Abstract
The past few decades have seen revolutions in the applications of nanomaterials in different walks of science. One of the significant applications in healthcare is the use of nanoparticles (NP) in killing both free floating and biofilm forming bacteria. Several nanoparticles like CuO, Fe3O4, TiO2, ZnO, MgO and Al2O3 NPs have been proven to achieve this feature with varying efficacies. A more advanced and efficient way to disrupt bacterial biofilms is the use of nanocomposite (NC) materials to eliminate bacteria. Along with various metal oxides, materials like graphene and chitosan can also be used to create various types of NC. One of the biggest advantages of NP and NC over antibiotics is their ability to circumvent the problem of bacterial resistance. The mechanisms by which NC disrupts biofilms, synthesis and characterization of NC and their relative advantages and limitations are discussed in this chapter. With the ever-increasing incidences of diseases caused by multidrug resistant and biofilm forming bacteria, there is an urgent need to devise materials like nanocomposites with a broader spectrum of action.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction to Biofilms
Bacterial infections are becoming a huge threat to mankind due to the emergence of multi-drug-resistant bacteria. These organisms deploy several strategies to defend the effects of drugs by possessing R plasmids, multi-drug efflux pumps, integrons, transposons or type IV secretion systems (Alavi and Karimi 2018). Another predominant reason that helps a few bacterial species to develop resistance against several drugs is the ability to form biofilms. A biofilm is a group of cells held together by a mesh-like framework given by proteins, exopolysaccharides, DNA and lipopeptides in the matrix (Nirwati et al. 2019). Some of the most common organisms that have the ability to form biofilms and are extensively responsible in causing nosocomial infections in patients are Gram-negative organisms such as Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli and Gram-positive organisms such as Staphylococcus aureus and Streptococcus pyogenes (Khan et al. 2017). When bacteria are protected within a biofilm, they tolerate the antibiotics, host defence system and any form of stress quite easily (Sharma et al. 2019). Depletion or unavailability of nutrients, slow growth rate, low penetration power of antibiotics and dormancy of cells are few characteristic features seen in a biofilm that impart resistance against various drugs (Stewart 2002). The most common infections caused by biofilm formers are sinusitis, cystic fibrosis, chronic obstructive pulmonary disease, otitis media and chronic wounds (van Tilburg Bernardes et al. 2015). Cells in a biofilm have been observed to be 10–1000 times more resistant to antibiotics when compared to the planktonic cells (Sharma et al. 2019). When a biofilm is disrupted, the cells are dispersed to their original planktonic state and become susceptible to the same antibiotics that they were resistant to, when they were enclosed in the biofilm (Berlanga et al. 2017). The scenario gets challenging when biofilms are established on medical devices such as catheters and implants which prove that bacteria can form biofilms on both biotic and abiotic surfaces. They are able to stay together as a community predominantly because of the presence of appendages like pili and flagella or in some other cases, electrostatic attraction or van der Waal’s forces (Paluch et al. 2020). The first step in biofilm formation is attachment of cells to either the host tissue or any abiotic surface. Secondly, they are capable of producing certain signalling molecules that further alter gene expression in the favour of the bacterial species (Ivanova et al. 2020).
However, biofilms have positive aspects too. For example, they act as biological controls against pathogens infecting plants, as biofertilizers for improved production of crops, to mitigate hazardous pollutants from the environment, for treating waste water and to prevent corrosion. Although biofilms are helpful in agricultural and industrial domains, research is predominantly being done to combat the problems caused by biofilm-forming bacteria in the medical field. Biofilms are considered to be a master plan strategized by bacteria to survive in environments that give them too little to sustain (Muhammad et al. 2020). They achieve this by mere cell-to-cell communication with the neighbouring bacteria. This communication is scientifically termed as quorum sensing.
5.1.1 Quorum Sensing
Bacterial cells make use of a phenomenon called quorum sensing to detect the number of organisms in the surrounding. They produce chemical molecules called auto inducers (AIs) that facilitate in measuring the cell density in a given site. In Gram-negative organisms, AIs are acyl homoserine lactones (AHLs), and Gram-positive organisms on the contrary produce short oligopeptides as their AIs. These signalling molecules diffuse from the cell to the exterior in a natural process. When many cells are present in a specific location, the concentration of AIs in the surrounding is more than the concentration of AIs within the cells. Therefore, to strike a balance, some AIs diffuse back into the cells and thereby increase the concentration of AIs within the cells to collectively affect the transcription of few genes that are responsible for virulence, sporulation, biofilm formation, bioluminescence, antibiotic production or competence in bacteria (Rutherford and Bassler 2012).
Biofilms are indirectly responsible for promoting multiple drug resistance in several bacterial species as they bring cells close to each other, making it extremely easy for conjugation to occur. R plasmids present in a specific strain/species can be passed on to the surrounding cells via horizontal gene transfer (HGT), thereby making them resistant to various drugs even in the planktonic stage (Madsen et al. 2012). These are the main reasons which makes it imperative to find a novel solution to tackle the menace created by biofilm formers.
Instead of disrupting the bacterial biofilm, scientists have been working towards preventing the communication between bacteria. This has been a practical move and has been found to be efficient due to molecules known as quorum quenchers. The target molecules here are the AIs without a doubt. They work in one or more ways against AIs. Enzymes like AHL lactonase and AHL acylases specifically hydrolyse homoserine lactone rings and amide bonds, respectively. Enzymes like oxidoreductases work a little differently by modifying the AHL molecules, so that they lose their function. The third strategy is to use inductor antagonists that can compete with AHLs for the binding site on the receptor or can non-competitively bind to the receptor to block the signal cascade reactions lined up (Paluch et al. 2020). For example, halogenated furanones isolated from marine red algae have structural similarity to the signalling molecules and have been classified under quorum sensing inhibitors (QSIs) (Hayat et al. 2019). In the last two cases, the auto inducers are not destroyed, but the ultimate goal of preventing biofilm formation is achieved.
5.2 Nanoparticles
The field of nanotechnology started establishing itself when inventions such as the scanning tunnel microscope and atomic force microscopes were made, as they play a major role in imaging surfaces at the atomic level (Ferdous and Nemmar 2020). Gold, platinum and silver nanoparticles (AgNPs) have gained utmost importance in the field of medical research. Specifically pertaining to silver, a lot of experiments are being done as it has been effective against a broad spectrum of pathogens since ancient times. Silver has been a part of several ointments and other topical agents to prevent any forms of bacterial infections on open wounds and burns (Mohanta et al. 2020). Considering their efficacy, nanotechnology is undoubtedly one of the best ways to target biofilms with the help of AgNPs or nanocomposites. The advantage nanoparticles have when compared to any other method used against biofilms is their tremendously small size that provides a large surface area, high reactivity and the ability to easily penetrate through the biofilm matrix. Their large surface area makes them a very good drug carrier (Qayyum and Khan 2016). Numerous physical and chemical methods to synthesize these particles had gained popularity until scientists analysed the harmful impact on the environment. In the recent past, eco-friendly methods have been identified to produce AgNPs. Once synthesized, they are also combined with polymers to form nanocomposites (Awad et al. 2015).
5.2.1 Synthesis of Nanoparticles
Mostly, silver nitrate (AgNO3) solution is used as the precursor molecule of silver. Rarely, silver wires and silver sulphate are in use as well. A reducing agent and a stabilizing agent are mandatory to synthesize AgNPs. The reducing agent is added to AgNO3 solution to convert silver ions into elemental silver. Once, elemental silver starts forming, there is a visible colour change in the solution as well as agglomeration of these particles. Agglomeration reduces the surface exposure of nanoparticles drastically, and therefore, to prevent it from happening, it is a must to make use of a stabilizing agent. But the biggest disadvantage of using these reducing agents and stabilizers is their deleterious effects on the environment. Hence, scientists have recently started using greener protocols to synthesize nanoparticles (Iravani et al. 2014).
5.2.1.1 Green Synthesis of Nanoparticles
Greener alternatives for the synthesis of AgNPs focus on the use of actinomycete (Hamed et al. 2020), bacteria, fungi or plants as the source of enzymes required for the reduction of silver. Fungi are more beneficial when compared to bacteria as they produce extracellular enzymes in bulk amounts which helps in reducing the steps involved in downstream processing. For example, the white rot fungi Pycnoporus sp. can be grown in malt extract for about 5 days at 32 ° C in an orbital shaker incubator. After 5 days of incubation, the mycelium should be filtered out using a Whatman filter paper, and equal amounts of AgNO3 solution should be added at 1 mM concentration to the filtrate. The solution must be further kept in dark conditions inside a shaker incubator and later characterized using UV-Vis spectroscopy. The exact mechanism behind this method is not very clear. However, it is assumed that the fungal cell produces an extracellular enzyme called NADH dependent nitrate reductase which reduces the silver ions when they come in contact with the fungal cell walls. To be sure of the synthesis of AgNPs, a positive control (AgNO3 solution and deionized water) and a negative control (AgNO3 solution) can be kept in the same conditions (Gudikandula and Charya Maringanti 2016).
Plants are another source of multiple secondary metabolites that can be used for the production of AgNPs. Different parts of the plants can be procured, dried and powdered using a blender. The coarse powder must then be added into deionized water and sonicated. The solution should be filtered and about 10 ml of the filtrate should be added to 90 ml of AgNO3 solution. This solution is incubated overnight at 60 °C. Gradually a visible colour change is observed which indicates the formation of AgNPs (Mohanta et al. 2020).
5.2.2 Characterization of Nanoparticles
There are umpteen number of methods used to characterize silver nanoparticles. The parameters taken into consideration for their characterization are size, surface charge, shape and distribution (Carvalho et al. 2018). A surface plasmon band is a characteristic feature of silver nanoparticles that can be observed using UV-Vis spectrophotometer. In an experiment conducted by Krishna Gudikandula et al., a comparison was drawn between the surface plasmon band obtained with chemically synthesized silver nanoparticles and biologically synthesized silver nanoparticles to give an absorption peak at 430 and 420 nm, respectively. These readings were typical of silver nanoparticles (Gudikandula and Charya Maringanti 2016). Dynamic light scattering (DLS) spectroscopy is another method used to confirm the synthesis of nanoparticles. When light is incident on a particle, the light reflects at a specific angle and the angle between the incident light and reflected light can be calculated over time. Bigger particles reflect the incident light slowly, whereas smaller particles such as the nanoparticles reflect the incident light so quickly that it gets difficult to calculate the photon correlation with respect to time. This is repeated several times to get a precise value (Carvalho et al. 2018).
5.3 Nanocomposites
The solutions to various problems existing in the medical field today are nanocomposites. Nanocomposites are substances made of materials that possess multiple phases, and each of those phases has up to three dimensions in nanometre size. Nanocomposites can be broadly classified into three types: metal matrix nanocomposite (MMNC), ceramic matrix nanocomposite (CMNC) and polymer matrix nanocomposite (PMNC) (Omanović-Mikličanin et al. 2020).
Metal matrix nanocomposites (MMNCs): Metal matrix nanocomposites comprise ductile metal in which nanosized reinforcement is fixed. Due to its high ductility, strength, and toughness, they are extensively used in the aerospace industries.
Ceramic matrix nanocomposites (CMNCs): In ceramic matrix composites, one or more ceramic phases are added to augment the chemical stability and resist wear and tear. However, ceramic matrix composites have a disadvantage. They are extremely brittle and therefore not in much demand in the industrial domain. To overcome this, CMNCs were developed which were tougher than ceramic matrix composites. Polymer matrix nanocomposites (PMNCs): PMNCs are constructed with the help of fillers which are also called nanofillers. These nanofillers are broadly divided into 1D (linear), 2D (layered) and 3D (powdered) forms. The best example of 1D, 2D and 3D forms are carbon nanotubes, montmorillonite and silver nanoparticles (Omanović-Mikličanin et al. 2020).
5.3.1 Preparation of Nanocomposites
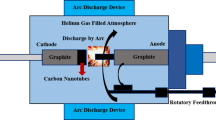
During the synthesis of nanocomposites, at least one dimension of the various layers of inorganic or organic material are fillers. These fillers must be less than 100 nm in size (Fawaz and Mittal 2014). There are several methods by which nanocomposites can be synthesized. In situ polymerization method is mainly used to synthesize nanocomposites made of graphite. Graphite does not have charged groups naturally present on its surface, and therefore ionic interaction between graphite and the polymer would be difficult. However, the expanded graphite has pores big enough (2–10μm) such that the polymer material remains embedded within the pores even after the solvent has been extracted (Fu et al. 2019).
In the solution mixing method, the nanofillers are allowed to swell up in a solvent in which the polymer is soluble. The solvents used can be water, chloroform, toluene, etc. The nanofillers in use are most of the time layered silicates. When these silicates are mixed with polymers vigorously, the polymers intercalate between the layered silicates by displacing the solvent. Perfect mixing is obtained by magnetic stirring or ultrasonication followed by slow evaporation of the solvent and casting of the nanocomposite (Rane et al. 2018).
Sol-gel technique is used extensively in ceramic engineering (Shahjahan 2017). A widely used substance in this technique is tetra ethyl ortho silicate (TEOS) as it is highly efficient in forming networks (Owens et al. 2016). Sol is a substance that has solid materials distributed in a solution. They are in a colloidal state. They undergo slow hydrolysis reactions and thereafter polymerize to form a gel. The crystals grow which allows the polymers to seep between the various layers, thus forming nanocomposites (Khan et al. 2016).
5.3.2 Characterization of Nanocomposites
Various methods like FT-IR, TEM, TGA and XRD are used to characterize nanocomposites. FT-IR spectra reveal a lot about the functional groups present in a given sample based on the bond stretching observed as peaks. The peaks of AgNPs should not be showing any peaks at the carbonyl frequency region assuring the absence of the stabilizing agents like acetate added during the synthesis of nanocomposites. TEM has the ability to image the synthesized materials on a nanometre scale. The dispersion quality of nanoparticles can also be observed. It is important to confirm that a homogenous mixture is obtained such that there are no agglomerates of nanoparticles. These factors can be clearly visualized using a TEM image (Puggal et al. 2016). XRD makes use of wide-angle X-ray diffraction to check the crystalline nature, exfoliation and intercalation of polymers between nanoclay layers and dispersion of the nanoparticles within the polymer matrix. These parameters can be calculated using Bragg’s law and Scherrer’s law (El-Sheikhy and Al-Shamrani 2015). TGA can also be used to characterize NC. When polymers contain substances such as nanotubes and montmorillonite, the temperature at which thermal degradation happens is increased, i.e. thermal stability is enhanced. This is widely seen in polymethyl methacrylate (PMMA), polydimethylsiloxane (PDMS), polyamide and polypropylene. The reason for greater thermal stability is because of char formation as suggested by many researchers. The permeability is reduced, and the char thus formed, blocks the outward movement of the decomposed products on degradation. This quality is a characteristic feature of nanocomposites when compared to polymers without nanoparticles (Corcione and Frigione 2012).
5.3.3 Antibiofilm Activity of Nanocomposites
Two materials that are gaining a lot of importance in the field of nanotechnology are polyvinyl alcohol (PVA) and chitosan (CS) for their very good level of biodegradability and biocompatibility. In a study done by Omnia M Abdallah et al., PVA and CS were mixed with biologically synthesized AgNPs to test their antimicrobial and antibiofilm activities. Both the solutions had 0.1% of AgNPs. Once the solutions were thoroughly mixed, they were casted into a petri dish each and further placed in a desiccator. To remove any further residual water or solvent molecules, the plates were kept at 60 °C. These nanofilms thus formed were used to check their antimicrobial, antibiofilm and cytotoxic effects. The films without the addition of AgNPs were set as controls (Abdallah et al. 2020).
5.3.3.1 Application in Biomedical Devices
Titanium is a material that has been extensively used in implants. Likewise, infections have been unavoidable even after sterilizing and disinfecting the implant before surgeries (Corrêa et al. 2015). In a study conducted by Secenti et al., 20 New Zealand rabbits were deliberately injected with bacteria at the surgical site on iliac crests. One group had screws coated with AgNPs using the sol gel technique, and the other group had titanium screws without AgNPs. After a duration of 28 days, the rabbits were sacrificed, following which the screws and adjacent bones were tested for biofilm formation with the help of TEM and SEM. Observations concluded that AgNP-coated screws did not entertain biofilm formation, whereas the uncoated screws favoured biofilm formation drastically (Sivolella et al. 2012).
Another important medical scenario observed in the field of dentistry is stomatitis. It is a condition majorly caused by the organism Candida albicans that colonizes the rough edges of the inner surfaces of complete dentures. Predominantly, geriatric prosthetic wearers succumb to stomatitis because of reduced motor dexterity, memory loss, and cognitive impairment. However, to overcome this problem various experiments were carried out to modify the material used for dentures to enhance antimicrobial activity as most antifungal agents are not very effective against cells in a biofilm. Poly methyl methacrylate acrylic (PMMA) resin that comprises 1μg/ml AgNP has been found to reduce the adherence of Candida spp. on the denture and thereby inhibit biofilm formation. In addition, the modified denture did not show any forms of cytotoxic or genotoxic effects (Corrêa et al. 2015).
There have been various such materials used as nanocomposites that have been successful against biofilm-forming pathogens. A few of them have been summarized in Fig. 5.1.
5.3.4 Scope of Nanocomposites as Biofilm Disrupting Agents
The multifunctional properties of nanocomposites make them ideal candidates for sustainable therapeutic agents against bacterial biofilms either directly or by conjugating with antimicrobial agents. Conjugation of polymeric nanomaterials in drug delivery has been prevalent in the medical field over the past few decades (Kumar et al. 2018; Umesh et al. 2018). Nanocomposites can be successfully employed for the delivery of phytochemical compounds specifically to biofilms, thereby solving the issue associated with hydrophobicity that limits their accessibility to the biofilms (Barros and Casey 2020). Enhancement of antibiofilm activity of nanocomposites can be carried out through surface functionalization with active metabolites through either covalent or noncovalent conjugation (Pathak 2019). Nanocomposites represent multiphase systems and are visualized as alternatives to overcome the limitations of microcomposites and monolithics and have the potential to become the materials of the future (Omanović-Mikličanin et al. 2020). Although the in-depth understanding regarding the effect of nanocomposites on human health and environment is not fully explored, recent research works suggest that functionalized nanocomposites can be less toxic to immune cells and thus offer a hope to be used as antibiofilm agents in in vivo conditions. In spite of having potential applications as antibiofilm agents in laboratory studies, the application of nanocomposites as therapeutic agents against biofilm-forming bacteria still faces a lot of hurdles. Translation potential of these nanocomposites from a lab scale to real-life therapeutic application is extremely challenging. Most of the reported applications of antibiofilm properties of nanocomposites were related to oral biofilm or periodontitis, the possibility of extending nanocomposites to hinder the biofilm-forming bacteria in respiratory and urogenital infection needs more focus. Another major constraint to solve is the production cost associated with nanocomposite synthesis (Ramasamy and Lee 2016). Green synthesis of nanocomposites with plant extracts was reported to have high antimicrobial activity along with the reduction in synthesis cost. This method also is eco-friendly as it reduces the usage of solvents, and the synthesized composites are likely to be biocompatible (Mondal et al. 2020). As the field of nanocomposite is relatively new and a multidisciplinary field encompassing science, technology and engineering, significant research and development in these sectors can truly revolutionize the application of nanocomposites against biofilm-forming pathogens.
5.3.5 Limitations of Nanocomposites as Biofilm Disrupting Agents
Most of the NC works by generating reactive oxygen species (ROS) which can hamper bacterial growth and multiplication. But it has been observed that ROS generation does not always directly cause cell death. Gene expression analysis has shown that ZnO NP can even inhibit the expression of oxidative stress genes (Kadiyala et al. 2018). The advantages of NC include their large surface area and high reactivity. These positive aspects can also lead to some side effects. Also chemically synthesized NPs can have toxicity issues due to the use of dangerous compounds during their synthesis some of which may remain in trace amounts in the NP and cause undesirable effects (Römling et al. 2005). In order to circumvent this problem, an environmentally friendly and less toxic approach called the “green synthesis” can be resorted to (Salem and Fouda 2020). Another aspect to be looked at is the mutagenicity of these nanostructures. Inadequate levels of NPs may not destroy the biofilms, but may induce mutations, which can lead to the emergence of “super mutants”. Tungsten oxide NP has been shown to directly interact with DNA and cause single-strand breaks. So even though the majority of the bacterial cells were killed, few remaining were found to be mutants (Thongkumkoon et al. 2014). The degree of horizontal gene transfer was also more pronounced with the use of aluminium oxide NPs with the evidence of a bacterium being conjugated to many other bacteria (Qiu et al. 2012). Despite the several promises they offer; nanocomposites have some limitations as well. Our primary intention in using nanocomposites is to eradicate the pathogenic microbes. But the non-specificity of various NC may lead to elimination of symbiotic organisms as well (Qayyum and Khan 2016). This can lead to disruptions in the normal microflora composition.
5.4 Conclusions and Outlook
Biofilms have been a serious problem in the health sector for a while. Bacteria evolving themselves into superbugs that are resistant to over 20 different drugs have come into existence, and it is definitely the need of the hour to find novel solutions. The use of nanoparticles and nanocomposites in the field of medicine is a promising tool. However, the application of these methods is currently restricted to only implants or other medical devices and topical agents. A solution must be found to deal with biofilm-forming pathogens that cause severe lung infections or urinary tract infections. A lot is yet to be unravelled in this emerging field of nanotechnology to overcome this challenge of multiple drug resistance completely.
References
Abdallah, O. M., EL-Baghdady, K. Z., Khalil, M. M. H., El Borhamy, M. I., & Meligi, G. A. (2020). Antibacterial, antibiofilm and cytotoxic activities of biogenic polyvinyl alcohol-silver and chitosan-silver nanocomposites. Journal of Polymer Research, 27(3), 74. https://doi.org/10.1007/s10965-020-02050-3.
Abdulkareem, E. H., Memarzadeh, K., Allaker, R. P., Huang, J., Pratten, J., & Spratt, D. (2015). Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. Journal of Dentistry, 43(12), 1462–1469. https://doi.org/10.1016/j.jdent.2015.10.010.
Alavi, M., & Karimi, N. (2018). Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag–TiO 2, TiO 2 –Ag, Ag–Cu and Cu–Ag nanocomposites against multi-drug-resistant bacteria. Artificial Cells, Nanomedicine, and Biotechnology, 46(Suppl 3), S399–S413. https://doi.org/10.1080/21691401.2018.1496923.
Awad, M. A., Mekhamer, W. K., Merghani, N. M., Hendi, A. A., Ortashi, K. M. O., Al-Abbas, F., & Eisa, N. E. (2015). Green synthesis, characterization, and antibacterial activity of silver/polystyrene nanocomposite. Journal of Nanomaterials, 2015, 1–6. https://doi.org/10.1155/2015/943821.
Barros, C. H. N., & Casey, E. (2020). A review of nanomaterials and technologies for enhancing the antibiofilm activity of natural products and phytochemicals. ACS Applied Nano Materials, 3(9), 8537–8556. https://doi.org/10.1021/acsanm.0c01586.
Berlanga, M., Gomez-Perez, L., & Guerrero, R. (2017). Biofilm formation and antibiotic susceptibility in dispersed cells versus planktonic cells from clinical, industry and environmental origins. Antonie Van Leeuwenhoek, 110(12), 1691–1704. https://doi.org/10.1007/s10482-017-0919-2.
Carvalho, P. M., Felício, M. R., Santos, N. C., Gonçalves, S., & Domingues, M. M. (2018). Application of light scattering techniques to nanoparticle characterization and development. Frontiers in Chemistry, 6, 237. https://doi.org/10.3389/fchem.2018.00237.
Corcione, C., & Frigione, M. (2012). Characterization of nanocomposites by thermal analysis. Materials, 5(12), 2960–2980. https://doi.org/10.3390/ma5122960.
Corrêa, J. M., Mori, M., Sanches, H. L., da Cruz, A. D., Poiate, E., & Poiate, I. A. V. P. (2015). Silver nanoparticles in dental biomaterials. International Journal of Biomaterials, 2015, 1–9. https://doi.org/10.1155/2015/485275.
El-Sheikhy, R., & Al-Shamrani, M. (2015). On the processing and properties of clay/polymer nanocomposites CPNC. Latin American Journal of Solids and Structures, 12(2), 385–419. https://doi.org/10.1590/1679-78251399.
Fawaz, J., & Mittal, V. (2014). Synthesis of polymer nanocomposites: Review of various techniques. In V. Mittal (Ed.), Synthesis techniques for polymer nanocomposites (pp. 1–30). New York: Wiley-VCH Verlag. https://doi.org/10.1002/9783527670307.ch1.
Ferdous, Z., & Nemmar, A. (2020). Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. International Journal of Molecular Sciences, 21(7), 2375. https://doi.org/10.3390/ijms21072375.
Fu, S., Sun, Z., Huang, P., Li, Y., & Hu, N. (2019). Some basic aspects of polymer nanocomposites: A critical review. Nano Materials Science, 1(1), 2–30. https://doi.org/10.1016/j.nanoms.2019.02.006.
Gudikandula, K., & Charya Maringanti, S. (2016). Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. Journal of Experimental Nanoscience, 11(9), 714–721. https://doi.org/10.1080/17458080.2016.1139196.
Gunputh, U., Le, H., Besinis, A., Tredwin, C., & Handy, R. (2019). Multilayered composite coatings of titanium dioxide nanotubes decorated with zinc oxide and hydroxyapatite nanoparticles: Controlled release of Zn and antimicrobial properties against Staphylococcus aureus. International Journal of Nanomedicine, 14, 3583–3600. https://doi.org/10.2147/IJN.S199219.
Hamed, A. A., Kabary, H., Khedr, M., & Emam, A. N. (2020). Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Advances, 10(17), 10361–10367. https://doi.org/10.1039/C9RA11021F.
Hayat, S., Muzammil, S., Shabana, A. B., Siddique, M. H., Saqalein, M., & Nisar, M. A. (2019). Quorum quenching: Role of nanoparticles as signal jammers in Gram-negative bacteria. Future Microbiology, 14(1), 61–72. https://doi.org/10.2217/fmb-2018-0257.
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of silver nanoparticles: Chemical, physical and biological methods. Research in Pharmaceutical Sciences, 9(6), 385–406.
Ivanova, A., Ivanova, K., Tied, A., Heinze, T., & Tzanov, T. (2020). Layer-by-layer coating of aminocellulose and quorum quenching acylase on silver nanoparticles synergistically eradicate bacteria and their biofilms. Advanced Functional Materials, 30(24), 2001284. https://doi.org/10.1002/adfm.202001284.
Kadiyala, U., Turali-Emre, E. S., Bahng, J. H., Kotov, N. A., & VanEpps, J. S. (2018). Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale, 10(10), 4927–4939. https://doi.org/10.1039/C7NR08499D.
Khan, W. S., Hamadneh, N. N., & Khan, W. A. (2016). Polymer nanocomposites – Synthesis techniques, classification and properties. In Science and applications of tailored nanostructures. Portland: One Central Press (OCP).
Khan, H. A., Baig, F. K., & Mehboob, R. (2017). Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific Journal of Tropical Biomedicine, 7(5), 478–482. https://doi.org/10.1016/j.apjtb.2017.01.019.
Kumar, S., Sarita Nehra, M., Dilbaghi, N., Tankeshwar, K., & Kim, K.-H. (2018). Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Progress in Polymer Science, 80, 1–38.
Madsen, J. S., Burmølle, M., Hansen, L. H., & Sørensen, S. J. (2012). The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunology & Medical Microbiology, 65(2), 183–195. https://doi.org/10.1111/j.1574-695X.2012.00960.x.
Mohanta, Y. K., Biswas, K., Jena, S. K., Hashem, A., Abd-Allah, E. F., & Mohanta, T. K. (2020). Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Frontiers in Microbiology, 11, 1143. https://doi.org/10.3389/fmicb.2020.01143.
Mondal, P., Anweshan, A., & Purkait, M. K. (2020). Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere, 259, 127509. https://doi.org/10.1016/j.chemosphere.2020.127509.
Muhammad, M. H., Idris, A. L., Fan, X., Guo, Y., Yu, Y., Jin, X., Qiu, J., Guan, X., & Huang, T. (2020). Beyond risk: Bacterial biofilms and their regulating approaches. Frontiers in Microbiology, 11, 928. https://doi.org/10.3389/fmicb.2020.00928.
Muñoz-Bonilla, A., Cerrada, M., Fernández-García, M., Kubacka, A., Ferrer, M., & Fernández-García, M. (2013). Biodegradable polycaprolactone-titania nanocomposites: Preparation, characterization and antimicrobial properties. International Journal of Molecular Sciences, 14(5), 9249–9266. https://doi.org/10.3390/ijms14059249.
Muthuchamy, M., Govindan, R., Shine, K., Thangasamy, V., Alharbi, N. S., Thillaichidambaram, M., Khaled, J. M., Wen, J.-L., & Alanzi, K. F. (2020). Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumoniae. Carbohydrate Polymers, 230, 115646. https://doi.org/10.1016/j.carbpol.2019.115646.
Necula, B. S., Fratila-Apachitei, L. E., Zaat, S. A. J., Apachitei, I., & Duszczyk, J. (2009). In vitro antibacterial activity of porous TiO2–Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomaterialia, 5(9), 3573–3580. https://doi.org/10.1016/j.actbio.2009.05.010.
Nirwati, H., Sinanjung, K., Fahrunissa, F., Wijaya, F., Napitupulu, S., Hati, V. P., Hakim, M. S., Meliala, A., Aman, A. T., & Nuryastuti, T. (2019). Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proceedings, 13(S11), 20. https://doi.org/10.1186/s12919-019-0176-7.
Noreen, Z., Khalid, N. R., Abbasi, R., Javed, S., Ahmad, I., & Bokhari, H. (2019). Visible light sensitive Ag/TiO2/graphene composite as a potential coating material for control of campylobacter jejuni. Materials Science and Engineering: C, 98, 125–133. https://doi.org/10.1016/j.msec.2018.12.087.
Omanović-Mikličanin, E., Badnjević, A., Kazlagić, A., & Hajlovac, M. (2020). Nanocomposites: A brief review. Health and Technology, 10(1), 51–59. https://doi.org/10.1007/s12553-019-00380-x.
Owens, G. J., Singh, R. K., Foroutan, F., Alqaysi, M., Han, C.-M., Mahapatra, C., Kim, H.-W., & Knowles, J. C. (2016). Sol–gel based materials for biomedical applications. Progress in Materials Science, 77, 1–79. https://doi.org/10.1016/j.pmatsci.2015.12.001.
Paluch, E., Rewak-Soroczyńska, J., Jędrusik, I., Mazurkiewicz, E., & Jermakow, K. (2020). Prevention of biofilm formation by quorum quenching. Applied Microbiology and Biotechnology, 104(5), 1871–1881. https://doi.org/10.1007/s00253-020-10349-w.
Pathak, Y. V. (Ed.). (2019). Surface modification of nanoparticles for targeted drug delivery (1st ed.). New York: Springer. https://doi.org/10.1007/978-3-030-06115-9.
Puggal, S., Dhall, N., Singh, N., & Litt, M. S. (2016). A review on polymer nanocomposites: Synthesis, characterization and mechanical prop. Indian Journal of Science and Technology, 9(4). https://doi.org/10.17485/ijst/2016/v9i4/81100.
Qayyum, S., & Khan, A. U. (2016). Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. MedChemComm, 7(8), 1479–1498. https://doi.org/10.1039/C6MD00124F.
Qiu, Z., Yu, Y., Chen, Z., Jin, M., Yang, D., Zhao, Z., Wang, J., Shen, Z., Wang, X., Qian, D., Huang, A., Zhang, B., & Li, J.-W. (2012). Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proceedings of the National Academy of Sciences of the United States of America, 109(13), 4944–4949. https://doi.org/10.1073/pnas.1107254109.
Ramasamy, M., & Lee, J. (2016). Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. BioMed Research International, 2016, 1–17. https://doi.org/10.1155/2016/1851242.
Rane, A. V., Kanny, K., Abitha, V. K., & Thomas, S. (2018). Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of inorganic nanomaterials (pp. 121–139). Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-08-101975-7.00005-1.
Römling, U., Gomelsky, M., & Galperin, M. Y. (2005). C-di-GMP: The dawning of a novel bacterial signalling system: C-di-GMP signalling in bacteria. Molecular Microbiology, 57(3), 629–639. https://doi.org/10.1111/j.1365-2958.2005.04697.x.
Rutherford, S. T., & Bassler, B. L. (2012). Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine, 2(11), a012427–a012427. https://doi.org/10.1101/cshperspect.a012427.
Salem, S. S., & Fouda, A. (2020). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biological Trace Element Research, 199, 344. https://doi.org/10.1007/s12011-020-02138-3.
Shahjahan, M. (2017). Synthesis and characterization of silver nanoparticles by sol-gel technique. Nanoscience and Nanometrology, 3(1), 34. https://doi.org/10.11648/j.nsnm.20170301.16.
Sharma, D., Misba, L., & Khan, A. U. (2019). Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrobial Resistance & Infection Control, 8(1), 76. https://doi.org/10.1186/s13756-019-0533-3.
Sivolella, S., Stellini, E., Brunello, G., Gardin, C., Ferroni, L., Bressan, E., & Zavan, B. (2012). Silver nanoparticles in alveolar bone surgery devices. Journal of Nanomaterials, 2012, 1–12. https://doi.org/10.1155/2012/975842.
Stewart, P. S. (2002). Mechanisms of antibiotic resistance in bacterial biofilms. International Journal of Medical Microbiology, 292(2), 107–113. https://doi.org/10.1078/1438-4221-00196.
Thongkumkoon, P., Sangwijit, K., Chaiwong, C., Thongtem, S., Singjai, P., & Yu, L. D. (2014). Direct nanomaterial-DNA contact effects on DNA and mutation induction. Toxicology Letters, 226(1), 90–97. https://doi.org/10.1016/j.toxlet.2014.01.036.
van Tilburg Bernardes, E., Lewenza, S., & Reckseidler-Zenteno, S. (2015). Current research approaches to target biofilm infections. Postdoc Journal: A Journal of Postdoctoral Research and Postdoctoral Affairs, 3(6), 36–49. https://doi.org/10.14304/surya.jpr.v3n6.5.
Umesh, M., Priyanka, K., Thazeem, B., & Preethi, K. (2018). Biogenic PHA nanoparticle synthesis and characterization from Bacillus subtilis NCDC0671 using orange peel medium. International Journal of Polymeric Materials and Polymeric Biomaterials, 67(17), 996–1004. https://doi.org/10.1080/00914037.2017.1417284.
Conflicts of Interest
All the authors have declared that there is no conflict of interest for publishing this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Santhosh, S.K., Sarojini, S., Umesh, M. (2021). Anti-Biofilm Activities of Nanocomposites: Current Scopes and Limitations. In: Pal, K. (eds) Bio-manufactured Nanomaterials. Springer, Cham. https://doi.org/10.1007/978-3-030-67223-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-67223-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67222-5
Online ISBN: 978-3-030-67223-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)