Abstract
Biomaterials play a fundamental role in disease management and the improvement of health care. In recent years, there has been a significant growth in the diversity, function, and number of biomaterials used worldwide. Yet, attachment of pathogenic microorganisms onto biomaterial surfaces remains a significant challenge that substantially undermines their clinical applicability, limiting the advancement of these systems. The emergence and escalating pervasiveness of antibiotic-resistant bacterial strains makes the management of biomaterial-associated nosocomial infections increasingly difficult. The conventional post-operative treatment of implant-caused infections using systemic antibiotics is often marginally effective, further accelerating the extent of antimicrobial resistance. Methods by which the initial stages of bacterial attachment and biofilm formation can be restricted or prevented are therefore sought. The surface modification of biomaterials has the potential to alleviate pathogenic biofouling, therefore preventing the need for conventional antibiotics to be applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whether they are intended to sustain functions and physiological processes critical to life, to restore or preserve a level of activity, for diagnosis of disease, treatment delivery, tissue engineering, or to be used as a part of an elective aesthetic procedure to improve contour and visual appearance, biomaterials play an important role in disease management and in the advancement of health care (Tan et al. 2011; Van Vlierberghe et al. 2011; Ansari and Husain 2012). Their applications range from being coatings on tablets and capsules in pharmaceutical preparations, to being essential components of extracorporeal devices such as contact lenses or kidney dialyzers, and indwelling devices and implants (Dorozhkin 2011; Wagoner and Herschler 2011). Their chemical composition and physical and biological properties are equally diverse, with incessant advancements in technology being driven by the increasing need to care for an ageing population, with its associated need for extended medical care (Hoppe et al. 2011; Khan and Sefton 2011; Lewis 2011; Saito et al. 2011; Shadanbaz and Dias 2012). An increasing human life expectancy places a strong emphasis on the need for the long-term, stable performance of permanent devices and biomaterials used to replace host tissues and organs (Cardoso et al. 2011; Gioe et al. 2011; Kitao et al. 2011; Zhao et al. 2011). Concurrently, bio-resorbable materials are gaining importance for the facilitation of host tissue restoration. This is done as an alternative to failed tissue replacement, hence overcoming any limitations that might be imposed by the use of foreign structures (Bendrea et al. 2011; Naderi et al. 2011). These limitations include poor biocompatibility, causing inflammation, inadequate tissue integration, and non-haemocompatibility, and time-dependent deterioration of properties (Donald 2011; Sun et al. 2011).
The development of infections in or around the indwelling biomaterial devices, such as orthopaedic prostheses, urinary tract and cardiovascular catheters, intraocular lenses and dentures, continues to be a key issue that hinders the utilisation of these devices (Hanssen 2002; Montanaro et al. 2007). While evidently detrimental to the healing process and overall well-being of the patient, the attachment of bacterial cells, together with the ensuing formation of a biofilm, may also impede the performance of the device onto which they colonise (Liu et al. 2004; Stobie et al. 2008). Schematization of the four-stage universal growth cycle of a biofilm with common characteristics, including initiation, maturation, maintenance, and dissolution is presented in Fig. 1. The functions that the biofilm performs are vast, ranging from acting as a physical defence barrier against phagocytic predation and preventing cell detachment under normal flow conditions, to working as a selective permeability barrier (Costerton et al. 1999; Subbiahdoss et al. 2009). Physical and chemical permeability ensures the effective transport of organic molecules and ions to cells at distance from the surface of the biofilm, binding and concentrating nutrients close to cells to ensure the survival of the cell population (Decho 2000). The very same mechanism limits the diffusion of agents that are damaging to the bacteria, including the systemic antibiotics routinely used to treat post-operative infections, and antibacterial agents leaching out from the biomaterial matrix. In some instances, the inability for conventional antibiotic therapies to make ready contact with the bacteria leads to only partial suppression of the infection, resulting in the development of chronic infections (Pavithra and Mukesh 2008). Elderly and immune-compromised patients are particularly susceptible to the development of sepsis, in some cases resulting in lethal consequences. Furthermore, continuous exposure of bacteria to high doses of conventional antimicrobials exerts a selective pressure on these microorganisms, positively affecting their ability to resist those broad-spectrum antibiotics (Andersson and Hughes 2011; Freire-Moran et al. 2011). It is not surprising then, that treating biomaterial-associated infections can be complicated, often demanding the physical (surgical) removal of the infected device.
The temporal evolution of a biofilm. Schematic representation of the characteristic steps associated with the formation of a biofilm, including initiation (I), maturation (II and III), maintenance (IV), and dissolution (V). Pseudomonas aeruginosa cells present during these steps are represented in the second row of images, the first three images obtained using DAPI, followed by chromosomal GFP, then a LIVE/DEAD BacLight kit, as observed with confocal microscopy. The S. aureus attachment is presented in the third row, each obtained using scanning electron microscopy. (From Bordi and de Bentzmann 2011, used with permission)
Clearly, follow-up surgeries resulting in the requirement for extended hospital care are of no benefit to either the patient or the already overburdened healthcare system. Therefore, rather than addressing the consequence of bacterial colonisation, scientists are searching for methods by which the initial stages of bacterial attachment can be prevented. This can be achieved via a synthesis of novel materials and composites with advantageous characteristics integrated directly into the material matrix. A more economically attractive approach, however, is to enhance materials that are already being used by the medical industry (Raynor et al. 2009). Presently, numerous surface modification techniques are under intense investigation for their ability to effectively impart biocompatibility and biofunctionality onto a variety of biomaterials, while preserving their valuable bulk properties and physical dimensionality (Fu et al. 2011; Morent et al. 2011; Solouk et al. 2011; Vasilev et al. 2011; Wu et al. 2011a). In this review, we will concentrate on the methods currently being used to manipulate the nanoscale surface architecture with a view to imparting bactericidal activity onto the surface, and the effect of these modifications on bacterial attachment and biofilm formation. We will first give a brief overview of the current opinion regarding the factors that contribute to controlling cell–surface interactions, with particular regard to the influence of the surface micro- and nanotopographies on this process. We will then review several instances where the deterministic modification of a biomaterial surface has been successfully employed in order to mitigate the extent of bacterial attachment and biofilm formation. Finally, interesting developments in the area of bactericidal biomaterial coatings will be discussed.
Cell-surface dynamics
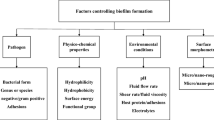
Although concentrated research efforts have been made in order to understand cell-surface dynamics, many of the facets of these mechanisms remain unclear. There is a particular scarcity of theoretical and experimental evidence on this topic on the nanometer and atomic length scales. Recently, the advancement of manufacturing and characterisation techniques has equipped researchers with the necessary tools for the effective investigation of the physico-chemical, mechanical, and biological cues that affect cell–surface interface dynamics (Desmet et al. 2009; Parreira et al. 2011). The fundamental behaviour of living cells, including their adherence to biomaterials, has been found to be intimately linked to the surface properties of such a material and the characteristics of the medium that separates the cells and the substrate surface. The latter can either promote or impede cell attachment, in part by influencing the characteristics of the biomaterial surface, and by applying selective pressure on the bacterial cells (Fig. 2) (Döring et al. 2011; Lee et al. 2011). Phenotypic plasticity in response to environmental stimuli include turning regulatory genes on and off, thus regulating the size, shape, metabolic rate, and the substances that the cells excrete. In addition, these stimuli can cause the production of diverse progeny with distinct phenotypes via hypermutation and localized increased mutation rates (Dobrzyński et al. 2011; Lele et al. 2011). Chemical composition, temperature, nutrient availability, presence of other colonisers, the concentration of harmful substances (antimicrobials, metabolic by-products, and ions), and predation by host immune cells are some of the environmental factors that will directly impact the interface dynamics (Ardehali et al. 2002; Liu et al. 2011; Saldarriaga Fernández et al. 2011). For instance, the flow conditions around a substrate surface have a profound influence on the biofilm initiation, development, and subsequent stability, by affecting the rate at which bacterial cells are delivered to the surface, the time they reside in close proximity to the surface, and the probability for the cell detachment (Busscher et al. 2010). A significant decrease in desorption probabilities have been demonstrated for bacteria subjected to several minutes of direct cell − surface contact, owing to the exponential increase in the adhesion forces, namely, acid–base interactions, amongst cells and between cells and the abiotic surface (Andrews 2009; Busscher et al. 2010). Similarly, the acidity and ionic strength of the physiological solution have been shown to profoundly influence the degree of hydrophobicity of the respective bacterial cell and abiotic target surfaces, and as such on the strength of the electrostatic interactions within biofilm architecture (Pavithra and Mukesh 2008). Even though a precise understanding of the role that the medium plays is essential for adequate cell–surface interface characterisation and indeed, it can be effectively controlled for in vitro three-dimensional tissue formation, it is not the case for in vivo biomaterial utilisation.
a Formation of a biofilm by Staphylococcus aureus on polyvinylchloride in response to various charged molecules and glycosaminoglycans. The asterisk shows the statistical difference (p < 0.01) from a saline-only control. b Effect of heparin, which is frequently used as an anticoagulant coating for catheters, on the adherence kinetics of S. aureus. Sodium heparin (1,000 U/ml, 10 % vol/vol) stimulated biofilm formation of S. aureus on polystyrene and polyvinylchloride. Microscopic images of the S. aureus biofilms incubated with and without heparin. Scanning electron micrographs of 12-h biofilm (c and d, ×12,500 magnification, bar = 10 μm). Phase-contrast microscopy of 4-h biofilms on polystyrene (e and f, bar = 20 μm; the arrow indicates a phase-bright microcolony), and epifluorescent microscopy (g and h, Styo-9 fluorescent staining, 250-ms exposure, ×400 magnification). Epifluorescent microscopy (i and j, ×100 magnification, calcofluor staining) of 5-h biofilms. (From Shanks et al. 2005, used with permission)
Over the past few years, a variety of advanced nanotechnological tools have become available that allow for a high degree of manufacturing control over the surface characteristics of biomaterials. These have the potential to be used to effectively modulate the cell response (Fu et al. 2011; Morent et al. 2011; Solouk et al. 2011; Vasilev et al. 2011; Wu et al. 2011a). It is well established that both the stability of cell attachment and the dynamics of the colonisation process differ with surface architecture, chemical composition, and surface energy of the material, and how these compare to the bacterium-specific factors. The bacterium-specific factors include the hydrophobicity, surface energy, and electrophoretic mobility of the microorganism, and the existence of extracellular appendages, such as pili and flagella (Donlan 2002; Dorobantu et al. 2009). The nature of extracellular polymeric substances (EPS), the level of secretion, and the manner in which these undergo changes in spatial conformation and structural arrangement in response to the nanoscopic features of the abiotic surface also influence cell–surface interactions (Cappella and Dietler 1999). Although obtaining a complete description of an EPS biochemical profile would be a challenge, most EPS is known to be comprised of a wide range of polysaccharides, proteins, glycoproteins, and glycolipids, and in some cases, substantial amounts of extracellular DNA (Flemming et al. 2007; Flemming and Wingender 2010). Recently, the structural and functional diversity of bacterial extracellular polysaccharides, together with their effect on the colonisation, survival and pathogenicity of bacteria, have been reviewed (Bazaka et al. 2011b). The paper highlighted the difficulties associated with obtaining a detailed physico-chemical analysis of bacterial extracellular carbohydrates, owing to the diversity of sugar monomers, linkages, and unique structures present in the carbohydrate fraction of the EPS, and the complexities associated with the isolation of individual polysaccharides from environmental biofilms. The biochemical composition of the EPS is also dependent on the maturity of the microbial community, as well as the local physico-chemical environment (Shenga et al. 2010). Jiao et al. (2010) showed that the EPS obtained from a mid-developmental stage and a mature acidophilic microbial biofilm were both qualitatively and quantitatively different, with more than twice as much EPS being present in the mature biofilm. The presence and relative concentrations of carbohydrates, metals, proteins, and minor quantities of DNA and lipids, were also different between the two biofilm stages.
Pseudomonas aeruginosa is a model biofilm-forming bacteria, with many extrapolations commonly being made from observations of P. aeruginosa biofilm behavior and properties to biofilms in general (Flemming et al. 2007). However, recent studies have shown that such an extrapolation may be highly misleading. Even within a single species of bacteria, there is a high degree of extracellular polysaccharide diversity. For instance, a polyanion polysaccharide, alginate, is the best-characterized component of P. aeruginosa biofilms. The other two biofilm-related polysaccharides produced by the microorganism are Psl and Pel. Early studies of mucoid P. aeruginosa biofilms positively correlated the surface-dependent induction of algC expression with attachment stability, with cells not exhibiting algC up-expression shown to be less capable of remaining at a surface under flow conditions (Davies and Geesey 1995). Subsequent studies demonstrated that non-mucoid P. aeruginosa strains, which are thought to be the first to colonize cystic fibrosis patients, were not reliant on alginate for biofilm development (Petrova and Sauer 2012). Similar specificity has been demonstrated for Pel, which is crucial for maintaining cell–cell interactions; it acts as a primary structural scaffold for the PA14 biofilm community (Colvin et al. 2011). Deletion of pelB in this strain resulted in a severe biofilm deficiency due to an impaired extent of monolayer formation, with little effect on the ability of PA14 cells for biofilm initiation being observed. On the other hand, in PAO1 strain, Psl was demonstrated to be the primary structural polysaccharide for biofilm maturity, with deletion of pel operon having no effect on PAO1 attachment or biofilm development (Colvin et al. 2011). Disruption of pslA and pslB, however, severely compromised the initiation of biofilm formation by a P. aeruginosa PAO1 strain, suggesting that expression of psl plays a notable role in cell–surface and intercellular interactions (Ma et al. 2006). Other polysaccharides produced by Pseudomonas spp., such as levan and other yet-to-be-identified exopolysaccharides, may have an equally significant role in biofilm formation (Laue et al. 2006).Extracellular DNA has been shown to contribute to biofilm formation by PAO1 P. aeruginosa isolates (Whitchurch et al. 2002; Yang et al. 2007), as well as by other bacterial species (Vilain et al. 2009); however, the role of eDNA changed as biofilms evolved. It is not surprising then, that genotypically distinct bacteria will have notably different attachment preferences towards a surface.
By reconfiguring the spatial distribution of chemical functionalities and nanoscale morphological features on the surface of the biomaterials, it is possible to attenuate the attachment, propagation, and biofilm formation. Potential uses for such surfaces extend beyond preventing bacterial attachment to indwelling biomaterial devices and other clinically relevant surfaces. The effect of surface hydrophobicity as a function of both surface chemistry and topography on the attachment preferences of different bacterial species has been discussed by Bazaka et al. (2011d). This is due to focal adhesion sites of the microorganism being in the range comparable to that of the nanoscale features of the abiotic surface (Brunetti et al. 2010), and due to the fact that surface attachment is a fundamental stage in biofilm development and in the formation of chemical signalling pathways amongst bacterial cells (Hochbaum and Aizenberg 2010; Petrova and Sauer 2011). The environmental signals and signaling pathways that regulate biofilm formation, the components of the biofilm matrix, and the mechanisms and regulation of biofilm dispersal, have been reviewed by Karatan and Watnick (2009). A cell-to-cell communication system, quorum sensing, is believed to regulate motility, adhesion, cell-to-cell aggregation, and biofilm formation, as well as virulence and metabolic activity in several bacterial species (Bjellanda et al. 2012).
The surfaces of several animals and plants have been studied for their behaviour, since they have evolved an ability to prevent surface fouling even in environments that are highly populated by biofilm-forming microorganisms. The skin of whales, sharks, and dolphins remain clean of bacteria, owing to a favourable fusion of surface chemistry and micro- and nanoscopic topographies. Similarly, the hydrophobic chemistry of Nelumbo nucifera (lotus) leaf is augmented by a two-layer morphology, resulting in a superhydrophobic surface with low adhesion (Rios et al. 2006, 2007; Dodiuk et al. 2007; Bhushan et al. 2008). Materials that mimic these naturally occurring surfaces are proving equally useful in effectively averting bacterial fouling without the need to use bactericidal agents and by providing a research platform for investigating the cell–surface dynamics. The former is important since it provides the means of responsible management of disease, particularly in the context of rapidly evolving antibiotic resistant pathogenic strains (Baum et al. 2002; Chung et al. 2007; Fadeeva et al. 2011). The latter is essential for intelligent surface design, as it provides researchers with an understanding of how the individual surface characteristics of an abiotic material interact with the chemical and topographical particulars of surfaces of living cells (Malkin and Plomp 2011).
It is important to note that alteration of individual properties, such as only the nanoscopic topography or chemical functionality, is often difficult to achieve (Whitehead et al. 2010). First described by Wenzel (1949), there exists an intimate link between the surface chemistry and topography of a substrate, which becomes increasingly important as we approach nanometer and sub-nanometer scale regimes. The relationship states that a chemically hydrophilic surface will become increasingly more hydrophilic as the surface roughness is increased. By the same reasoning, the wetting ability of a hydrophobic surface decreases with increasing surface roughness. Hence, the wetting behaviour and surface energy of an abiotic surface are likely to change in response to alterations in the chemistry and/or topography of a surface. As a result, decoupling the contribution of a single variable, such as surface roughness, from a bacterial response that is invoked by a complex of material surface characteristics is challenging. In part, this is due to the paucity of data existing for the fundamental behaviour of cells in a response to physical environment at nanoscopic length scale (Anselme et al. 2010; Bazaka et al. 2011a). Finally, most surface modifications are directed towards optimisation for a given application, frequently modulating both surface chemistry and topography.
Nanotopography and surface nanostructuring
As is the case with other external environmental stimuli, there is little doubt that micro and nanoscale topographies have an effect on the attachment behaviour and metabolic activity of microorganisms (Bos et al. 1999). Yet, the extent to which bacterial attachment and subsequent biofilm formation is affected by the surface nanotopography remains a subject of dispute (Anselme et al. 2010). Similarly, there are vast differences in opinion regarding the length scale at which the influence is most profound (An et al. 1995; Boulangé-Petermann et al. 1997; Medilanski et al. 2002; Whitehead et al. 2005, 2006). In the literature, a number of early studies determined surface topography to be a comparatively insignificant factor in bacterial adhesion, with microorganisms observed to have little predilection for topographical cues (An et al. 1995; Bos et al. 1999; Scheuerman et al. 1998). Subsequent investigations reported a feature–cell size correspondence, where features of the size comparable to the size of the bacterium allowed for maximisation of the bacteria–surface contact area, hence increasing the microorganism’s binding potential (Katsikogianni and Missirlis 2004). The same reckoning was applied to the interpretation of reduced bacterial attachment to surfaces with topographic attributes of smaller dimension than that of the cell (Edwards and Rutenberg 2001). Regularity of the nanoscopic features of the surface was also shown to alter bacterial attachment preferences (Díaz et al. 2007; Rowan et al. 2002; Rozhok et al. 2006; Whitehead et al. 2005). Surfaces with regularly distributed pits of 1 and 2 μm were demonstrated to enhance the extent of attachment of P. aeruginosa and Staphylococcus aureus, whereas topographies characterised by irregularly scattered 0.2 and 0.5 μm did not instil the same effect (Whitehead et al. 2005). The attachment patterns and growth of pathogenic P. fluorescens and S. aureus were influenced by the presence of defined trenches, within which cells preferred to align and grow (Diaz et al. 2007; Harris et al. 2004). On the other hand, a random cell distribution was observed for smooth surfaces (Scheuerman et al. 1998; Diaz et al. 2007). Similarly, an aligned attachment of Escherichia coli cells was detected for surfaces with 1.3-μm-wide and 120-nm-deep microgrooves (Díaz et al. 2007) but not for topographies containing grooves of 50 nm in height and period of 1.6 μm (Ploux et al. 2009).
Our own investigations into the role that surface properties play in bacterial cell adhesion, proliferation, and biofilm development have shown that bacterial interactions with materials are influenced by the level of surface roughness and unique topographical peculiarities of that surface. A variety of topographically distinct glass and polymer substrates seeded with cells of taxonomically different bacteria were investigated, with results showing enhanced attachment and increased secretion of EPS for nanosmooth surfaces irrespective of the microorganism species being used in the study (Ivanova et al. 2008; Mitik-Dineva et al. 2008, 2009). In addition to being a major determinant of virulence for numerous pathogenic bacteria, EPS impede antibody opsonisation and phagocytosis, and induce inflammation and aberrant complement activation that can be damaging to host tissues (Bazaka et al. 2011b). Importantly, these promote the colonisation of tissues and surfaces by enabling cell adhesion and co-aggregation via dipole interactions, covalent or ionic bonding, steric interactions, and hydrophobic association. Components of free EPS can be released onto unfriendly surfaces, thus pre-conditioning the target for subsequent colonisation. Indeed, Staleya guttiformis cells were observed to excrete EPS to facilitate attachment to poly(tert-butyl methacrylate) polymeric surfaces via the creation of biopolymer network (Ivanova et al. 2008). The size and distribution of topographical peculiarities were also demonstrated to be significant, with optical fibres etched with regular features attracting lesser levels of bacterial attachment compared to unmodified irregular topographies (Mitik-Dineva et al. 2010).
Significant research efforts have been devoted to exploring how metallic surface nanotopographies, such as those of a titanium biomaterial, influence bacterial cell–surface dynamics. Excellent mechanical properties, biocompatibility, and environmental stability make titanium and titanium alloys a biomaterial of choice for a broad range of medical applications, including orthopaedic and dental implants, cardiac valves and in the microsurgical restoration of middle ear function. Yet, similar to other implantable materials, titanium surfaces are susceptible to bacterial colonisation that is highly detrimental to the performance of the implant. Furthermore, a reduction in the average grain size of titanium, imparted in order to improve mechanical strength, enhance the viability of mice fibroblast cells and accelerate the proliferation of mice preosteoblastic cells (Estrin et al. 2009; Valiev et al. 2008), was also found to be susceptible to a significant increase in levels of attachment of bacteria (Truong et al. 2010a). Despite being chemically similar to the pristine titanium, nanostructuring of the surface enhanced the hydrophilicity of the material. The complex morphology of the nanostructured titanium surface was designed to combine nanoscale smoothness with evenly spaced 100- to 200-nm micro features. Despite some differences, S. aureus, P. aeruginosa, and E. coli cell attachment was found to have increased appreciably on nanostructured titanium compared to that of the nano-rougher untreated sample, accompanied by contemporaneous increase in the production of EPS and changes in cell morphology (Truong et al. 2009). The result is contradictory to the finding of other researchers that showed that preferential cell attachment occurred on surfaces that were more hydrophobic (Gottenbos et al. 2001). These results suggested that surface nanotopography was indeed the major contributor to the observed cell–surface dynamics (Truong et al. 2010b). Subsequent experiments using magnetron sputter thin titanium films with corresponding surface roughness parameters of R q 1.6, 1.2, and 0.7 nm confirmed an augmented attachment response by S. aureus and P. aeruginosa, with 2- to 3-fold increases in the number of retained cells and an elevated level of EPS secretion being observed (Ivanova et al. 2010; Truong et al. 2010c). Interestingly, the attachment of S. epidermidis to titanium surfaces with an average roughness between 0.43 and 1.25 nm was not influenced by the degree of roughness (An et al. 1995).
Given the importance of titanium as a biomaterial, there is a distinct need to design nanostructured metallic surfaces that effectively limit bacterial attachment and formation of complex biofilm communities, and hence reduce the potential for implant-associated infections and inflammations. Surface structuring, as a means to achieve antifouling surfaces, has been identified as an attractive solution for the long-term prevention of bacterial adhesion. The approach takes its inspiration from a number of naturally occurring superhydrophobic surfaces that possess water-repellent, self-cleaning and anti-icing properties due to a favourable combination of a low intrinsic surface free-energy and a hierarchical structural configuration (Webb et al. 2011). Such complex hierarchical nanotopography can minimize the contact area between an abiotic surface and the physiological fluid containing bacterial cells, extending the maximum water contact angles of 120° that can be achieved on a flat surface. A number of fabrication techniques have been developed to mimic these superhydrophobic surfaces, including vacuum-ultraviolet lithography, e-beam lithography, soft lithography, template lithography, templating from anodised alumina, replica moulding, and microwave plasma-enhanced chemical vapour deposition micropatterning (Fadeeva et al. 2009). Recently, superhydrophobic (θ W = 166°) titanium surfaces mimicking the surface of the lotus leaf were successfully fabricated using a femtosecond laser-based micro- and nanostructuring (Fadeeva et al. 2011). The resulting surfaces were highly effective against P. aeruginosa, greatly reducing the colonization propensity of the microorganism, whereas attachment of S. aureus cells was enhanced by the presence of the superimposed nano- and microtopography. The morphological dissimilarity, with P. aeruginosa being a larger rod-shaped and S. aureus being a smaller spherical cell, led to differences in the surface contact area being achieved between the respective cell and the surface, further influenced by the ability of EPS to adequately anchor the cells to the surface. The vastly distinct attachment preferences of the two pathogens in response to the same surface suggest that there exists the possibility to design topographies that would selectively inhibit the attachment and growth of one cell type while encouraging the other. Structures that induce opposing response from human versus pathogenic cells, such as osteoblasts and Staphylococcus epidermidis (Colon et al. 2006) or E. coli K12 (Ploux et al. 2009), respectively, are of particular interest (Wu et al. 2011b). Since these have the capacity to favour the competitive colonization by eukaryotic over bacterial cells, such surfaces hold the potential to enhance biocompatibility and bacterial retardation of medical implants (Ploux et al. 2010).
Bactericidal coatings
Bioactive coatings are becoming an increasingly attractive solution for the prevention of biomaterial-associated infections, for they not only have the ability to inhibit bacterial adhesion, but also to eliminate the attached pathogenic cells that managed to overcome the antifouling property of the surface (Fu et al. 2005; Gabriel et al. 2007; Norowski and Bumgardner 2009; Zilberman and Elsner 2008). The latter is achieved by means of releasing antibacterial agents, such as antibiotics vancomycin, amoxicillin, and gentamicin, in a controlled time-resolved fashion, thus inhibiting bacterial growth at the implant site (Price et al. 1996; Stigter et al. 2004). However, with the quickly dwindling efficacy of many systemic antibiotics and antiseptics and the speedy rise of multi-resistant nosocomial bacteria (Madkour and Tew 2008), alternative antimicrobial agents are being investigated. These include silver ions (Kumar and Münstedt 2005; Zaporojtchenko et al. 2006), nitric oxide (Nablo et al. 2001), bioactive antibodies (Rojas et al. 2000), antimicrobial peptides (Shukla et al. 2010), and some naturally occurring broad-spectrum biocidal compounds, such as essential oils (Bazaka et al. 2011c,d; Low et al. 2011).
The strong bactericidal effect of silver ions against S. aureus, S. epidermidis, P. aeruginosa, E. coli and Klebsiella pneumonia among others is well documented (Birla et al. 2009; Rai et al. 2009). The antimicrobial activity of Ag-based materials is typically associated with their Ag+ content, where Ag+ is believed to interact with the cytoplasmic components and nucleic acids, to inhibit respiratory chain enzymes (Holt and Bard 2005), and to interfere with membrane permeability (Lok et al. 2007). In Vibrio cholera, low concentrations of Ag+ were shown to induce a massive proton leakage through the membrane, leading to a collapse of proton motive force (Dibrov et al. 2002). Tile inactivation of microorganism enzymes takes place via silver complexes with electron donors containing sulfur, phosphorous, oxygen or nitrogen, such as thiols, carboxylates, phosphates, hydroxyl, amines, imidazoles, indoles (Ahearn et al. 1995). Encouraged by the minimal detrimental effects to eukaryotic cells (Silver 2003; Agarwal et al. 2010), silver-containing coatings, nanoparticles, silver-exchanged zeolites, and hybrid silver composites with dendrimers and polymers are being intensely investigated (Marambio-Jones and Hoek 2010; Shao and Zhao 2010b; Li et al. 2011).
Electroless plating, sputtering, ion beam-assisted deposition, and chemical deposition are among the techniques employed to introduce silver onto the surface of the biomaterial (Sardella et al. 2006). Sol–gel processing was used to fabricated silver-doped organic–inorganic hybrid coatings of tetraethoxysilane- and triethoxysilane-terminated poly(ethylene glycol)-block-polyethylene, which at a weight ratio of 80:20 and a 5 wt.% silver salt were found to have notable activity against E. coli and S. aureus (Marini et al. 2007). Similar antibacterial activity against S. epidermidis and S. aureus was observed for co-sputtered silver-containing hydroxyapatite (Chen et al. 2006) and silver-coated polymers, where deposition was achieved by combining magnetron sputtering with a neutral atom beam plasma source (Dowling et al. 2001). Physical vapour co-deposition of titanium/silver hard combined coatings provided significant antimicrobial potency against S. epidermis and K. pneumonia strains andcytocompatibility with osteoblast and epithelial cells (Ewald et al. 2006). With mechanical performance similar to that of pure titanium, the application of TiAg coatings can impart antimicrobial activity on load-bearing endoprosthetic surfaces. Sol − gels containing titanium dioxide in combinations with silver were found to attenuate P. aeruginosa growth; however, the effect on S. aureus colonization was limited (Tarquinio et al. 2010).
The photocatalytic activity of titanium dioxide can be utilised to impart antiviral, antibacterial, and fungicidal properties, and effectively inhibit biofilm formation on the surface of TiO2 implants. Titanium dioxide is known to activate upon exposure to ultraviolet light, where irradiation of the surface promotes electrons from the valence band to the conduction band leaving a positively charged hole. As electrons and holes migrate, the potent oxidative species, such as ∙OH and O2·−, are produced (Ditta et al. 2008). In solution, these can further react to produce H2O2. These photocatalytic species are believed to attack the outer membrane of bacteria. It is generally believed that the surface bound hydroxyl radicals (·OH) play the main role in killing microorganisms, however its exact mode of action, whether they remains bound or diffuse into the bulk of the solution, the significance of other reactive oxygen species, such as H2O2 and O2·−, and the specific microorganism involved remains a subject of active debate (Cho et al. 2005). For instance, MS-2 phage were shown to be inactivated mainly by the free hydroxyl radical in the solution bulk, whereas E. coli cells were damaged by both the free and the surface-bound hydroxyl radicals, and other reactive species, namely O2·− and H2O2 (Ditta et al. 2008).As the bactericidal effect of illuminated TiO2 has been positively correlated to the rate of cell adsorption onto the titanium surface, the properties of the liquid medium will also affect the killing efficacy of the surface (Gogniat et al. 2006). Addition of silver or copper can further enhance the antibacterial effectiveness of the photocatalytic coating, even under weak UV irradiation and lower exposure time. Compromised by the oxidative species, the cytoplasmic membrane is susceptible to the effectual permeation by the Ag or Cu ions (Hashimoto et al. 2005). Once within the bacterial cell, the Cu ions are thought to bind to specific sites in the DNA, particularly guanosine residues, causing single-strand breakage and base modification (Yates et al. 2008). The redox couples of Cu0/Cu2+ and Ag0/Ag+ can cause the transfer of electron leading to the O −2 generation even under dark conditions, enhancing the bactericidal activities via the synergy of the oxidation role of the O −2 and the bacteriostatic action of antibacterial ions (Hu et al. 2007).
Thin TiN/Ag composite films prepared using pulsed magnetron spattering demonstrated an increase in P. aeruginosa and S. aureus inhibition, corresponding to an increase in silver content (Kelly et al. 2009). Heterogeneously distributed throughout the TiN matrix, the presence of the silver resulted in changes in the surface nanotopographies — surface features, grain sizes, and physicochemistry — with most profound changes observed at a silver concentration of 16.7 at.% (Whitehead et al. 2010). Recently, Ivanova et al. (2011) used magnetron sputtered nanoscopically thin silver films to show that while silver ion concentration may be the major determinant of bacterial viability, the extent of bacterial attachment and the patterns are largely affected by surface topography of the films (Ivanova et al. 2011). A strong correlation was also detected between total surface energy γ TOT of the silver coating and P. aeruginosa adhesion, with the number of adhered bacteria increasing linearly with γ TOT but decreasing linearly with an increasing electron donor component γ − (Shao and Zhao 2010a).
Plasma assisted micro- and nanofabrication methods are widely used to deposit a wide range of coatings, to pattern, and to selectively modify the properties of biomaterials (Hynek 2011). For deposition of nanocomposite coatings, plasma enhanced chemical vapour deposition is frequently employed to fabricate the organic matrix, in which particles are then introduced (Sardella et al. 2006). A combination of radiofrequency (RF) glow discharge and simultaneous sputtering from the silver RF electrode was used to deposit nanocomposite Ag/C x H y O z thin films characterized by sounds antibacterial properties (Favia et al. 2000). Similar plasma set up was used for the in situ formation of Ag nanoparticles within a functional hydrocarbon matrix, with the ability to control the concentrating of incorporated silver component (Körner et al. 2010, 2011). Silver nanoparticles bound to a plasma-deposited thin polymeric binding layer of allylamine were effective in preventing attachment of S. epidermidis and biofilm formation while supporting attachment and spreading of osteoblastic cells (Vasilev et al. 2009, 2010). Phosphine-stabilised silver maleimide complex deposited using plasma polymerization onto glass and non-woven polypropylene fabrics demonstrated cytocompatibility with Swiss mouse fibroblasts and human neonatal keratinocytes, while effectively limiting colonization by P. aeruginosa (Poulter et al. 2009).
Recently, RF plasma polymerization was used to fabricate thin film coatings from non-synthetic terpinen-4-ol, a major constituent of Melaleuca alternifolia essential oil attributed with the oil’s broad spectrum antibacterial, antiviral, antifungal, and anti-inflammatory activity (Bazaka et al. 2010). The antifouling and bactericidal properties of the coatings were found to be strongly dependent on the fabrication conditions. Coatings deposited at lower input power that favoured condensation and only limited monomer fragmentation retained most of the activity of the monomer, effectively preventing adhesion and biofilm formation of S. aureus, P. aeruginosa, and S. epidermidis. On the other hand, films fabricated at conditions that resulted in high degree of monomer fragmentation, promoted adhesion of the aforementioned pathogens. In addition to differences in surface chemistry and hydrophobicity, surface nanoarchitecture was found to be a contributing factor to bacterial attachment and biofilm formation, with non-active films being significantly smoother compared to the unmodified substrate and bactericidal counterparts (Bazaka et al. 2011c).
Concluding remarks and future prospects
This review demonstrates that there are surface engineering methods that hold great potential in the fabrication of substrates that can control microbial attachment and biofilm formation, particularly with regard to alleviating biomaterial associated infections. The presented physico-chemical modification methods have the advantages of high efficiency and minimal influence on the bulk properties of the biomaterials. Compared to the conventional antimicrobial-based methods for preventing and treating microbial colonisation, the approaches discussed in this review would be associated with low toxicity and minimal development of bacterial resistance. The latter would be afforded through modification of the bacterial cell–surface interactions rather than by simply killing the pathogen, hence lessening the selective pressure for antibiotic resistance. For the same reasons, such tailored surface modifications are an attractive means of mitigating the attachment and proliferation of bacterial strains that have already developed resistance to one or more currently available antibiotics. This area is of particular importance within the clinical setting, where nosocomial infections severely impede patient recovery and are recognised as the foremost cause of death in intensive care units (Livermore 2005). Lastly, these surfaces will provide scientists with a platform for in-depth investigation of the cell–surface interactions necessary for the effective management of microorganism colonisation in a variety of natural and engineered environments. With the ongoing advancement of manufacturing and characterisation technologies and significant research efforts dedicated to the area, nano-engineered surfaces with high degree of control at molecular and atomic length scales would evolve as a feasible alternative for ecological management of biofouling.
References
Agarwal A, Weis TL, Schurr MJ, Faith NG, Czuprynski CJ, McAnulty JF, Murphy CJ, Abbott NL (2010) Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 31:680–690
Ahearn DG, May LL, Gabriel MM (1995) Adherence of organisms to silver-coated surfaces. J Ind Microbiol Biot 15:372–376
An YH, Friedman RJ, Draughn RA, Smith EA, Nicholson JH, John JF (1995) Rapid quantification of staphylococci adhered to titanium surfaces using image analyzed epifluorescence microscopy. J Microbiol Methods 24:29–40
Andersson DI, Hughes D (2011) Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol Rev 35:901–911
Andrews SS (2009) Accurate particle-based simulation of adsorption, desorption and partial transmission. Phys Biol 6:046015
Ansari SA, Husain Q (2012) Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol Adv 30:512–523
Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L (2010) The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 6:3824–3846
Ardehali R, Shi L, Janatova J, Mohammad SF, Burns Gregory L (2002) The effect of apo-transferrin on bacterial adhesion to biomaterials. Artif Organs 26:512–520
Baum C, Meyer W, Stelzer R, Fleischer LG, Siebers D (2002) Average nanorough skin surface of the pilot whale (Globicephala melas, Delphinidae): considerations on the self-cleaning abilities based on nanoroughness. Mar Biol 140:653–657
Bazaka K, Jacob MV, Truong VK, Wang F, Pushpamali WA, Wang J, Ellis A, Berndt CC, Crawford RJ, Ivanova EP (2010) Effect of plasma-enhanced chemical vapour deposition on the retention of antibacterial activity of terpinen-4-ol. Biomacromolecules 11:2016–2026
Bazaka K, Crawford RJ, Ivanova EP (2011a) Do bacteria differentiate between degrees of nanoscale surface roughness? Biotechnol J 6:1103–1114
Bazaka K, Crawford RJ, Nazarenko EL, Ivanova EP (2011b) Bacterial extracellular polysaccharides. In: Linke D, Goldman A (eds) Bacterial adhesion, vol. 715. Advances in experimental medicine and biology. Springer, Netherlands, pp 213–226
Bazaka K, Jacob M, Truong VK, Crawford RJ, Ivanova EP (2011c) The effect of polyterpenol thin film surfaces on bacterial viability and adhesion. Polymers 3:388–404
Bazaka K, Jacob MV, Crawford RJ, Ivanova EP (2011d) Plasma assisted surface modification of organic biopolymers. Acta Biomater 7:2015–2028
Bendrea A-D, Cianga L, Cianga I (2011) Review paper: progress in the field of conducting polymers for tissue engineering applications. J Biomater Appl 26:3–84
Bhushan B, Koch K, Jung YC (2008) Nanostructures for superhydrophobicity and low adhesion. Soft Matter 4:1799–1804
Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48:173–179
Bjellanda AM, Søruma H, Tegegneb DA, Winther-Larsena HC, Willassenb NP, Hansen H (2012) LitR of Vibrio salmonicida is a salinity-sensitive quorum-sensing regulator of phenotypes involved in host interactions and virulence. Infect Immun 80:1681–1689
Bordi C, de Bentzmann S (2011) Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intens Care 1:19
Bos R, Van Der Mei HC, Busscher HJ (1999) Physico-chemistry of initial microbial adhesive interactions — its mechanisms and methods for study. FEMS Microbiol Rev 23:179–229
Boulangé-Petermann L, Rault J, Bellon-Fontaine MN (1997) Adhesion of Streptococcus thermophilus to stainless steel with different surface topography and roughness. Biofouling 11:201–216
Brunetti V, Maiorano G, Rizzello L, Sorce B, Sabella S, Cingolani R, Pompa PP (2010) Neurons sense nanoscale roughness with nanometer sensitivity. Proc Natl Acad Sci U S A 107:6264–6269
Busscher HJ, Norde W, Sharma PK, van der Mei HC (2010) Interfacial re-arrangement in initial microbial adhesion to surfaces. Curr Opin Colloid Interface Sci 15:510–517
Cappella B, Dietler G (1999) Force–distance curves by atomic force microscopy. Surf Sci Rep 34:5–104
Cardoso MV, de Almeida NA, Mine A, Coutinho E, Van Landuyt K, De Munck J, Van Meerbeek B (2011) Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust Dent J 56:31–44
Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, Ong JL (2006) In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 27:5512–5517
Cho M, Chung H, Choi W, Yoon J (2005) Different inactivation behaviours of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl Environ Microbiol 71:270–275
Chung KK, Schumacher JF, Sampson EM, Burne RA, Antonelli PJ, Brennan AB (2007) Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2:89–94
Colon G, Ward BC, Webster TJ (2006) Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res, Part A 78:595–604
Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, Parsek MR (2011) Thepel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 7:e1001264
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Davies DG, Geesey GG (1995) Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol 61:860–867
Decho AW (2000) Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 20:1257–1273
Desmet T, Morent R, Geyter ND, Leys C, Schacht E, Dubruel P (2009) Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromolecules 10:2351–2378
Diaz C, Cortizo MC, Schilardi PL, de Saravia SGG, de Mele MAFL (2007) Influence of the nano-micro structure of the surface on bacterial adhesion. Mater Res 10:11–14
Díaz C, Schilardi PL, Salvarezza RC, Lorenzo F, de Mele M (2007) Nano/microscale order affects the early stages of biofilm formation on metal surfaces. Langmuir 23:11206–11210
Dibrov P, Dzioba J, Gosink KK, Häse CC (2002) Chemiosmotic mechanism of antimicrobial activity of Ag(+) in Vibrio cholerae. Antimicrob Agents Chemother 46:2668–2670
Ditta IB, Steele A, Liptrot C, Tobin J, Tyler H, Yates HM, Sheel DW, Foster HA (2008) Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl Microbiol Biotechnol 79:127–133
Dobrzyński M, Bernatowicz P, Kloc M, Kubiak J (2011) Evolution of bet-hedging mechanisms in cell cycle and embryo development stimulated by weak linkage of stochastic processes. In: Kubiak JZ (ed) Cell cycle in development, vol 53. Results and problems in cell differentiation. Springer, Berlin, pp 11–30
Dodiuk H, Rios PF, Dotan A, Kenig S (2007) Hydrophobic and self-cleaning coatings. Polym Adv Technol 18:746–750
Donald LE (2011) Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: a tutorial review. Acta Biomater 7:31–56
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890
Döring G, Parameswaran IG, Murphy TF (2011) Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol Rev 35:124–146
Dorobantu LS, Bhattacharjee S, Foght JM, Gray MR (2009) Analysis of force interactions between AFM tips and hydrophobic bacteria using DLVO theory. Langmuir 25:6968–6976
Dorozhkin SV (2011) Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 1:3–56
Dowling DP, Donnelly K, McConnell ML, Eloy R, Arnaud MN (2001) Deposition of anti-bacterial silver coatings on polymeric substrates. Thin Solid Films 398–399:602–606
Edwards KJ, Rutenberg AD (2001) Microbial response to surface microtopography: the role of metabolism in localized mineral dissolution. Chem Geol 180:19–32
Estrin Y, Kasper C, Diederichs S, Lapovok R (2009) Accelerated growth of preosteoblastic cells on ultrafine grained titanium. J Biomed Mater Res, Part A 90A:1239–1242
Ewald A, Gluckermann S, Thull R, Gbureck U (2006) Antimicrobial titanium/silver PVD coatings on titanium. Biomed Engineer Online 5:22
Fadeeva E, Schlie S, Koch J, Ngezahayo A, Chichkov BN (2009) The hydrophobic properties of femtosecond laser fabricated spike structures and their effects on cell proliferation. Phys Status Solidi A 206:1348–1351
Fadeeva E, Truong VK, Stiesch M, Chichkov BN, Crawford RJ, Wang J, Ivanova EP (2011) Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 27:3012–3019
Favia P, Vulpio M, Marino R, d'Agostino R, Mota RP, Catalano M (2000) Plasma-deposition of Ag-containing polyethyleneoxide-like coatings. Plasmas Polym 5:1–14
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: The “House of biofilm cells”. J Bacteriol 189:7945–7947
Freire-Moran L, Aronsson B, Manz C, Gyssens IC, So AD, Monnet DL, Cars O (2011) Critical shortage of new antibiotics in development against multidrug-resistant bacteria—time to react is now. Drug Resist Updates 14:118–124
Fu J, Ji J, Yuan W, Shen J (2005) Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials 26:6684–6692
Fu R-H, Wang Y-C, Liu S-P, Huang C-M, Kang Y-H, Tsai C-H, Shyu W-C, Lin S-Z (2011) Differentiation of stem cells: strategies for modifying surface biomaterials. Cell Transplant 20:37–47
Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN (2007) Infectious disease: connecting innate immunity to biocidal polymers. Mater Sci Eng, R 57:28–64
Gioe T, Sharma A, Tatman P, Mehle S (2011) Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res 469:48–54
Gogniat G, Thyssen M, Denis M, Pulgarin C, Dukan S (2006) The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol Lett 258:18–24
Gottenbos B, Grijpma DW, van der Mei HC, Feijen J, Busscher HJ (2001) Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 48:7–13
Hanssen AD (2002) Managing the infected knee: as good as it gets. J Arthroplasty 17:98–101
Harris LG, Tosatti S, Wieland M, Textor M, Richards RG (2004) Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 25:4135–4148
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285
Hochbaum AI, Aizenberg J (2010) Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett 10:3717–3721
Holt KB, Bard AJ (2005) Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 44:13214–13223
Hoppe A, Güldal NS, Boccaccini AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32:2757–2774
Hu C, Guo J, Qu J, Hu X (2007) Efficient destruction of bacteria with Ti(IV) and antibacterial ions in co-substituted hydroxyapatite films. Appl Catal B Environ 73:345–353
Hynek B (2011) Nanocomposites and nanostructures based on plasma polymers. Surf Coat Technol 205(Supplement 2):S10–S14
Ivanova EP, Mitik-Dineva N, Wang J, Pham DK, Wright JP, Nicolau DV, Mocanasu RC, Crawford RJ (2008) Staleya guttiformis attachment on poly(tert-butylmethacrylate) polymeric surfaces. Micron 39:1197–1204
Ivanova EP, Truong VK, Wang JY, Berndt CC, Jones RT, Yusuf II, Peake I, Schmidt HW, Fluke C, Barnes D, Crawford RJ (2010) Impact of nanoscale roughness of titanium thin film surfaces on bacterial retention. Langmuir 26:1973–1982
Ivanova E, Hasan J, Truong V, Wang J, Raveggi M, Fluke C, Crawford R (2011) The influence of nanoscopically thin silver films on bacterial viability and attachment. Appl Microbiol Biotechnol 91:1149–1157
Jiao Y, Cody GD, Harding AK, Wilmes P, Schrenk M, Wheeler KE, Banfield JF, Thelen MP (2010) Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol 76:2916–2922
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347
Katsikogianni M, Missirlis YF (2004) Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria–material interactions. Eur Cells Mater 8:37–57
Kelly PJ, Li H, Whitehead KA, Verran J, Arnell RD, Iordanova I (2009) A study of the antimicrobial and tribological properties of TiN/Ag nanocomposite coatings. Surf Coat Technol 204:1137–1140
Khan OF, Sefton MV (2011) Endothelialized biomaterials for tissue engineering applications in vivo. Trends Biotechnol 29:379–387
Kitao T, Ando Y, Yoshikawa M, Kobayashi M, Kimura T, Ohsawa H, Machida S, Yokoyama N, Sakota D, Konno T, Ishihara K, Takatani S (2011) In vivo evaluation of the “tinypump” as a pediatric left ventricular assist device. Artif Organs 35:543–553
Körner E, Aguirre MH, Fortunato G, Ritter A, Rühe J, Hegemann D (2010) Formation and distribution of silver nanoparticles in a functional plasma polymer matrix and related Ag+ release properties. Plasma Process Polymer 7:619–625
Körner E, Rupper P, Lübben JF, Ritter A, Rühe J, Hegemann D (2011) Surface topography, morphology and functionality of silver containing plasma polymer nanocomposites. Surf Coat Technol 205:2978–2984
Kumar R, Münstedt H (2005) Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 26:2081–2088
Laue H, Schenk A, Li H, Lambertsen L, Neu TR, Molin S, Ullrich MS (2006) Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology 152:2909–2918
Lee B, Schjerling CK, Kirkby N, Hoffmann N, Borup R, Molin S, HØIby N, Ciofu O (2011) Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. APMIS 119:263–274
Lele UN, Baig UI, Watve MG (2011) Phenotypic plasticity and effects of selection on cell division symmetry in Escherichia coli. PLoS One 6:e14516
Lewis G (2011) Viscoelastic properties of injectable bone cements for orthopaedic applications: state-of-the-art review. J Biomed Mater Res, Part B 98B:171–191
Li W-R, Xie X-B, Shi Q-S, Duan S-S, Ouyang Y-S, Chen Y-B (2011) Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 24:135–141
Liu X, Chu PK, Ding C (2004) Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng, R 47:49–121
Liu Y, Wang J-C, Ren L, Tu Q, Liu W-M, Wang X-Q, Liu R, Zhang Y-R, Wang J-Y (2011) Microfluidics-based assay on the effects of microenvironmental geometry and aqueous flow on bacterial adhesion behaviors. J Pharm Anal 1:175–183
Livermore DM (2005) Minimising antibiotic resistance. Lancet Infect Dis 5:450–459
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PKH, Chiu JF, Che CM (2007) Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem 12:527–534
Low WL, Martin C, Hill DJ, Kenward MA (2011) Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Int J Antimicrob Agents 37:162–165
Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ (2006) Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188:8213–8221
Madkour AE, Tew GN (2008) Towards self-sterilizing medical devices: controlling infection. Polym Int 57:6–10
Malkin AJ, Plomp M (2011) High-resolution architecture and structural dynamics of microbial and cellular systems: insights from in vitro Atomic Force Microscopy. In: Kalinin SV, Gruverman A (eds) Scanning probe microscopy of functional materials. Springer, New York, pp 39–68
Marambio-Jones C, Hoek E (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Marini M, De Niederhausern S, Iseppi R, Bondi M, Sabia C, Toselli M, Pilati F (2007) Antibacterial activity of plastics coated with silver-doped organic–inorganic hybrid coatings prepared by sol–gel processes. Biomacromolecules 8:1246–1254
Medilanski E, Kaufmann K, Wick LY, Wanner O, Harms H (2002) Influence of the surface topography of stainless steel on bacterial adhesion. Biofouling 18:193–203
Mitik-Dineva N, Wang J, Mocanasu RC, Stoddart PR, Crawford RJ, Ivanova EP (2008) Impact of nano-topography on bacterial attachment. Biotechnol J 3:536–544
Mitik-Dineva N, Wang J, Truong VK, Stoddart P, Malherbe F, Crawford RJ, Ivanova EP (2009) Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol 58:268–273
Mitik-Dineva N, Wang J, Truong VK, Stoddart PR, Alexander MR, Albutt DJ, Fluke C, Crawford RJ, Ivanova EP (2010) Bacterial attachment on optical fibre surfaces. Biofouling 26:461–470
Montanaro L, Campoccia D, Arciola CR (2007) Advancements in molecular epidemiology of implant infections and future perspectives. Biomaterials 28:5155–5168
Morent R, De Geyter N, Desmet T, Dubruel P, Leys C (2011) Plasma surface modification of biodegradable polymers: a review. Plasma Process Polymer 8:171–190
Nablo BJ, Chen T-Y, Schoenfisch MH (2001) Sol–gel derived nitric-oxide releasing materials that reduce bacterial adhesion. J Am Chem Soc 123:9712–9713
Naderi H, Matin MM, Bahrami AR (2011) Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26:383–417
Norowski PA, Bumgardner JD (2009) Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res, Part B 88B:530–543
Parreira P, Magalhães A, Gonçalves IC, Gomes J, Vidal R, Reis CA, Leckband DE, Martins MCL (2011) Effect of surface chemistry on bacterial adhesion, viability, and morphology. J Biomed Mater Res, Part A 99A:344–353
Pavithra D, Mukesh D (2008) Biofilm formation, bacterial adhesion and host response on polymeric implants' issues and prevention. Biomed Mater 3:034003
Petrova OE, Sauer K (2011) SagS contributes to the motile-sessile switch and acts in concert with BfiSR to enable Pseudomonas aeruginosa biofilm formation. J Bacteriol 193:6614–6628
Petrova OE, Sauer K (2012) Sticky situations: key components that control bacterial surface attachment. J Bacteriol 194:2413–2425
Ploux L, Anselme K, Dirani A, Ponche A, Soppera O, Roucoules V (2009) Opposite responses of cells and bacteria to micro/nanopatterned surfaces prepared by pulsed plasma polymerization and UV-irradiation. Langmuir 25:8161–8169
Ploux L, Ponche A, Anselme K (2010) Bacteria/material interfaces: role of the material and cell wall properties. J Adhes Sci Technol 24:2165–2201
Poulter N, Munoz-Berbel X, Johnson AL, Dowling AJ, Waterfield N, Jenkins ATA (2009) An organo-silver compound that shows antimicrobial activity against Pseudomonas aeruginosa as a monomer and plasma deposited film. Chem Commun 7312–7314
Price JS, Tencer AF, Arm DM, Bohach GA (1996) Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res 30:281–286
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Raynor JE, Capadona JR, Collard DM, Petrie TA, Garcia AJ (2009) Polymer brushes and self-assembled monolayers: versatile platforms to control cell adhesion to biomaterials (Review). Biointerphases 4:FA3–FA16
Rios PF, Dodiuk H, Kenig S, McCarthy S, Dotan A (2006) The effects of nanostructure and composition on the hydrophobic properties of solid surfaces. J Adhes Sci Technol 20:563–587
Rios PF, Dodiuk H, Kenig S, McCarthy S, Dotan A (2007) Transparent ultra-hydrophobic surfaces. J Adhes Sci Technol 21:399–408
Rojas IA, Slunt JB, Grainger DW (2000) Polyurethane coatings release bioactive antibodies to reduce bacterial adhesion. J Contr Release 63:175–189
Rowan B, Wheeler MA, Crooks RM (2002) Patterning bacteria within hyperbranched polymer film templates. Langmuir 18:9914–9917
Rozhok S, Fan Z, Nyamjav D, Liu C, Mirkin CA, Holz RC (2006) Attachment of motile bacterial cells to prealigned holed microarrays. Langmuir 22:11251–11254
Saito N, Aoki K, Usui Y, Shimizu M, Hara K, Narita N, Ogihara N, Nakamura K, Ishigaki N, Kato H, Haniu H, Taruta S, Ahm Kim Y, Endo M (2011) Application of carbon fibers to biomaterials: a new era of nano-level control of carbon fibers after 30-years of development. Chem Soc Rev 40:3824–3834
Saldarriaga Fernández IC, Busscher HJ, Metzger SW, Grainger DW, van der Mei HC (2011) Competitive time- and density-dependent adhesion of staphylococci and osteoblasts on crosslinked poly(ethylene glycol)-based polymer coatings in co-culture flow chambers. Biomaterials 32:979–984
Sardella E, Favia P, Gristina R, Nardulli M, d'Agostino R (2006) Plasma-aided micro- and nanopatterning processes for biomedical applications. Plasma Process Polymer 3:456–469
Scheuerman TR, Camper AK, Hamilton MA (1998) Effects of substratum topography on bacterial adhesion. J Colloid Interface Sci 208:23–33
Shadanbaz S, Dias GJ (2012) Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater 8:20–30
Shanks RMQ, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA (2005) Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun 73:4596–4606
Shao W, Zhao Q (2010a) Effect of corrosion rate and surface energy of silver coatings on bacterial adhesion. Colloids Surf, B 76:98–103
Shao W, Zhao Q (2010b) Influence of reducers on nanostructure and surface energy of silver coatings and bacterial adhesion. Surf Coat Technol 204:1288–1294
Shenga GP, Yua HQ, Lib XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894
Shukla A, Fleming KE, Chuang HF, Chau TM, Loose CR, Stephanopoulos GN, Hammond PT (2010) Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 31:2348–2357
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341–353
Solouk A, Cousins BG, Mirzadeh H, Seifalian AM (2011) Application of plasma surface modification techniques to improve hemocompatibility of vascular grafts: a review. Biotechnol Appl Biochem 58:311–327
Stigter M, Bezemer J, de Groot K, Layrolle P (2004) Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Controlled Rel 99:127–137
Stobie N, Duffy B, McCormack DE, Colreavy J, Hidalgo M, McHale P, Hinder SJ (2008) Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol–gel coating. Biomaterials 29:963–969
Subbiahdoss G, Kuijer R, Grijpma DW, van der Mei HC, Busscher HJ (2009) Microbial biofilm growth vs. tissue integration: "The race for the surface" experimentally studied. Acta Biomater 5:1399–1404
Sun H, Meng F, Dias AA, Hendriks M, Feijen J, Zhong Z (2011) α-Amino acid containing degradable polymers as functional biomaterials: rational design, synthetic pathway, and biomedical applications. Biomacromolecules 12:1937–1955
Tan A, Yildirimer L, Rajadas J, De La Peña H, Pastorin G, Seifalian A (2011) Quantum dots and carbon nanotubes in oncology: a review on emerging theranostic applications in nanomedicine. Nanomedicine 6:1101–1114
Tarquinio KM, Kothurkar NK, Goswami DY, Sanders RC Jr, Zaritsky AL (2010) Levine AM (2010) Bactericidal effects of silver plus titanium dioxide-coated endotracheal tubes on Pseudomonas aeruginosa and Staphylococcus aureus. Int J Nanomed 5:177–183
Truong VK, Rundell S, Lapovok R, Estrin Y, Wang JY, Berndt CC, Barnes DG, Fluke CJ, Crawford RJ, Ivanova EP (2009) Effect of ultrafine-grained titanium surfaces on adhesion of bacteria. Appl Microbiol Biotechnol 83:925–937
Truong VK, Lapovok R, Estrin YS, Rundell S, Wang JY, Fluke CJ, Crawford RJ, Ivanova EP (2010a) The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 31:3674–3683
Truong VK, Wang J, Lapovok R, Estrin Y, Malherbe F, Berndt C, Crawford R, Ivanova E (2010b) Bacterial attachment response on titanium surfaces with nanometric topographic features. In: Bucak S (ed) Trends in colloid and interface science XXIII, vol 137. Progress in colloid and polymer science. Springer, Berlin, pp 41–45
Truong VK, Wang JY, Shurui W, Malherbe F, Berndt CC, Crawford RJ, Ivanova EP (2010c) Bacterial attachment response to nanostructured titanium surfaces. International Conference on Nanoscience and Nanotechnology, pp 253–256
Valiev RZ, Semenova IP, Latysh VV, Rack H, Lowe TC, Petruzelka J, Dluhos L, Hrusak D, Sochova J (2008) Nanostructured titanium for biomedical applications. Adv Eng Mater 10:B15–B17
Van Vlierberghe S, Dubruel P, Schacht E (2011) Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12:1387–1408
Vasilev K, Sah V, Anselme K, Ndi C, Mateescu M, Br D, Martinek P, Ys H, Ploux L, Griesser HJ (2009) Tunable antibacterial coatings that support mammalian cell growth. Nano Lett 10:202–207
Vasilev K, Sah VR, Goreham RV, Ndi C, Short RD, Griesser HJ (2010) Antibacterial surfaces by adsorptive binding of polyvinyl-sulphonate-stabilized silver nanoparticles. Nanotechnology 21:215102
Vasilev K, Griesser SS, Griesser HJ (2011) Antibacterial surfaces and coatings produced by plasma techniques. Plasma Process Polymer 8:1010–1023
Vilain S, Pretorius JM, Theron J, Brözel VS (2009) DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl Environ Microbiol 75:2861–2868
Wagoner Johnson AJ, Herschler BA (2011) A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater 7:16–30
Webb HK, Hasan J, Truong VK, Crawford RJ, Ivanova EP (2011) Nature inspired structured surfaces for biomedical applications. Curr Med Chem 18:3367–3375
Wenzel RN (1949) Surface roughness and contact angle. J Phys Colloid Chem 53:1466–1467
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
Whitehead KA, Colligon J, Verran J (2005) Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surf B 41:129–138
Whitehead KA, Rogers D, Colligon J, Wright C, Verran J (2006) Use of the atomic force microscope to determine the effect of substratum surface topography on the ease of bacterial removal. Colloids Surf B 51:44–53
Whitehead K, Kelly P, Li H, Verran J (2010) Surface topography and physicochemistry of silver containing titanium nitride nanocomposite coatings. J Vac Sci Technol B Microelectron Nanometer Struct Process Meas Phenom 28:180–187
Wu S, Liu X, Yeung A, Yeung KWK, Kao RYT, Wu G, Hu T, Xu Z, Chu PK (2011a) Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl Mater Interfaces 3:2851–2860
Wu Y, Zitelli JP, TenHuisen KS, Yu X, Libera MR (2011b) Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 32:951–960
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T (2007) Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153:1318–1328
Yates HM, Brook LA, Ditta IB, Evans P, Foster HA, Sheel DW, Steele A (2008) Photo-induced self-cleaning and biocidal behaviour of titania and copper oxide multilayers. J Photochem Photobiol A Chem 197:197–205
Zaporojtchenko V, Podschun R, Schürmann U, Kulkarni A, Faupel F (2006) Physico-chemical and antimicrobial properties of co-sputtered Ag–Au/PTFE nanocomposite coatings. Nanotechnology 17:4904
Zhao H, Yang Y, Yu G, Zhou J (2011) A systematic review of outcome and failure rate of uncemented Scandinavian total ankle replacement. Int Orthop 35:1751–1758
Zilberman M, Elsner JJ (2008) Antibiotic-eluting medical devices for various applications. J Contr Release 130:202–215
Acknowledgements
This study was supported in part by Australian Research Council (ARC) and Advanced Manufacturing CRC.K. B. is a recipient of an Australian Postgraduate Award (APA) and an Australian Institute of Nuclear Science and Engineering Postgraduate Research Award (AINSE PGRA).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bazaka, K., Jacob, M.V., Crawford, R.J. et al. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl Microbiol Biotechnol 95, 299–311 (2012). https://doi.org/10.1007/s00253-012-4144-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4144-7