cell membranes of the bacterial cells can also get damaged because of these sharp edges which cause the efflux of RNA [133]. This mechanism can occur in biofilms formed by both Gram-positive and Gram-negative bacterial species. The antibiofilm activities of graphene oxide nanoparticles may also arise from their reduced nano-dimensional walls [134]. It was seen that once biofilms of Staphylococcus aureus come in contact with these reduced walls, cell membrane damage occurs in the biofilm-forming bacterial cells which ultimately leads to their death [135]. The antibiofilm activities of graphene nanowires can be compared to the antibiofilm activities of single-walled carbon nanotubes [136]. Various research works also reported the antibiofilm activities of graphene nanoparticles with reduced nano-dimensional wires against Escherichia coli biofilms.
4.5 Applications of Graphene Oxide
Emission of toxic gases like carbon dioxide, carbon monoxide, ammonia, methane, nitrogen dioxide, nitric oxide; etc. from various industrial clouds of smoke has led to air pollution worldwide. Graphene oxide can serve as an effective candidate in the catalysis and conversion of various gases involved in air pollution occurring in different processing industries (Table 3). Due to the attachment of different oxygen groups at the edges and basal planes of graphene oxide, they have the capability to interact with different molecules which are either non-covalent or covalent [137]. Toxic gases are converted firstly by capturing the gases followed by their storage. Several reactions are carried out to catalyze the harmful gases and convert them or utilize them directly into other processes [138]. Apart from treating this air pollution issue, graphene oxides are also used to address water pollution problems. All over the world, graphene oxides are used either to absorb different pollutants or to convert them into other forms which are not very harmful. The water pollutants which are converted by graphene oxides include various heavy metal ions, organic dyes; etc. [139].
5 Carbon Quantum Dots
With the improvement in the field of nano-therapeutics; the application of nanoparticles and nanocomposites into antibiofilm research became a prime target for researchers for treating various biofilm-related infections [151]. Biomaterials like antimicrobial peptides or antibiofilm peptides, metal ions and radicals, small molecules, natural bioactive compounds; etc. were prepared in nano-dimensions and were tested against various bacterial biofilms [152]. Recently, carbon quantum dots have been explored for their antimicrobial activities against various nosocomial infections causing bacterial strains. Carbon quantum dots are extremely small in size and exert antibiofilm effectiveness against infections caused by bacterial biofilms.
5.1 Structural Chemistry
Carbon quantum dots have the capacity for the emission of photoluminescent waves in the near-infrared region (NIR) of the electromagnetic spectrum. This is why, carbon quantum dots have multiple applications in various fields such as drug delivery, drug repositioning, cancer therapy, bioimaging, photoacoustic imaging; etc. [153]. The effect of photoluminescence in carbon quantum dots can be tuned and ranged varying from deep UV region to near-infrared region. The photoluminescence in carbon quantum dots can undergo alterations on the basis of shape and size, modifications of edges and surfaces, various heteroatom doping, edge effects, QCE or quantum confinement effect, surface effect; etc. [154]. Apart from these, carbon quantum dots possess light stability and possess a resistance in response to photobleaching and photo-blinking. However, in the case of the traditional semiconductor quantum dots, resistance in response to photobleaching and photo-blinking is absent. In the case of the structural perspective of carbon quantum dots, they should ideally possess smaller sizes and shapes and should possess an increased volume-to-surface area ratio. This should be done for avoiding a huge number of defects on the surface [155]. There are different optical properties in carbon quantum dots that are dependent on the sizes of these quantum dots. These optical properties might vary based on the defective surfaces of the quantum dots and recombinant radioactive pathways caused by the varied volume-to-surface area ratio [156].
5.2 Mechanism of Synthesis
There are multiple methods through which carbon quantum dots can be synthesized. These include microwave irradiation, electrochemical carbonization, chemical ablation, treatment by hydrothermal method, and laser ablation (Fig. 7).
5.2.1 Top-Down Method
In the synthesis of carbon quantum dots through a top-down approach, various sources of carbon are used for example carbon rod, carbon soot, graphite; etc. The top-down synthesis process in the formation of carbon quantum dots can be performed by high-energy ball milling, laser ablation, and electrochemical routes [157]. For an instance, in earlier days, the processes used in the synthesis of carbon quantum dots involved the treatment of single-walled carbon nanotubes to purify them from arc discharge soot. This arc discharge soot undergoes oxidation with the help of nitric acid and then extraction is performed on it with sodium hydroxide. The generated product was moved apart through a gel electrophoresis treatment process. This differentiates the suspended product into three different classes of substances [158]. The first is the large carbon nanotubes which do not penetrate through the gel because of their size constraints. The second product is short in size, irregularly shaped, and provides a band that is slow-moving. The third product moves fast and possesses various fluorescent characteristics. These are carbon quantum dots [159].
Another example of a top-down method includes a laser ablation technique involving laser ablation on a carbon target along with the presence of argon as a carrier gas leading to the production of nanocomposites of carbon [160]. In this process, water vapor is applied to the chamber in which the reaction is taking place. The water vapor should be at a temperature of 900 ˚C and at a pressure of 75 kPa while it is applied to the reaction chamber. Carbon nanocomposites are formed and start accumulating. This aggregation of carbon nanocomposites undergoes treatment with nitric acid for 12 h which is followed by a process of reflux. Then the product was associated with some organic species, the purpose of which is to give the carbon quantum dots photoluminescent characteristics that ultimately undergo surface passivation [161].
5.2.2 Bottom-Up Method
The bottom-up process of synthesis is relatively easy for performance and is used for the synthesis of carbon quantum dots on an industrial scale. In this approach, the polymeric materials as well as the molecules undergo dehydration which ultimately leads to carbonization. Because of this, the production of carbon quantum dots takes place [162]. Various technologies such as combustion, CVD or chemical vapor deposition, ultrasonic assistance, hydrothermal, solvothermal, microwave assistance; etc. are included in the bottom-up approach for the synthesis of carbon quantum dots. An example of carbon quantum dots synthesis by bottom-up approach involves pyrolysis which consists of one step only [163].
5.3 Antibiofilm Activities
Compared to the other nano-bio materials, carbon quantum dots are unique in multiple features like high stability against light, better optical characteristics than other nanocomposites, lower levels of cytotoxicity inside the living system, good rate of aqueous solubility, relatively easier preparation methods and modification strategies, biocompatibility in the human body and lastly, permeability through the bacterial cell wall [164]. Among these, treatment by hydrothermal method perhaps is the easiest and most eco-friendly method, which can be done in just one step. Multiple researchers have suggested the effective preparation of carbon quantum dots from a single carbon source from species like Lactobacillus plantarum [165].
5.4 Mechanism of Antibiofilm Activities
Biofilm-forming bacterial species like Porphyromonas gingivalis, Streptococcus sanguinis, Streptococcus mutants; etc. are largely inhibited and even killed by quantum dots with aminoguanidine and Citrus limetta as the carbon sources [166]. Carbon quantum dots are also formed by using small drug molecules as the core and covering it in a complete meshwork of carbon (Fig. 8). The biggest application of quantum dots is the delivery of drugs at the exact target sites. Metronidazole is often used as a single source of carbon for the preparation of nano-dimensional carbon quantum dots [167]. These results in the creation of carbon dots that show a high level of photo-luminescence and are extremely non-toxic. These are also extremely specific in their antibiofilm activities. The majority of their antibiofilm activities are shown against biofilm-forming obligative anaerobic bacteria [168]. For more specificity against biofilms formed by Gram-negative bacterial species, tinidazole is also used in place of metronidazole as the carbon source in the preparation of carbon quantum dots. These quantum dots are extremely effective as they can effectively undergo penetration into the biofilm for killing the bacterial species because of their nano-dimensional size [169].
5.5 Applications of Carbon Quantum Dots
The carbon quantum dots which share some level of similarity with semiconductor quantum dots show a number of biomedical applications (Table 4). These are stretched from designing probes which are used in bioimaging, to drug delivery, photovoltaic cells, CL or chemiluminescence, photocatalysis, gene deliveries in various biological applications, ECL or electrochemiluminescence, fluorescent inks, LEDs or light emitting diodes, optical sensors; etc. In the last decade, carbon quantum dots have come out to be suitable candidates in sensor systems where LOD or limit of detection is an important parameter of consideration. The range of LOD can be precise from nanomolar to picomolar to even femtomolar level [170]. There are different advantages of using carbon quantum dots. This is mainly because of the easy and relatively simple synthesis of carbon quantum dots, easier functionalization of functionalization, high quantum yield and stability of carbon quantum dots, almost no toxicity, and high brightness of carbon quantum dots; etc. [171]. These are precisely the reasons why carbon quantum dots are preferred so much over other semiconductor quantum dots, which are relatively more expensive and generates toxicity as well.
5.5.1 Carbon Nanoparticles and Nanocomposites as Biosensors to Detect Biofilm
Biosensors could be a promising tool in studying biofilm formation and screening of anti-biofilm drugs. Literature has reported the use of graphene-modified carbon microelectrode arrays to assess the growth of the biofilm of S. mutans [182]. In a study Jayathilake et al. coupled glucose oxidase to a multiwalled carbon nanotube to produce a dual-tip microsensor, which could sense the adsorption of glucose above the biofilm boundary by Scanning Electrochemical Microscopy [183]. Though there has been a plethora of reports on carbon nanoparticles and nanocomposites as biosensors, very little is known about their activity in assessing biofilm growth. This scientific agenda is still a virgin area where a further in-depth investigation is required.
6 Cytotoxic Effects of Carbon Nanoparticles and Nanocomposites in the Living System
Various toxicological parameters especially the cytotoxicity effects on the living system are the main points of concern before applying carbon nanoparticles for carriers of drug delivery or as drugs in our bodies to eradicate infections caused by bacterial biofilms [184]. The optimization of the concentrations in which the carbon nanocomposites are applied in our body is of utmost importance and should be done correctly. The majority of the research works fail in this optimization, which is why carbon nanotubes and nanocomposites still possess a lot of limitations. For the same reason, The United States Food and Drug Administration has yet not given approval for the usage of carbon nanotubes [185]. Many kinds of the literature show that the toxicity level of these nanotubes is way over the thresholds of biosafety limits; whereas other works show that though the nanotubes possess various levels of biocompatibility, some of these are enough biocompatible for application in the living system. On human cells, single-walled nanotubes and multi-walled nanotubes have different cytotoxic results as they are structurally very different. Other parameters which influence these cytotoxic results include the purification of nanotubes, how long and perfectly spherical the nanotubes are, the moieties of functionalization contained by the nanotubes and their reactivities, the applied concentration of the nanotubes; etc. [186]. Various research works have suggested that carbon nanotubes exert induction of oxidative stress which can be harmful to the living system. These nanotubes can successfully eradicate biofilm infections from the heart and blood vessels however, these processes may lead to several cardiovascular diseases, pulmonary inflammations, spleen and liver inflammations; etc. as side effects [187].
Some research work confirms that carbon nanotubes in conjugation with titanium dioxide nanoparticles or silver nanoparticles result in the reduction of the cytotoxic effects of these nanotubes on living cells [188]. Another approach to decreasing this cytotoxic effect was to form polymeric matrices with these carbon nanotubes. This decreases cytotoxicity to a great extent. But although we have all these results, the cytotoxicity of carbon nanotubes is still a very big issue for its application in the human body and more research should be conducted to resolve or reduce its cytotoxicity on living systems [189].
7 Conclusions, Outlook and Future Aspects
The utilization of nanoparticles (NPs) is regarded as a propitious approach for combating the issue of bacterial biofilms. The inefficacy of antibiotic resistance mechanisms against nanoparticles is responsible for this phenomenon. In view of this, nanoparticles have been progressively employed as viable antimicrobial agents or as conduits for augmenting the efficacy of prevailing antibiotics. Carbon-based nanoparticles and nanocomposites have been shown to be promising anti-biofilm agents in in vitro assays. In this era of antibiotic resistance, such an alternative would be of great inclusion in the medical field.
A large number of research studies have been carried out previously, that report on the effectiveness of the antibiofilm property of carbon nanoparticles. The shape and size of the said nanoparticles possess major roles in displaying the antibiofilm activity of the carbon nanoparticles. The carbon nanoparticles especially the SWCNT, fullerenes, GO nanoparticles; etc. were seen to possess extreme effectiveness against bacterial biofilms. The mechanism of their inhibition might follow a cascade of steps as follows [190]. At first, the biofilm layer disruption will take place meaning the breakdown of the EPM/EPS or extracellular polymeric matrix or substances will take place first. This will be followed by the inhibition of the bacterial biofilm cells. This can be achieved by either killing the bacterial cells or by inhibiting the growth of bacterial cells by changing their respiratory chain pathway or enzymatic activities involved in that pathway [191]. Apart from changing the respiratory chain pathway, these nanoparticles can also impose changes or inhibit different pathways or the enzymes involved in those specific pathways like interacting physically with cell membranes, inhibiting energy metabolism, formation of aggregates of carbon nanoparticles inside the bacterial cells, disrupting the cellular membranes and cell walls of the bacteria forming a biofilm. Because of their effectiveness as antibiofilm agents, they have huge scope and applications in the medical and biological sectors [192]. But we should keep in mind that before applying them, these nanoparticles must undergo a process of subsequent purification and specific functionalization. If it’s applied in any type of physiological medium, then the solubility of these nanoparticles should also be increased. Again, because the amalgamation of carbon nanostructures and metal nanoparticles leading to the formation of carbon nanocomposites possess high antibiofilm effectiveness, they should also be considered for their application in medical and biological sectors or even inside the patient’s body [193]. But, in the end, we can say that as the number of studies on carbon nanoparticles is so less and as very few numbers of literature are available on this topic, and also as very few research works have been carried out on their antibiofilm activities, we need more and more research and literature on carbon nanoparticles and their antibiofilm activities to understand their effectiveness vividly [194].
Though recently, there has been apprehension regarding the plausible health implications of synthesized carbon nanoparticles, compounded by our restricted comprehension of the modus operandi of these nanoparticles in concert with host cells, and the resulting biological pathways that are influenced. Despite the extensive research conducted on the evaluation of diverse nanoparticles in the context of their anti-biofilm efficacy, limited investigations have been undertaken to elucidate the mechanisms through which these nanoparticles interact with the complex biofilm structures. The consequentiality of nanoparticles’ capability to access the biofilm proficiently is pivotal in terms of accomplishing the successful eradication of biofilms.
In conclusion, despite the existence of numerous challenges that must be addressed to achieve successful translation into clinical applications, the substantial ongoing endeavours in the development and evaluation of carbon nanoparticle-based therapeutics have the potential to introduce essential tools for the management of patients for whom conventional antibiotic treatments have proven ineffective. Additionally, these endeavours could establish a pioneering platform for intelligent treatments of antibiotic-resistant biofilm infections.
Data Availability
Not applicable.
References
D. Bocia˛ga, W. Jakubowski, P. Komorowski, A. Sobczyk-Guzenda, A. Je˛drzejczak, D. Batory, Olejnik, A
Surface characterization and biological evaluation of silver-, Incorporated DLC coatings fabricated by hybrid RF PACVD/MS method. Mater. Sci. Eng. C 63, 462–474 (2016). [CrossRef] [PubMed]
Y. Liu, P. Guo, X. He, L. Li, A. Wang, H. Li, Developing transparent copper-doped diamond-like carbon films for marine antifouling applications. Diam. Relat. Mater. 69, 144–151 (2016). [CrossRef]
C.A. Love, R.B. Cook, T.J. Harvey, P.A. Dearnley, R.J.K. Wood, Diamond like carbon coatings for potential application in biological implants—A review. Tribol Int. 63, 141–150 (2013). [CrossRef]
W.-C. Lan, S.-F. Ou, M.-H. Lin, K.-L. Ou, M.-Y. Tsai, Development of silver-containing diamond-like carbon for biomedical applications. Part I: microstructure characteristics, mechanical properties and antibacterial mechanisms. Ceram. Int. 39, 4099–4104 (2013). [CrossRef]
M. Cloutier, S. Turgeon, Y. Busby, M. Tatoulian, J.J. Pireaux, D. Mantovani, Controlled distribution and clustering of silver in Ag-DLC Nanocomposite Coatings using a hybrid plasma Approach. ACS Appl. Mater. Interfaces. 8, 21020–21027 (2016). [CrossRef] [PubMed]
G. Dearnaley, J.H. Arps, Biomedical applications of diamond-like carbon (DLC) coatings: a review. Surf. Coat. Technol. 200, 2518–2524 (2005). [CrossRef]
R. Zhang, J. Zhao, Y. Yang, A novel diamond-like carbon film. Surf. Interfaces. 7, 1–5 (2017). [CrossRef]
L. Swiatek, A. Olejnik, J. Grabarczyk, A. Jedrzejczak, A. Sobczyk-Guzenda, M. Kaminska, W. Jakubowski, W. Szymanski, D. Bociaga, Multi-doped diamond like-carbon coatings (DLC-Si/Ag) for biomedical applications fabricated using the modified chemical vapour deposition method. Diam. Relat. Mater. 67, 54–62 (2016). [CrossRef]
L.L. Zhang, Q. Yang, Y. Tang, L. Yang, C. Zhang, Y. Hu, X. Cui, Synthesis and characterization of boron incorporated diamond-like carbon thin films. Thin Solid Films. 589, 457–464 (2015). [CrossRef]
J. Luo, Y.Q. Fu, H. Le, J.A. Williams, S. Spearing, W. Milne, Diamond and diamond-like carbon MEMS. J. Micromech Microeng. 17, S147–S163 (2007). [CrossRef]
N. Dwivedi, S. Kumar, H.K. Malik, Strange hardness characteristic of hydrogenated diamond-like carbon thin film by plasma enhanced chemical vapor deposition process. Appl. Phys. Lett. 102, 011917 (2013). [CrossRef]
T.B. Santos, A.A. Vieira, L.O. Paula, E.D. Santos, P.A. Radi, S. Khouri, H.S. Maciel, R.S. Pessoa, L. Vieira, Flexible camphor diamond-like carbon coating on polyurethane to prevent Candida albicans biofilm growth. J. Mech. Behav. Biomed. Mater. 68, 239–246 (2017). [CrossRef] [PubMed]
S.I. Hosseini, Z. Javaherian, D. Minai-Tehrani, R. Ghasemi, Z. Ghaempanah, M.A. Firouzjah, B. Shokri, Antibacterial properties of fluorinated diamond-like carbon films deposited by direct and remote plasma. Mater. Lett. 188, 84–87 (2017). [CrossRef]
T. Fadel, Realizing the Promise of Carbon Nanotubes: Challenges, Opportunities, and the Pathway to Commercialization. 2015. Available online: https://www.nano.gov/sites/default/files/pub_resource/2014_nni_cnt_tech_meeting_report.pdf (accessed on 8 September 2017)
; : Socio-Environmental Systems (SES) Research. Fullerene, SES Research, U.S.A. Houston, 2017; Available online: https://www.sesres.com/fullerene/ (accessed on 8 September 2017)
K. Norinaga, O. Deutschmann, Detailed kinetic modeling of gas-phase reactions in the chemical vapor deposition of carbon from light hydrocarbons. Ind. Eng. Chem. Res. 46, 3547–3557 (2007). [CrossRef]
L. Sun, C. Tian, M. Li, X. Meng, L. Wang, R. Wang, J. Yin, H. Fu, From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 1, 6462–6470 (2013). [CrossRef]
E. Ruiz-Hitzky, M. Darder, F.M. Fernandes, E. Zatile, F.J. Palomares, P. Aranda, Supported graphene from natural resources: Easy preparation and applications. Adv. Mater. 23, 5250–5255 (2011). [CrossRef] [PubMed]
S.S. Kawale, S. Bhardwaj, D. Kshirsagar, C. Bhosale, M. Sharon, M. Sharon, Thin Films of Carbon Nanomaterial from Natural Precursor by Hot Wire CVD. Fuller. Nanotub Carbon Nanostruct. 19, 540–549 (2011). [CrossRef]
M.V. Jacob, R.S. Rawat, B. Ouyang, K. Bazaka, D.S. Kumar, D. Taguchi, M. Iwamoto, R. Neupane, O.K. Varghese, Catalyst-Free plasma enhanced growth of Graphene from Sustainable sources. Nano Lett. 15, 5702–5708 (2015). [CrossRef] [PubMed]
I. Malek, C. Schaber, T. Heinlein, J. Schneider, S. Gorb, R. Schmitz, Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 4, 5228–5235 (2016). [CrossRef]
S. Yick, A. Mai-Prochnow, I. Levchenko, J. Fang, M.K. Bull, M. Bradbury, A.B. Murphy, K.K. Ostrikov, The effects of plasma treatment on bacterial biofilm formation on vertically-aligned carbon nanotube arrays. RSC Adv. 5, 5142–5148 (2015). [CrossRef]
S.-R. Chae, Y. Watanabe, M.R. Wiesner, Comparative photochemical reactivity of spherical and tubular fullerene nanoparticles in water under ultraviolet (UV) irradiation. Water Res. 45, 308–314 (2011). [CrossRef] [PubMed]
O. Akhavan, M. Abdolahad, Y. Abdi, S. Mohajerzadeh, Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon. 47, 3280–3287 (2009). [CrossRef]
S. Darbari, Y. Abdi, F. Haghighi, S. Mohajerzadeh, N. Haghighi, Investigating the antifungal activity of TiO2 nanoparticles deposited on branched carbon nanotube arrays. J. Phys. D Appl. Phys. 44, 245401 (2011). [CrossRef]
O. Akhavan, M. Abdolahad, Y. Abdi, S. Mohajerzadeh, Silver nanoparticles within vertically aligned multi-wall carbon nanotubes with open tips for antibacterial purposes. J. Mater. Chem. 21, 387–393 (2011). [CrossRef]
R. Egerton, P. Li, M. Malac, Radiation damage in the TEM and SEM. Micron. 35, 399–409 (2004). [CrossRef] [PubMed]
V.H. Crespi, N.G. Chopra, M.L. Cohen, A. Zettl, S.G. Louie, Anisotropic electron-beam damage and the collapse of carbon nanotubes. Phys. Rev. B 54, 5927–5931 (1996). [CrossRef]
D. Lolla, J. Gorse, C. Kisielowski, J. Miao, P.L. Taylor, G.G. Chase, D.H. Reneker, Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale. 8, 120–128 (2016). [CrossRef] [PubMed]
L. Meng, C. Fu, Q. Lu, Advanced technology for functionalization of carbon nanotubes. Prog Nat. Sci. 19, 801–810 (2009). [CrossRef]
L. Gu, P.G. Luo, H. Wang, M.J. Meziani, Y. Lin, L.M. Veca, L. Cao, F. Lu, X. Wang, R.A. Quinn, Single-walled carbon nanotube as a unique scaffold for the multivalent display of sugars. Biomacromolecules. 9, 2408–2418 (2008). [CrossRef] [PubMed]
O. Akhavan, R. Azimirad, S. Safa, Functionalized carbon nanotubes in ZnO thin films for photoinactivation of bacteria. Mater. Chem. Phys. 130, 598–602 (2011). [CrossRef]
H.Z. Zardini, M. Davarpanah, M. Shanbedi, A. Amiri, M. Maghrebi, L. Ebrahimi, Microbial toxicity of ethanolamines—multiwalled carbon nanotubes. J. Biomed. Mater. Res. Part. A 102, 1774–1781 (2014). [CrossRef] [PubMed]
K. Varshney, Carbon nanotubes: a review on synthesis, properties and applications. Int. J. Eng. Res. 2, 660–677 (2014)
V.K.K. Upadhyayula, V. Gadhamshetty, Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: a review. Biotechnol. Adv. 28, 802–816 (2010). [CrossRef] [PubMed]
J.R. Lawrence, M.J. Waiser, G.D.W. Swerhone, J. Roy, V. Tumber, A. Paule, A.P. Hitchcock, J.J. Dynes, D.R. Korber, Effects of fullerene (C60), multi-wall carbon nanotubes (MWCNT), single wall carbon nanotubes (SWCNT) and hydroxyl and carboxyl modified single wall carbon nanotubes on riverine microbial communities. Environ. Sci. Pollut Res. 23, 10090–10102 (2016). [CrossRef] [PubMed]
S. Liu, L. Wei, L. Hao, N. Fang, M.W. Chang, R. Xu, Y. Yang, Y. Chen, Sharper and faster “nano darts” kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano. 3, 3891–3902 (2009). [CrossRef] [PubMed]
J. Zhu, J. Wang, J. Hou, Y. Zhang, J. Liu, Van der B. Bruggen, Graphene-based antimicrobial polymeric membranes: a review. J. Mater. Chem. A 5, 6776–6793 (2017). [CrossRef]
R. Zhou, H. Gao, Cytotoxicity of graphene: recent advances and future perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 452–474 (2014). [CrossRef] [PubMed]
H.M. Hegab, A. ElMekawy, L. Zou, D. Mulcahy, C.P. Saint, M. Ginic-Markovic, The controversial antibacterial activity of graphene-based materials. Carbon. 105, 362–376 (2016). [CrossRef]
A.V. Titov, P. Král, R. Pearson, Sandwiched graphene—membrane superstructures. ACS Nano. 4, 229–234 (2009). [CrossRef] [PubMed]
M. Dallavalle, M. Calvaresi, A. Bottoni, M. Melle-Franco, F. Zerbetto, Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces. 7, 4406–4414 (2015). [CrossRef] [PubMed]
De A.C.C. Leon, On the antibacterial mechanism of graphene oxide (GO) Langmuir—Blodgett films. Chem. Commun. 51, 2886–2889 (2015)
L. Hui, J.-G. Piao, J. Auletta, K. Hu, Y. Zhu, T. Meyer, H. Liu, L. Yang, Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces. 6, 13183–13190 (2014). [CrossRef] [PubMed]
O. Akhavan, E. Ghaderi, A. Esfandiar, Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 115, 6279–6288 (2011). [CrossRef] [PubMed]
O. Akhavan, Photocatalytic reduction of graphene oxides hybridized by ZnO nanoparticles in ethanol. Carbon. 49, 11–18 (2011). [CrossRef]
V.C. Sanchez, A. Jachak, R.H. Hurt, A.B. Kane, Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem. Res. Toxicol. 25, 15–34 (2011). [CrossRef] [PubMed]
F. Perreault, De A.F. Faria, S. Nejati, M. Elimelech, Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano. 9, 7226–7236 (2015). [CrossRef] [PubMed]
S. Romero-Vargas Castrillón, F. Perreault, De A.F. Faria, M. Elimelech, Interaction of graphene oxide with bacterial cell membranes: insights from force spectroscopy. Environ. Sci. Technol. Lett. 2, 112–117 (2015). [CrossRef]
S. Liu, T.H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong, Y. Chen, Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 5, 6971–6980 (2011). [CrossRef] [PubMed]
O. Akhavan, E. Ghaderi, Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 4, 5731–5736 (2010). [CrossRef] [PubMed]
S. Liu, M. Hu, T.H. Zeng, R. Wu, R. Jiang, J. Wei, L. Wang, J. Kong, Y. Chen, Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir. 28, 12364–12372 (2012). [CrossRef] [PubMed]
W. Jiang, H. Mashayekhi, B. Xing, Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 157, 1619–1625 (2009). [CrossRef] [PubMed]
J.A. Lemire, J.J. Harrison, R.J. Turner, Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 11, 371–384 (2013). [CrossRef] [PubMed]
J. Ma, J. Zhang, Z. Xiong, Y. Yong, X. Zhao, Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 21, 3350–3352 (2011). [CrossRef]
N. Hussain, A. Gogoi, R.K. Sarma, P. Sharma, A. Barras, R. Boukherroub, R. Saikia, P. Sengupta, M.R. Das, Reduced graphene oxide nanosheets decorated with au nanoparticles as an effective bactericide: investigation of biocompatibility and leakage of sugars and proteins. ChemPlusChem. 79, 1774–1784 (2014). [CrossRef]
L. Yu, Y. Zhang, B. Zhang, J. Liu, Enhanced antibacterial activity of silver nanoparticles/halloysite nanotubes/graphene nanocomposites with sandwich-like structure. Sci. Rep 2014, 4, 4551. Available online: https://www.nature.com/articles/srep04551 (accessed on 8 September 2017). [CrossRef] [PubMed]
S. Kellici, J. Acord, A. Vaughn, N.P. Power, D.J. Morgan, T. Heil, S.P. Facq, G.I. Lampronti, Calixarene assisted Rapid Synthesis of Silver-Graphene Nanocomposites with enhanced antibacterial activity. ACS Appl. Mater. Interfaces. 8, 19038–19046 (2016). [CrossRef] [PubMed]
S. Zhu, J. Guo, J. Dong, Z. Cui, T. Lu, C. Zhu, D. Zhang, J. Ma, Sonochemical fabrication of Fe3O4 nanoparticles on reduced graphene oxide for biosensors. Ultrason. Sonochem. 20, 872–880 (2013). [CrossRef] [PubMed]
R. Li, N.D. Mansukhani, L.M. Guiney, Z. Ji, Y. Zhao, C.H. Chang, C.T. French, J.F. Miller, M.C. Hersam, A.E. Nel, Identification and optimization of Carbon Radicals on Hydrated Graphene Oxide for ubiquitous Antibacterial Coatings. ACS Nano. 10, 10966–10980 (2016). [CrossRef] [PubMed]
L. Yu, Y. Zhang, B. Zhang, J. Liu, H. Zhang, C. Song, Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 447, 452–462 (2013). [CrossRef]
J. Peng, W. Gao, B.K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L.B. Alemany, X. Zhan, G. Gao, Graphene quantum dots derived from carbon fibers. Nano Lett. 12, 844–849 (2012). [CrossRef] [PubMed]
M.G. Hahm, A. Leela Mohana Reddy, D.P. Cole, M. Rivera, J.A. Vento, J. Nam, H.Y. Jung, Y.L. Kim, N.T. Narayanan, D.P. Hashim, Carbon nanotube—Nanocup hybrid structures for high power supercapacitor applications. Nano Lett. 12, 5616–5621 (2012). [CrossRef] [PubMed]
K.S. Novoselov, V. Fal, L. Colombo, P. Gellert, M. Schwab, K. Kim, A roadmap for graphene. Nature. 490, 192–200 (2012). [CrossRef] [PubMed]
X. Cao, Y. Shi, W. Shi, G. Lu, X. Huang, Q. Yan, Q. Zhang, H. Zhang, Preparation of novel 3D graphene networks for supercapacitor applications. Small. 7, 3163–3168 (2011). [CrossRef] [PubMed]
H. Li, X. He, Y. Liu, H. Huang, S. Lian, S.-T. Lee, Z. Kang, One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon. 49, 605–609 (2011). [CrossRef]
Q. Cao, J.A. Rogers, Ultrathin Films of single-walled Carbon Nanotubes for Electronics and Sensors: a review of fundamental and Applied aspects. Adv. Mater. 21, 29–53 (2009). [CrossRef]
W. Choi, I. Lahiri, R. Seelaboyina, Y.S. Kang, Synthesis of Graphene and its applications: a review. Crit. Rev. Solid State Mater. Sci. 35, 52–71 (2010). [CrossRef]
Y. Wu, X. Lin, M. Zhang, Carbon nanotubes for thin film transistor: Fabrication, properties, and applications. J. Nanomater 2013, 2013, 1–16. [CrossRef]
D.S. Hecht, L. Hu, G. Irvin, Emerging transparent electrodes based on Thin Films of Carbon Nanotubes, Graphene, and metallic nanostructures. Adv. Mater. 23, 1482–1513 (2011). [CrossRef] [PubMed]
X. Wang, L. Zhi, K. Müllen, Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 8, 323–327 (2008). [CrossRef] [PubMed]
D.H. Seo, Z.J. Han, S. Kumar, K. Ostrikov, Structure-Controlled, Vertical Graphene-Based, Binder-Free Electrodes from Plasma-Reformed Butter Enhance Supercapacitor performance. Adv. Energy Mater. 3, 1316–1323 (2013). [CrossRef]
D.H. Seo, S. Pineda, S. Yick, J. Bell, Z.J. Han, K.K. Ostrikov, Plasma-enabled sustainable elemental lifecycles: honeycomb-derived graphenes for next-generation biosensors and supercapacitors. Green. Chem. 17, 2164–2171 (2015). [CrossRef]
E.C. Stancu, A.-M. Stanciuc, S. Vizireanu, C. Luculescu, L. Moldovan, A. Achour, G. Dinescu, Plasma functionalization of carbon nanowalls and its effect on attachment of fibroblast-like cells. J. Phys. D Appl. Phys. 47, 1–10 (2014). [CrossRef]
S. Goenka, V. Sant, S. Sant, Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control Release. 173, 75–88 (2014). [CrossRef] [PubMed]
K. Yang, S. Zhang, G. Zhang, X. Sun, S.-T. Lee, Z. Liu, Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 10, 3318–3323 (2010). [CrossRef] [PubMed]
Y. Chen, A. Star, S. Vidal, Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 42, 4532–4542 (2013). [CrossRef] [PubMed]
S.M. Dizaj, A. Mennati, S. Jafari, K. Khezri, K. Adibkia, Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 5, 19–23 (2015)
H. Ji, H. Sun, X. Qu, Antibacterial applications of graphene-based nanomaterials: recent achievements and challenges. Adv. Drug Deliv Rev. 105, 176–189 (2016). [CrossRef] [PubMed]
I.N. Kholmanov, M.D. Stoller, J. Edgeworth, W.H. Lee, H. Li, J. Lee, C. Barnhart, J.R. Potts, R. Piner, D. Akinwande, Nanostructured hybrid transparent conductive films with antibacterial properties. ACS Nano. 6, 5157–5163 (2012). [CrossRef] [PubMed]
K. Prasad, G. Lekshmi, K. Ostrikov, V. Lussini, J. Blinco, M. Mohandas, K. Vasilev, S. Bottle, K. Bazaka, K. Ostrikov, Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5431540/ (accessed on 8 September 2017). [CrossRef] [PubMed]
J. Li, G. Wang, H. Zhu, M. Zhang, X. Zheng, Z. Di, X. Liu, X. Wang, Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep 2014, 4, 4359. Available online: http://www.nature.com/articles/srep04359 (accessed on 8 September 2017). [CrossRef] [PubMed]
D. Bitounis, H. Ali-Boucetta, B.H. Hong, D.H. Min, K. Kostarelos, Prospects and challenges of graphene in biomedical applications. Adv. Mater. 25, 2258–2268 (2013). [CrossRef] [PubMed]
H. Liu, L. Zhang, M. Yan, J. Yu, Carbon Nanostructures in Biology and Medicine. J. Mater. Chem. B 5, 6437–6450 (2017). [CrossRef]
G. Goncalves, P. Marques, M. Vila, Graphene-Based Materials in Health and Environment (Springer, Berlin, Germany, 2017)
T. Mocan, C.T. Matea, T. Pop, O. Mosteanu, A.D. Buzoianu, S. Suciu, C. Puia, C. Zdrehus, C. Iancu, L. Mocan, Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 74, 3467–3479 (2017). [CrossRef] [PubMed]
L. Botta, B.M. Bizzarri, M. Crucianelli, R. Saladino, Advances in biotechnological synthetic applications of carbon nanostructured systems. J. Mater. Chem. B 5, 6490–9510 (2017). [CrossRef]
V. Kleandrova, V. Luan, F. Speck-Planche, A. Cordeiro, Review of structures containing Fullerene-C60 for delivery of Antibacterial Agents. Multitasking Model for Computational Assessment of Safety Profiles. Curr. Bioinform. 10, 565–578 (2015). [CrossRef]
S. Szunerits, A. Barras, R. Boukherroub, Antibacterial applications of nanodiamonds. Int. J. Environ. Res. Public. Health. 13, 413 (2016). [CrossRef] [PubMed]
Q. Chen, Z. Ma, G. Liu, H. Wei, X. Xie, Antibacterial activity of cationic cyclen-functionalized fullerene derivatives: membrane stress. Dig. J. Nanomater Biostruct (DJNB). 11, 753–761 (2016)
K.J. Moor, C.O. Osuji, J.-H. Kim, Antimicrobial photodynamic therapy with fulleropyrrolidine: Photoinactivation mechanism of Staphylococcus aureus, in vitro and in vivo studies. Appl. Microbiol. Biotechnol. 99, 4031–4043 (2015)
K.J. Moor, C.O. Osuji, J.-H. Kim, Dual-functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces. 8, 33583–33591 (2016). [CrossRef] [PubMed]
S. Kang, M. Pinault, L.D. Pfefferle, M. Elimelech, Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 23, 8670–8673 (2007). [CrossRef] [PubMed]
S. Kang, M. Herzberg, D.F. Rodrigues, M. Elimelech, Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [CrossRef] [PubMed]
S. Aslan, C.Z. Loebick, S. Kang, M. Elimelech, L.D. Pfefferle, Van P.R. Tassel, Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale. 2, 1789–1794 (2010). [CrossRef] [PubMed]
L.R. Arias, L. Yang, Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir. 25, 3003–3012 (2009). [CrossRef] [PubMed]
S. Gurunathan, J.W. Han, A.A. Dayem, V. Eppakayala, J.-H. Kim, Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 7, 5901–5914 (2012). [CrossRef] [PubMed]
Y. Tu, M. Lv, P. Xiu, T. Huynh, M. Zhang, M. Castelli, Z. Liu, Q. Huang, C. Fan, H. Fang, Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 8, 594–601 (2013). [CrossRef] [PubMed]
C. Zhao, B. Deng, G. Chen, B. Lei, H. Hua, H. Peng, Z. Yan, Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 9, 963–973 (2016). [CrossRef]
K. Myllymaa, J. Levon, V.-M. Tiainen, S. Myllymaa, A. Soininen, H. Korhonen, E. Kaivosoja, R. Lappalainen, Y.T. Konttinen, Formation and retention of staphylococcal biofilms on DLC and its hybrids compared to metals used as biomaterials. Colloids Surf. B Biointerfaces. 101, 290–297 (2013). [CrossRef] [PubMed]
S.N. Robertson, D. Gibson, W.G. MacKay, S. Reid, C. Williams, R. Birney, Investigation of the antimicrobial properties of modified multilayer diamond-like carbon coatings on 316 stainless steel. Surf. Coat. Technol. 314, 72–78 (2017). [CrossRef]
X. Su, Q. Zhao, S. Wang, A. Bendavid, Modification of diamond-like carbon coatings with fluorine to reduce biofouling adhesion. Surf. Coat. Technol. 204, 2454–2458 (2010). [CrossRef]
K. Honglertkongsakul, P.W. May, B. Paosawatyanyong, Electrical and optical properties of diamond-like carbon films deposited by pulsed laser ablation. Diam. Relat. Mater. 19, 999–1002 (2010). [CrossRef]
S. Skariyachan, A. Parveen, S. Garka, Nanoparticle fullerene (C60) demonstrated stable binding with antibacterial potential towards probable targets of drug resistant Salmonella typhi—A computational perspective and in vitro investigation. J. Biomol. Struct. Dyn. 34, 1–20 (2016). [CrossRef] [PubMed]
S. Dostalova, A. Moulick, V. Milosavljevic, R. Guran, M. Kominkova, K. Cihalova, Z. Heger, L. Blazkova, P. Kopel, D. Hynek et al., Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5. Mon Chem. Chem. Mon. 147, 905–918 (2016). [CrossRef]
S.K. Sharma, L.Y. Chiang, M.R. Hamblin, Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [CrossRef] [PubMed]
A. Rondags, W.Y. Yuen, M.F. Jonkman, B. Horváth, Fullerene C60 with cytoprotective and cytotoxic potential: prospects as a novel treatment agent in Dermatology? Exp. Dermatol. 26, 220–224 (2017). [CrossRef] [PubMed]
J. Naddeo, M. Ratti, S.M. O’Malley, J.C. Griepenburg, D.M. Bubb, E.A. Klein, Antibacterial Properties of Nanoparticles: a comparative review of chemically synthesized and laser-generated particles. Adv. Sci. Eng. Med. 7, 1044–1057 (2015). [CrossRef]
J. Boonstra, J.A. Post, Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 337, 1–13 (2004). [CrossRef] [PubMed]
M. Ishaq, K. Bazaka, K. Ostrikov, Pro-apoptotic NOXA is implicated in atmospheric-pressure plasma-induced melanoma cell death. J. Phys. D Appl. Phys. 48, 464002 (2015). [CrossRef]
M. Ishaq, K. Bazaka, K. Ostrikov, Intracellular effects of atmospheric-pressure plasmas on melanoma cancer cells. Phys. Plasmas. 22, 122003 (2015). [CrossRef]
R. Zhou, R. Zhou, X. Zhang, J. Zhuang, S. Yang, K. Bazaka, K. Ostrikov, Effects of atmospheric-pressure N2, He, Air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep 2016, 6, 32603. Available online: https://www.nature.com/articles/srep32603 (accessed on 8 September 2017). [CrossRef] [PubMed]
R. Zhou, R. Zhou, X. Zhang, J. Li, X. Wang, Q. Chen, S. Yang, Z. Chen, K. Bazaka, K. Ostrikov, Synergistic effect of atmospheric pressure plasma and TiO2 photocatalysis on inactivation of Escherichia coli cells in aqueous media. Sci. Rep 2016, 6, 39552. Available online: http://www.nature.com/articles/srep39552?WT.feed_name=subjects_physical-sciences (accessed on 8 September 2017). [CrossRef] [PubMed]
E. Navarro, A. Baun, R. Behra, N.B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P.H. Santschi, L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 17, 372–386 (2008). [CrossRef] [PubMed]
Z. Markovic, V. Trajkovic, Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials. 29, 3561–3573 (2008). [CrossRef] [PubMed]
J. Fang, D.Y. Lyon, M.R. Wiesner, J. Dong, Alvarez. Effect of a Fullerene Water suspension on bacterial phospholipids and membrane phase behavior. Environ. Sci. Technol. 41, 2636–2642 (2007). [CrossRef] [PubMed]
Y.J. Tang, J.M. Ashcroft, D. Chen, G. Min, C.-H. Kim, B. Murkhejee, C. Larabell, J.D. Keasling, F.F. Chen, Charge-Associated Effects of Fullerene derivatives on Microbial Structural Integrity and Central Metabolism. Nano Lett. 7, 754–760 (2007). [CrossRef] [PubMed]
T. Mashino, D. Nishikawa, K. Takahashi, N. Usui, T. Yamori, M. Seki, T. Endo, M. Mochizuki, Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 13, 4395–4397 (2003). [CrossRef] [PubMed]
T. Mashino, N. Usui, K. Okuda, T. Hirota, M. Mochizuki, Respiratory chain inhibition by fullerene derivatives: hydrogen peroxide production caused by fullerene derivatives and a respiratory chain system. Bioorg. Med. Chem. 11, 1433–1438 (2003). [CrossRef]
P.-C. Hsu, D. Jefferies, S. Khalid, Molecular Dynamics Simulations predict the Pathways via which pristine Fullerenes penetrate bacterial membranes. J. Phys. Chem. B 120, 11170–11179 (2016). [CrossRef] [PubMed]
K. Krishnamoorthy, M. Veerapandian, L.-H. Zhang, K. Yun, S.J. Kim, Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 116, 17280–17287 (2012). [CrossRef]
C. Yang, J. Mamouni, Y. Tang, L. Yang, Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir. 26, 16013–16019 (2010). [CrossRef] [PubMed]
H.J. Johnston, G.R. Hutchison, F.M. Christensen, S. Peters, S. Hankin, K. Aschberger, Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 4, 207–246 (2010). [CrossRef] [PubMed]
N. Saifuddin, A. Raziah, A. Junizah, Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem 2012, 2013. Available online: https://www.hindawi.com/journals/jchem/2013/676815/ (accessed on 8 September 2017). [CrossRef]
S.C. Smith, D.F. Rodrigues, Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon. 91, 122–143 (2015). [CrossRef]
S. Rhiem, End of Life Cycle Assessment for Carbon Nanotube (CNT) Containing Composites: Release of CNT and Ecotoxicological Consequences. Hochschulbibliothek der Rheinisch-Westfälischen Technischen Hochschule Aachen. 2014. Available online: http://publications.rwth-aachen.de/record/444979/files/5137.pdf (accessed on 8 September 2017)
H. Xu, S. Zhang, S.M. Anlage, L. Hu, G. Grüner, Frequency-and electric-field-dependent conductivity of single-walled carbon nanotube networks of varying density. Phys. Rev. B 2008, 77, 075418. Available online: http://www.physics.ucla.edu/research/Gruner_Group/pubs/pdf/pr_freq_depend.pdf (accessed on 8 September 2017). [CrossRef]
V. Skákalová, V. Vretenár, L. Kopera, P. Kotrusz, C. Mangler, M. Meško, J.C. Meyer, M. Hulman, Electronic transport in composites of graphite oxide with carbon nanotubes. Carbon. 72, 224–232 (2014). [CrossRef]
C.D. Vecitis, K.R. Zodrow, S. Kang, M. Elimelech, Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 4, 5471–5479 (2010). [CrossRef] [PubMed]
J. Smotlacha, R. Pincak, Electronic Properties of Carbon Nanostructures (arXiv, 2016)
S. Yang, Y.C. Huang, C.H. Luo, Y.C. Lin, J.W. Huang, C.P.J. Chuang, C.J. Chen, W. Fang, C.Y. Chuang, Inactivation efficiency of Bioaerosols using Carbon Nanotube plasma. CLEAN. Soil. Air Water. 39, 201–205 (2011). [CrossRef]
D. Lolla, M. Lolla, A. Abutaleb, H.U. Shin, D.H. Reneker, G.G. Chase, Fabrication, polarization of Electrospun Polyvinylidene Fluoride Electret fibers and effect on capturing Nanoscale Solid Aerosols. Materials. 9, 671 (2016). [CrossRef] [PubMed]
W. Xu, M. Kranz, S. Kim, M. Allen, Micropatternable elastic electrets based on a PDMS/carbon nanotube composite. J. Micromech Microeng. 20(104003–1–), 104003–104007 (2010). [CrossRef]
K. Bazaka, R.J. Crawford, E.L. Nazarenko, E.P. Ivanova, Bacterial extracellular polysaccharides. Bacterial Adhesion; Springer: Dordrecht, The Netherlands; Houten, The Netherlands, 2011; 213–226
K. Bazaka, M.V. Jacob, R.J. Crawford, E.P. Ivanova, Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 95, 299–311 (2012). [CrossRef] [PubMed]
K. Rahme, N. Dagher, Chemistry Routes for Copolymer Synthesis containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics, 11(7) (2019)
M. Ashrafi, M. Hamadanian, A.R. Ghasemi, F.J. Kashi, Improvement mechanical and Antibacterial Properties of Epoxy by Polyethylene glycol and Ag/CuO nanoparticles. Polym. Compos., (2019)
Y. Wang, H. Song, G. Wang, X. Yang, J. Wang, H. Wei, 131 I-labeled PEG and folic acid cofunctionalized graphene quantum dots for tumor-targeted imaging. J. Radioanal. Nucl. Chem. 319(3), 1119–1125 (2019)
D. Selli, M. Tawfilas, M. Mauri, R. Simonutti, Di C. Valentin, Optimizing PEGylation of TiO2 nanocrystals through a combined experimental and computational study. Chem. Mater. 31(18), 7531–7546 (2019)
S. Chen, Y. Quan, Y.-L. Yu, J.-H. Wang, Graphene quantum dot/silver nanoparticle hybrids with oxidase activities for antibacterial application. ACS Biomaterials Science & Engineering. 3(3), 313–321 (2017)
J. Yang, G. Gao, X. Zhang, Y.-H. Ma, X. Chen, F.-G. Wu, One-step synthesis of carbon dots with bacterial contact-enhanced fluorescence emission: fast gram-type identification and selective Gram-positive bacterial inactivation. Carbon. 146, 827–839 (2019)
Y. Xu, E. Hirata, Y. Iizumi, N. Ushijima, K. Kubota, S. Kimura, Y. Maeda, T. Okazaki, Single-walled Carbon Nanotube membranes accelerate active Osteogenesis in Bone defects: potential of guided bone regeneration membranes. ACS Biomaterials Science & Engineering. 8(4), 1667–1675 (2022). https://doi.org/10.1021/acsbiomaterials.1c01542
Z. Guoqiang Liu, X. Xu, Y. Dai, Y. Zeng, X. Wei, L.-T. He, De Novo Design of Entropy-Driven Polymers resistant to bacterial attachment via multicomponent reactions. J. Am. Chem. Soc. 143(41), 17250–17260 (2021). https://doi.org/10.1021/jacs.1c08332
M. Huan Xu, H. Shen, W. Shang, S. Xu, H.-R. Zhang, D. Yang, M.H. Zhou, Osteoconductive and antibacterial poly(lactic acid) fibrous membranes impregnated with Biobased Nanocarbons for biodegradable bone regenerative scaffolds. Ind. Eng. Chem. Res. 60(32), 12021–12031 (2021). https://doi.org/10.1021/acs.iecr.1c02165
M.C. Proner, Alessandra Cristina de Meneses, Andrea Azevedo Veiga, Helga Schlüter, Débora de Oliveira, Marco Di Luccio. Industrial cooling Systems and Antibiofouling strategies: a Comprehensive Review. Ind. Eng. Chem. Res. 60(8), 3278–3294 (2021). https://doi.org/10.1021/acs.iecr.0c05985
R.Ã. Damián, M. Sartori, A. Bertuola, E. Miñán, M.C. Gonik, Gonzalez, Mónica Fernández Lorenzo de Mele. Environmentally Induced Changes of Commercial Carbon Nanotubes in Aqueous Suspensions. Adaptive behavior of Bacteria in Biofilms. ACS Omega. 6(8), 5197–5208 (2021). https://doi.org/10.1021/acsomega.0c05114
T. Victor, H.M. Noronha, Camilla, J.C. Camargos, G. Jackson, Antonio, A.J. Souza Filho, C.A. Paula, F. Rezende, Andreia, Faria. Physical membrane-stress-mediated Antimicrobial Properties of Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 9(8), 3203–3212 (2021). https://doi.org/10.1021/acssuschemeng.0c08317
Rashin Namivandi-Zangeneh, E.H.H. Wong, Cyrille Boyer. Synthetic antimicrobial polymers in combination therapy: tackling Antibiotic Resistance. ACS Infect. Dis. 7(2), 215–253 (2021). https://doi.org/10.1021/acsinfecdis.0c00635
R. Alyssa, B.P. Deline, C.L. Frank, R. Smith, Leslie, N. Sigmon, Alexa, M.J. Wallace, D.G. Gallagher, D.P. Jr. Goodwin, D. Durkin, Howard Fairbrother. Influence of oxygen-containing functional groups on the Environmental Properties, Transformations, and toxicity of Carbon Nanotubes. Chem. Rev. 120(20), 11651–11697 (2020). https://doi.org/10.1021/acs.chemrev.0c00351
N. Amin Valiei, J.-F. Lin, G. Bryche, M. McKay, P.G. Canva, D. Charette, C. Nguyen, Moraes, Nathalie Tufenkji. Hydrophilic mechano-bactericidal Nanopillars Require External Forces to rapidly kill Bacteria. Nano Lett. 20(8), 5720–5727 (2020). https://doi.org/10.1021/acs.nanolett.0c01343
A. Jiajie Qian, R.F. Martinez, M.R. Marek, H. Nagorzanski, E.T. Zhi, D.W. Furlong, H. Kolpin, Gregory, M. LeFevre, David, Cwiertny. Polymeric Nanofiber-Carbon Nanotube Composite Mats as fast-equilibrium Passive Samplers for Polar Organic Contaminants. Environ. Sci. Technol. 54(11), 6703–6712 (2020). https://doi.org/10.1021/acs.est.0c00609
F. Chen, X. Ding, Y. Jiang, Y. Guan, D. Wei, A. Zheng, Permanent antimicrobial poly(vinylidene fluoride) prepared by Chemical Bonding with Poly(hexamethylene guanidine). ACS Omega. 5(18), 10481–10488 (2020). https://doi.org/10.1021/acsomega.0c00626
A. Jordan, A.L. Carver, R.P. Simpson, N. Rathi, A.G. Normil, M.D. Lee, K.A. Force, C.E. Fiocca, M. Maley, Kara, A.L. DiJoseph, A. Goldstein, Amin, L. Attari, Haley, J.G. O’Malley, N.Ã.M. Zaccaro, C.A. McCampbell, J.E. Wentz, M. Long, Lilly, J. McQueen, Francis, B.K. Sirch, M.E. Johnson, M.L. Divis, S.L. Chorney, M. DiStefano, Holly, B.L. Yost, E.A. Greyson, K. Cid, C.J. Lee, M. Yhap, D.L. Dong, B.E. Thomas Banks, Regan B. Newman, Jailene Rodriguez, A.T. Segil, J.A. Siberski, A.L. Lobo, Mark D. Ellison. Functionalized Single-Walled Carbon Nanotubes and Nanographene Oxide to Overcome Antibiotic Resistance in Tetracycline-Resistant Escherichia coli. ACS Applied Nano Materials 2020, 3 (4), 3910–3921. https://doi.org/10.1021/acsanm.0c00677
Y. Qiaofei Pan, W. Cao, D. Xue, W.L. Zhu, Picosecond Laser-Textured Stainless Steel Superhydrophobic Surface with an antibacterial adhesion property. Langmuir. 35(35), 11414–11421 (2019). https://doi.org/10.1021/acs.langmuir.9b01333
Y. Cai, X.-S. Wu, Y. Luo, M.-J. Su, G.-W. Chu, B.-C. Sun, J.-F. Chen, Plasma-Assisted Rotating Disk Reactor toward Disinfection of Aquatic Microorganisms. Industrial & Engineering Chemistry Research 2019, 58 (31), 13977–13986. https://doi.org/10.1021/acs.iecr.9b02562
F. Wu, Y. You, X. Zhang, H. Zhang, W. Chen, Y. Yang, D. Werner, S. Tao, X. Wang, Effects of various Carbon Nanotubes on Soil Bacterial Community Composition and structure. Environ. Sci. Technol. 53(10), 5707–5716 (2019). https://doi.org/10.1021/acs.est.8b06909
B. Oruc, H. Unal, Fluorophore-decorated Carbon Nanotubes with enhanced Photothermal Activity as Antimicrobial Nanomaterials. ACS Omega. 4(3), 5556–5564 (2019). https://doi.org/10.1021/acsomega.9b00099
S. Zhang, Q. Ren, H. Qi, S. Liu, Y. Liu, Adverse Effects of fine-particle exposure on joints and their surrounding cells and Microenvironment. ACS Nano. 13(3), 2729–2748 (2019). https://doi.org/10.1021/acsnano.8b08517
Y. Yue Qiao, H. Ping, B. Zhang, F. Zhou, Y. Liu, T. Yu, W. Xie, D. Li, Y. Zhong, K. Zhang, H.A. Yao, Santos, Min Zhou. Laser-Activatable CuS Nanodots to treat Multidrug-Resistant Bacteria and release copper ion to accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces. 11(4), 3809–3822 (2019). https://doi.org/10.1021/acsami.8b21766
H. Li, J. Huang, Y. Song, M. Zhang, H. Wang, F. Lu, H. Huang, Y. Liu, X. Dai, Z. Gu, Z. Yang, Ruhong Zhou, Zhenhui Kang. Degradable Carbon dots with broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces. 10(32), 26936–26946 (2018). https://doi.org/10.1021/acsami.8b08832
P. Denver, M.D. Linklater, V.A. Volder, M. Baulin, S. Werner, M. Jessl, L. Golozar, S. Maggini, E. Rubanov, S. Hanssen, P. Juodkazis, Elena, Ivanova. High aspect ratio nanostructures kill Bacteria via Storage and Release of Mechanical Energy. ACS Nano. 12(7), 6657–6667 (2018). https://doi.org/10.1021/acsnano.8b01665
A. Douglas, R. Carter, M. Li, Pint. Toward small-diameter Carbon Nanotubes synthesized from captured Carbon Dioxide: critical role of Catalyst Coarsening. ACS Appl. Mater. Interfaces. 10(22), 19010–19018 (2018). https://doi.org/10.1021/acsami.8b02834
D.G. Jr. Goodwin, S. Adeyemi, L. Adeleye, K.T. Sung, R.M. Ho, J. Burgess, Elijah, Petersen. Detection and quantification of Graphene-Family Nanomaterials in the Environment. Environ. Sci. Technol. 52(8), 4491–4513 (2018). https://doi.org/10.1021/acs.est.7b04938
N. Monika Mortimer, D. Devarajan, P.A. Li, Holden, Multiwall Carbon Nanotubes Induce more pronounced transcriptomic responses in Pseudomonas aeruginosa PG201 than Graphene, Exfoliated Boron Nitride, or Carbon Black. ACS Nano. 12(3), 2728–2740 (2018). https://doi.org/10.1021/acsnano.7b08977
P. Parasmani Pageni, Y.P. Yang, Y. Chen, M. Huang, T. Bam, M. Zhu, B.C. Nagarkatti, A.W. Benicewicz, Decho, and Chuanbing Tang. Charged metallopolymer-grafted silica nanoparticles for Antimicrobial Applications. Biomacromolecules. 19(2), 417–425 (2018). https://doi.org/10.1021/acs.biomac.7b01510
A. Prateek Khare, S. Singh, A. Verma, A.K. Bhati, Sonker, Kumud Malika Tripathi, and Sumit Kumar Sonkar. Sunlight-Induced Selective Photocatalytic degradation of Methylene Blue in Bacterial Culture by Pollutant Soot Derived nontoxic graphene nanosheets. ACS Sustain. Chem. Eng. 6(1), 579–589 (2018). https://doi.org/10.1021/acssuschemeng.7b02929
W.N. Arthur, A.L.R. Sloan, J. Santana-Pereira, M.R. Goswami, Liles, A. Virginia, Davis. Single-walled Carbon Nanotube Dispersion in Tryptic Soy Broth. ACS Macro Lett. 2017, 6 (11), 1228–1231. https://doi.org/10.1021/acsmacrolett.7b00656
B. Hongya Geng, J. Yao, K. Zhou, G. Liu, W. Bai, Y. Li, G. Song, M. Shi, Doi, J. Wang, Size fractionation of Graphene Oxide Nanosheets via controlled directional freezing. J. Am. Chem. Soc. 2017, 139 (36), 12517–12523. https://doi.org/10.1021/jacs.7b05490
N. Yadav, A. Dubey, S. Shukla, C.P. Saini, G. Gupta, R. Priyadarshini, Graphene Oxide-Coated Surface: inhibition of bacterial biofilm formation due to specific surface–interface interactions. ACS Omega. 2(7), 3070–3082 (2017). https://doi.org/10.1021/acsomega.7b00371
T.I. Kim, B. Kwon, J. Yoon, I.-J. Park, G.S. Bang, YongKeun, Y.-S. Park, Seo, and Sung-Yool Choi. Antibacterial Activities of Graphene Oxide–Molybdenum Disulfide Nanocomposite Films. ACS Applied Materials & Interfaces 2017, 9 (9), 7908–7917. https://doi.org/10.1021/acsami.6b12464
K.K. Yaqi You, H. Das, C.-W. Guo, M. Chang, J.W. Navas-Moreno, P. Chan, S.R. Verburg, X. Poulson, B. Wang, Xing, Y. Yang, Microbial Transformation of Multiwalled Carbon Nanotubes by Mycobacterium vanbaalenii PYR-1. Environ. Sci. Technol. 51(4), 2068–2076 (2017). https://doi.org/10.1021/acs.est.6b04523
C. Chuanxiong Nie, L. Cheng, J. Ma, Deng, C. Zhao, Mussel-inspired Antibacterial and Biocompatible Silver–Carbon Nanotube Composites: Green and Universal Nanointerfacial Functionalization. Langmuir. 32(23), 5955–5965 (2016). https://doi.org/10.1021/acs.langmuir.6b00708
M. Leanne, E.M. Gilbertson, Z.S. Albalghiti, F.Ã. Fishman, C. Perreault, J.D. Corredor, M. Posner, L.D. Elimelech, Pfefferle, B. Julie, Zimmerman. Shape-dependent surface reactivity and antimicrobial activity of Nano-Cupric Oxide. Environ. Sci. Technol. 50(7), 3975–3984 (2016). https://doi.org/10.1021/acs.est.5b05734
P. Yi, J.J. Pignatello, M. Uchimiya, C. Jason, White. Heteroaggregation of Cerium Oxide Nanoparticles and Nanoparticles of Pyrolyzed Biomass. Environ. Sci. Technol. 49(22), 13294–13303 (2015). https://doi.org/10.1021/acs.est.5b03541
François, Perreault, Andreia Fonseca de Faria, Siamak Nejati, and Menachem Elimelech. Antimicrobial Properties of Graphene Oxide Nanosheets: why size matters. ACS Nano. 9(7), 7226–7236 (2015). https://doi.org/10.1021/acsnano.5b02067
D.G. Jr. Goodwin, K.M. Marsh, I.B. Sosa, J.B. Payne, J.M. Gorham, E.J. Bouwer, Fairbrother. Interactions of microorganisms with polymer nanocomposite Surfaces containing oxidized Carbon Nanotubes. Environ. Sci. Technol. 49(9), 5484–5492 (2015). https://doi.org/10.1021/acs.est.5b00084
L. Anee Mohanty, L. Wei, Y. Lu, Chen, B. Cao, Impact of sublethal levels of single-wall Carbon Nanotubes on Pyoverdine Production in Pseudomonas aeruginosa and its environmental implications. Environ. Sci. Technol. Lett. 2(4), 105–111 (2015). https://doi.org/10.1021/acs.estlett.5b00057
R.-V. Santiago, Castrillón, François Perreault, Andreia Fonseca de Faria, and Menachem Elimelech. Interaction of Graphene Oxide with bacterial cell membranes: insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2(4), 112–117 (2015). https://doi.org/10.1021/acs.estlett.5b00066
G. Justin, S.A. Clar, S. Gustitus, C.A. Youn, K.J. Silvera Batista, Ziegler, J. Jean Claude, Bonzongo. Unique toxicological behavior from single-wall Carbon Nanotubes separated via selective adsorption on hydrogels. Environ. Sci. Technol. 49(6), 3913–3921 (2015). https://doi.org/10.1021/es505925m
Sudarshan Kurwadkar Xiaoqi, (Zhang Forrest Mitchell David Ramirez. Introduction. 1–16 (2015). https://doi.org/10.1021/bk-2015-1198.ch001
Y. Jin Song, D. Li, D. Ke, X.-E. Wang, Zhang, Situ graphene-modified carbon microelectrode array biosensor for biofilm impedance analysis. Electrochim. Acta. 403, 0013–4686 (2022). https://doi.org/10.1016/j.electacta.2021.139570
N.M. Jayathilake, D. Koley, Glucose microsensor with covalently immobilized glucose oxidase for probing bacterial glucose uptake by scanning electrochemical microscopy. Anal. Chem. 92, 3589–3597 (2020). https://doi.org/10.1021/acs.analchem.9b04284
F. Shu, Situ, A. Cristina, Samia. Highly efficient Antibacterial Iron Oxide@Carbon Nanochains from Wüstite Precursor Nanoparticles. ACS Appl. Mater. Interfaces. 6(22), 20154–20163 (2014). https://doi.org/10.1021/am505744m
H.S. Bradley Beless, Rifai, F. Debora, Rodrigues. Efficacy of Carbonaceous materials for Sorbing Polychlorinated Biphenyls from Aqueous Solution. Environ. Sci. Technol. 48(17), 10372–10379 (2014). https://doi.org/10.1021/es502647n
F. Yvonne Ligaya, C.M. Musico, P. Santos, Maria Lourdes, Dalida, F. Debora, Rodrigues. Surface modification of membrane filters using Graphene and Graphene Oxide-Based nanomaterials for bacterial inactivation and removal. ACS Sustain. Chem. Eng. 2(7), 1559–1565 (2014). https://doi.org/10.1021/sc500044p
H. Haitao Wang, W. Ma, Zheng, Dingding An, and Chongzheng na. Multifunctional and recollectable Carbon Nanotube Ponytails for Water Purification. ACS Appl. Mater. Interfaces. 6(12), 9426–9434 (2014). https://doi.org/10.1021/am501810f
R. Julia Wehling, R.N. Dringen, M. Zare, Maas, K. Rezwan, Bactericidal activity of partially oxidized nanodiamonds. ACS Nano. 8(6), 6475–6483 (2014). https://doi.org/10.1021/nn502230m
B. Zhu, X. Xia, N. Xia, S. Zhang, X. Guo, Modification of fatty acids in membranes of Bacteria: implication for an adaptive mechanism to the toxicity of Carbon Nanotubes. Environ. Sci. Technol. 48(7), 4086–4095 (2014). https://doi.org/10.1021/es404359v
Peng Yi and Kai Loon Chen, Influence of Solution Chemistry on the release of Multiwalled Carbon Nanotubes from silica surfaces. Environ. Sci. Technol. 47(21), 12211–12218 (2013). https://doi.org/10.1021/es403133r
M. Haiyan Yang, Tong, H. Kim, Effect of Carbon Nanotubes on the Transport and Retention of Bacteria in Saturated Porous Media. Environ. Sci. Technol. 47(20), 11537–11544 (2013). https://doi.org/10.1021/es4022415
M. Leanne, R.C. Pasquini, A.Ã.D. Sekol, L.D. Taylor, Pfefferle, Zimmerman. Realizing comparable oxidative and cytotoxic potential of single- and Multiwalled Carbon Nanotubes through Annealing. Environ. Sci. Technol. 47(15), 8775–8783 (2013). https://doi.org/10.1021/es401786s
D. Liu Shi, M.U. Shi, D.E. Nollert, Resasco, A. Striolo, Single-walled Carbon Nanotubes do not Pierce Aqueous Phospholipid Bilayers at Low Salt Concentration. J. Phys. Chem. B 117(22), 6749–6758 (2013). https://doi.org/10.1021/jp4039336
Peng Yi and Kai Loon Chen, Interaction of Multiwalled Carbon Nanotubes with supported lipid bilayers and vesicles as Model Biological membranes. Environ. Sci. Technol. 47(11), 5711–5719 (2013). https://doi.org/10.1021/es4002604
Funding
No funding.
Author information
Authors and Affiliations
Contributions
D.M and M.S have drafted the manuscript. D.L has helped in drawing the figures and tables. A.G, and M.N have done the necessary corrections and editing.
Corresponding author
Ethics declarations
Competing Interests
None to declare.
Ethics Approval
Not applicable.
Consent to Participate
The authors have the consent to participate.
Consent to Publish
The authors have the consent to publish.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukherjee, D., Sil, M., Goswami, A. et al. Antibiofilm Activities of Carbon-Based Nanoparticles and Nanocomposites: A Comparative Review. J Inorg Organomet Polym 33, 3961–3983 (2023). https://doi.org/10.1007/s10904-023-02732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02732-7