Abstract
A series of α-cycloamine substituted 2,2’-bipyridines 3ae’-3ce’ was obtained via the one-pot approach based on ipso-substitution of a cyano-group in 1,2,4-triazines, followed by aza-Diels–Alder reaction in good yields. Photophysical properties, including fluorosolvatochromism, were studied for 3ae’-3ce’ and were compared with α-unsubstituted 2,2’-bipyridines. In addition, dipole moments differences in ground and excited states were calculated by both Lippert-Mataga equation and DFT studies and were compared to each other. The correlation between the size of cycloamine unit and the dipole moments differences value (based on Lippert-Mataga equation) was observed. In addition charge transfer indices (DCT, Λ, H and t) were calculated to demonstrate influence of molecular structure on the intramolecular charge transfer degree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragments of cyclic amines (cycloamines) play a versatile role as units of heterocyclic compounds. They act as pharmacophore units in many drugs [1], with cycloamine units might play a key role in the biological activity of the targeted compounds. For example, the presence of piperidine derivatives significantly enhanced hTRPV1 antagonistic activities [2], piperazine derivatives can be used as pharmacophores for cholinesterase inhibitors [3], while due to azepan moiety it is possible to provide urokinase inhibition activity, as well as antimetastasis activity [4]. Morpholine and thiomorpholine are important pharmacophore units as well [5,6,7,8]. Many cycloamine containing heterocycles have found an application as pesticides, herbicides, antifungal agents, etc. [9,10,11,12,13]. Some of pyrrolidine and morpholine containing quinoline derivatives form a quinoline-DNA complex using calf thymus DNA [14]. 4,7-Dipyrrolidinyl-1,10-phenanthroline can be used as a ligand for N -arylation in an aqueous medium, unlike pyrrolidine-free 1,10-phenanthroline derivatives [15]. Cyclic amines have found great application in the synthesis of luminescent molecules, especially in donor–acceptor ones [16,17,18]. Introducing of cycloamines into 2,2’-bipyridine resulted in amine structure dependent solvatochromism, solid state fluorescence and halochromic fluorescence switching due to the donor–acceptor nature of the molecule [19]. It was also possible to obtain luminescent tests for formaldehyde, which can be found in living cells, animals and brain tissues [20]. In addition, modification of d-luciferin with a fragment of azetidine, azepan and thiomorpholine yielded higher photon flux compare to original d-luciferin [21].
Among the existing preparation methods for obtaining cycloamine containing heterocycles, a Pd-catalyzed Buchwald-Hartwig amination is an outstanding one [22, 23]. It is a versatile and efficient amination tool, however, requiring pre-functionalization of a heterocycle with a halogen atom. In addition, there are many examples of nucleophilic substitution reactions of various leaving groups [24,25,26,27,28]. Particularly, construction of bipyridines bearing an amine residue at α-position via cross-coupling reactions [29], as well as direct functionalization of 2,2'-bipyridine [30] or its N-oxide [31] by amine residues. It is worth noting the group of Professor Belskaya, who proposed one-pot approach for the insertion of various cycloamines at the C(5) position in 1,2,3-triazoles during its construction [32].
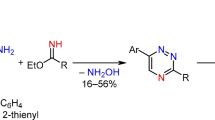
In addition, the approach based on preparation of 2,2'-bipyridines via their 1,2,4-triazine analogs [33,34,35], consisting of the nucleophilic substitution of hydrogen or easily leaving groups in 1,2,4-triazine followed by transformation of the triazine ring into the pyridine one, has to be mentioned. As part of this strategy, 2,2'-bipyridines containing carborane [36], alcohols [37], anilines [38], etc. at the α-position were previously obtained. It is necessary to note the single example of the preparation of α-pyrrolidine-2,2’-bipyridine derivative described by our research group [39]. In this case, the aza-Diels–Alder reaction was realized in pressure flask under increased pressure and temperature conditions, since it could not be carried out under milder conditions. In this work, we used this approach to obtain a series of 5-aryl-2,2'-bipyridines containing various cyclic amines residues at the C6 position of pyridine, and studied their photophysical properties.
Experimental
General Information
Unless otherwise indicated, all common reagents and solvents were used from commercial suppliers (Sigma-Aldrich, Acros Organics or Alfa Aesar) without further purification. All workup and purification procedures were carried out using analytical-grade solvents. 1H NMR and 13C NMR spectra were recorded at room temperature at 400 and 100 MHz, respectively, on a Bruker DRX-400 spectrometer using CDCl3 or DMSO-d6 as the solvent. 13C NMR DEPT 135 spectra were recorded at room temperature at 151 MHz on Bruker AVANCE NEO spectrometer using CDCl3 as the solvent Hydrogen chemical shifts were referenced to the hydrogen resonance of the corresponding solvent (DMSO-d6, δ = 2.50 ppm or CDCl3, δ = 7.26 ppm). Carbon chemical shifts were referenced to the carbon resonances of the solvent (CDCl3, δ = 77.16 ppm). Peaks were labeled as singlet (s), doublet (d), triplet (t), doublet of doublets (dd), doublet of doublets of doublets (ddd), and multiplet (m). Mass spectra were recorded on a MicrOTOF-Q II (Bruker Daltonics), electrospray as a method of ionization. Elemental analysis was performed on a PerkinElmer PE 2400 elemental analyzer. Melting points were obtained with Stuart SMP10 apparatus and are uncorrected. UV–vis absorption spectra were recorded on a Shimadzu UV-1800 spectrophotometer, and emission spectra were measured on a Horiba FluoroMax-4 by using quartz cells with a 1 cm path length at room temperature. Absolute quantum yields of the luminescence of target compounds in solution were measured by using the integrating sphere Quanta-φ of the Horiba FluoroMax 4 at room temperature. The quantum chemical calculations were carried out at the B3LYP/6-31G* level of theory with the help of the Gaussian-09 [40] program package. No symmetry restrictions were applied during the geometry optimization procedure. The hole-electron analysis was carried out in Multiwfn program (version 3.7) [41]. The Cartesian atomic coordinates for all optimized model structures are presented in the attached xyz-files.
Synthesis and Characterization
5-Cyano-6-aryl-3-(pyridine-2-yl)-1,2,4-triazines were prepared according to the literature [42].
Typical Procedure for the Synthesis of Corresponding Cycloamine-containing 2,2’-bipyridines 3aa’-3ce’
A mixture of a corresponding 5-cyano-1,2,4-triazine 1a-c (0.5 mmol, 1 eq.) and a corresponding cycloamine (0.5 mmol, 1 eq.) was mixed in a pressure flask at 150 ℃ under argon for 8 h. Then 2,5-norbornadien (4 eq.) and 1,2-dichlorobenzene (10 mL) were introduced. The reaction mixture was mixed at 225 ℃ under argon for 8 h. Then extra portion of 2,5-norbornadien (4 eq.) was introduced and the reaction mixture was mixed at 225 ℃ under argon for another 8 h. The solvent was removed under reduced pressure, and a solid was triturated by acetonitrile. The resulted precipitate was filtrated and recrystallized from acetonitrile.
Synthesis of 4-phenyl-1-(pyridin-2-yl)-3-(pyrrolidin-1-yl)-6,7-dihydro-5H-cyclopenta[c]pyridine 4
A mixture of 5-cyano-6-phenyl-1,2,4-triazine 1a (130 mg, 0.5 mmol, 1 eq.) and pyrrolidine (0.5 mmol, 0.04 mL, 1 eq.) was mixed in a pressure flask at 150 ℃ under argon for 8 h. Then 1-morpholinecyclopentene (0.4 ml, 5 eq.) was introduced and the reaction mixture was stirred at 200 ℃ for 2 h. After that extra portion of 1-morpholinecyclopentene (0.4 ml, 5 eq.) was introduced and the reaction mixture was stirred at 200 ℃ for another 2 h. The reaction mixture was triturated by acetonitrile (4 mL) and the precipitate was filtrated. The product was recrystallized from acetonitrile.
Results and Discussion
5-Cyano-6-aryl-3-(pyridine-2-yl)-1,2,4-triazines were used as starting compounds 1a-c obtained by the described procedure [42]. Interaction of 1a-c with cyclic amines was realized via ipso-substitution of the cyano group and performed under solvent-free conditions at 150 ℃ in a pressure flask (Scheme 1). The resulted 1,2,4-triazines 2aa'-2ce' were used on the next step without isolation and purification with the one-pot approach. The target 2,2’-bipyridines 3aa'-3ce' were formed as a result of the aza-Diels–Alder reaction using 2,5-norbornadiene as a dienophile at 225 ℃ in a 1,2-dichlorobenzene under argon atmosphere (Scheme 1). It is worth noting the possibility of using of 1-morpholinecyclopentene as a dienophile at this step under solvent-free conditions at 200 ℃ to obtain product 4. Overall yields for this one-pot two-step approach were in range of 60–75% for 3aa’-3ce’ and 62% for 4.
The structures of the target compounds were confirmed by 1H and 13C NMR spectroscopy, mass spectrometry and elemental analysis.
Photophysical Studies
The photophysical properties of the obtained fluorophores 3aa’-3ce’ were studied (Fig. 1, Table 1). UV/Vis absorption spectra contained two absorption bands with maxima in the ranges of 250–273 nm and 338–360 nm, which can be attributed to the corresponding π-π* and n-π* transitions. The maxima of the longest wavelength absorption bands changed in the range of 12–15 nm with a change in the size and nature of the cycloamine unit, however, no relationship was observed in this case. In addition, no differences were observed between morpholine and thiomorpholine containing fluorophores, while shifts in absorption maxima were detected in case of pyrrolidine, piperidine and azepane containing ones. As expected, the introduction of the methyl or methoxy groups into the phenylene fragment did not lead to significant changes in absorption spectra. It is necessary to note the bathochromic shift of absorption maxima for 2,2'-bipyridines 3aa'-3ce' in comparison with α-unsubstituted 2,2'-bipyridines, i.e., without a cycloamine unit [43]. In this case, the difference was up to 59 nm, and the reason could be attributed to the contribution of the lone pair of electrons of the nitrogen atom of the cycloamine fragment to the n-π* transition.
Introduction of cycloamine units influenced significantly on the fluorescence of 3aa'-3ce'. In most cases, the spectra were represented by one intense and wide emission band in the range of 350–600 nm with a maxima varying in the range of 432–456 nm (Fig. 1, Table 1), which are 45–97 nm redshifted than the fluorescence maxima for similar α-unsubstituted 2,2'-bipyridines [43]. No relationship was observed between the size and nature of the cycloamine unit and fluorescence; there was only a moderate change in the emission maxima in the range of 8–36 nm. In addition, the introduction of cycloamine fragments increased the Stokes shift values. It is worth noting a significant increase in the luminescence quantum yields values upon the introduction of cycloamine fragments in the series 5-phenyl- and 5-tolyl-2,2'-bipyridines, especially when comparing 5-phenyl-6-(azepan-1-yl)-2,2'-bipyridine 3a,b' (86.1%) with 5-phenyl-2,2'-bipyridine (3.2%, [43]). In the case of 4-methoxyphenyl derivatives, the quantum yields of compounds 3ca'-3ce ' were lower than for 4-methoxyphenyl-2,2'-bipyridine (89.0%, [43]).
Fluorosolvatochromism
Compounds 3aa'-3ce' are representatives of D-A push–pull fluorophores since they consist of a cycloamine donor (D) fragment and the 2,2'-bipyridine acceptor (A) one. Therefore, they should exhibit intramolecular charge separation (or intermolecular charge transfer, ICT) during photoexcitation with appearance of long-wavelength emission band. To confirm this, absorption and fluorescence spectra were obtained for the fluorophores 3aa'-3ce' in solvents of different polarity (Table S1). The UV/Vis spectra, as expected, showed only minor changes with peaks varying within a few nanometers for all 3aa'-3ce' fluorophores. The fluorescence spectra turned out to be more dependent on the solvent polarity, and the expected bathochromic shift of the emission maxima was observed with increasing solvent polarity. The correlation between emission maxima and solvent polarity and the Dimroth/Reichardt [44, 45] and Kosover [46, 47] scales (based on the empirical parameters Z and E T (30), respectively) turned out to be linear (Fig. 2, Tables S2-S4) for all fluorophores except 3ca' and 3cb' (R2 = 0.25 and 0.50, respectively). In these cases, the emission maxima in the most polar methanol turned out to be blue-shifted due to proticity of methanol.
Moreover, the differences in dipole moments in the ground and excited states (Δμ) for fluorophores 3aa'-3ce' were calculated by Lippert-Mataga equation [48,49,50] (Eqs. 1–3) and corresponding plots (Fig. 3).
Equation (1). Lippert-Mataga equation, where νA and νF are the wavenumbers (cm−1) of the absorption and emission, respectively; h is Planck's constant; c is the speed of light in vacuum; a is the radius of the cavity in which the fluorophore resides (Onsager radius [51]), μE and μG are the excited and ground state dipole moment, respectively.
Equation (2). Onsager radius [51], where VvdW – van der Waals volume.
Equation (3). Theoretical calculations of van der Waals volume, where, NB is the number of bonds, RA is the number of aromatic rings, and RNA is the number of non-aromatic rings.
For all the fluorophores, Δμ values were less 10D that could be explained by weak cycloamine donor unit and, therefore, weak charge separation in excited state. However, an unexpected correlation was determined for fluorophores 3aa’-3ac’, 3ba’-3bc’ and 3ca’-3 cc’, that is the larger cycloamine unit, the greater Δμ value (Table 2) with the single exception – compound 3bc’ with the Δμ value of 6.91 D. This correlation was not obvious due to the presence of σ-orbital overlapping only between CH2-units that had to have no influence on charge separation state. For compounds with morpholine and thiomorpholine units (3ad’-3ae’, 3bd’-3be’ and 3 cd’-3ce’), this correlation was not discovered due to the role of heteroatom (O or S). Comparison of Δμ values calculated by Lippert-Mataga equation with the ones calculated by B3LYP/6-31G* level of theory with the help of Gaussian-09 (Table S5) reveals differences in Δμ values (Table 2). Moreover, no clear correlation between the size of cycloamine unit and Δμ value was observed according to DFT calculations. These differences could be explained by the role of the solvents in Lippert-Mataga equation that was not included in DFT calculations.
DFT Studies and Charge Transfer Indices
As a next step, HOMO–LUMO spatial distributions, energy gap values ΔE, as well as oscillator strength for all 3ae’-3ce’ fluorophores were calculated by B3LYP/6-31G* level of theory with the help of Gaussian-09 (Table 2, Figs. S1-S3). The HOMO-orbitals were distributed mainly on the cycloamine unit, central pyridine and the aromatic substituent, while the LUMO-orbitals distributed only along the 2,2’-bipyridine domain. The lowest ΔE correspond to morpholine- and thiomorpholine-containing molecules 3ad’, 3ae’, 3bd’, 3be’, 3 cd’ and 3ce’ associated with the most energetically favorable conjugated structures. Meanwhile, the largest values of the oscillator strength correspond to S0-S1 π-π* transitions of morpholine-containing compounds 3ad’, 3bd’ and 3 cd’, which also confirms the high degree of charge transfer (Table 2).
To gain a deeper understanding of the influence of molecular structure on the intramolecular charge transfer degree, additional calculations of charge transfer indices (CT-indices) have been carried out. These DCT, Λ, H and t indices were initially proposed by Le Bahers et al. [52] and adapted by Lu and Chen [41]. Based on their work, the respective indices (DCT, Λ, H and t) have been calculated for all fluorophores (Eqs. S1-S9, ESI) and are presented in Table 2. Thus, the analysis of CT-indices made it possible to predict a significant overlap between the centroids of the positive donor cycloamine fragment and the negative acceptor 2,2’-bipyridine domain of the D-A fluorophores. Based on the combination of high DCT values close to H values, the lowest Λ index and t > 0, it was possible to obtain a series of the most promising compounds with intramolecular charge transfer: 3 cd’, 3ce’, 3bd’, 3be’, 3ad’ and 3ae’, which correlates with the experimental values of the Stokes shift and dipole moment difference calculated by the Lippert-Mataga mathematical model. Therefore, DFT calculations and the values of CT indices allowed not only to compile a series, but to arrange it according to the significance of CT fluorophores: 3 cd > 3bd > 3ad > 3ce > 3be > 3ae.
Conclusion
In summary, a series of α-cycloamine substituted 2,2’-bipyridines 3ae’-3ce’ has been obtained via the one-pot approach based on ipso-substitution of a cyano-group in 1,2,4-triazines, followed by aza-Diels–Alder reaction in good yields. Studies of photophysical properties demonstrated positive influence of cycloamine unit both on absorption and emission maxima, as well as luminescence quantum yields compare to α-unsubstituted 2,2’-bipyridines. Fluorophores 3ae’-3ce’ demonstrated red-shifted emission (fluorosolvatochromism) with increasing of solvent polarity. Dipole moments differences in ground and excited states were calculated by both Lippert-Mataga equation and DFT studies, and are in range of 6.55–8.09 D and 1.91–3.11 D, respectively. An unexpected correlation was determined for fluorophores 3aa’-3ac’, 3ba’-3bc’ and 3ca’-3 cc’, that is the larger cycloamine unit, the greater Lippert-Mataga Δμ value with the single exception (3bc’). In addition, CT-indices (DCT, Λ, H and t) were calculated. Thus, these data confirm charge-separation in fluorophores 3ae’-3ce’, while the method of their synthesis could find a potential application in design and construction of functional materials.
Data Availability
All relevant data are presented in the manuscript and the supplementary file.
References
Vitaku E, Smith DT, Njardarson JT (2014) Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA Approved Pharmaceuticals. J Med Chem 57:10257–10274. https://doi.org/10.1021/jm501100b
Kim MS, Ryu H, Kang DW et al (2012) 2-(3-Fluoro-4-methylsulfonylaminophenyl)propanamides as Potent Transient Receptor Potential Vanilloid 1 (TRPV1) Antagonists: Structure-Activity Relationships of 2-Amino Derivatives in the N -(6-Trifluoromethylpyridin-3-ylmethyl) C-Region. J Med Chem 55:8392–8408. https://doi.org/10.1021/jm300780p
Mohamed T, Yeung JCK, Rao PPN (2011) Development of 2-substituted-N-(naphth-1-ylmethyl) and N-benzhydrylpyrimidin-4-amines as dual cholinesterase and Aβ-aggregation inhibitors: Synthesis and biological evaluation. Bioorg Med Chem Lett 21:5881–5887. https://doi.org/10.1016/j.bmcl.2011.07.091
Buckley BJ, Aboelela A, Majed H et al (2021) Systematic evaluation of structure–property relationships and pharmacokinetics in 6-(hetero)aryl-substituted matched pair analogs of amiloride and 5-(N, N-hexamethylene)amiloride. Bioorg Med Chem 37:116116. https://doi.org/10.1016/j.bmc.2021.116116
Zhang F, Bhat S, Gabelli SB et al (2013) Pyridinylquinazolines selectively inhibit human methionine aminopeptidase-1 in Cells. J Med Chem 56:3996–4016. https://doi.org/10.1021/jm400227z
Kumari A, Singh RK (2020) Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR). Bioorg Chem 96:103578. https://doi.org/10.1016/j.bioorg.2020.103578
Kireev D, Chrétien J, Raevsky O (1995) Molecular modeling and quantitative structure-activity studies of anti-HIV-1 2-heteroarylquinoline-4-amines. Eur J Med Chem 30:395–402. https://doi.org/10.1016/0223-5234(96)88249-3
Motoyama M, Doan T, Hibner-Kulicka P et al (2021) Synthesis and structure-photophysics evaluation of 2- N -Amino-quinazolines: Small molecule fluorophores for solution and solid state. Chem Asian J 16:2087–2099. https://doi.org/10.1002/asia.202100534
Urch CJ, Salmon R, Lewis T et al (1996) Bicyclic amines as insecticides. WO 1996037494 A
Goeghova M, Zambach W, Muehlebach M et al (2009) Spiroheterocyclic pyrrolidine dione derivatives useful as pesticides. WO 2009049851 A1
Duke SO, Cantrell CL, Meepagala KM et al (2010) Natural toxins for use in pest management. Toxins (Basel) 2:1943–1962. https://doi.org/10.3390/toxins2081943
Meng L, Zhao H, Zhao S et al (2019) Inhibition of yeast-to-hypha transition and virulence of Candida albicans by 2-alkylaminoquinoline derivatives. Antimicrob Agents Chemother 63. https://doi.org/10.1128/AAC.01891-18
Fokas D, Coffen DL, Ryan WJ (1999) Spiro[pyrrolidine-2,3’-oxindole] compounds and methods of use
Bonacorso HG, Rodrigues MB, Iglesias BA et al (2018) New 2-(aryl/heteroaryl)-6-(morpholin-4-yl/pyrrolidin-1-yl)-(4-trifluoromethyl)quinolines: synthesis via Buchwald-Hartwig amination, photophysics, and biomolecular binding properties. New J Chem 42:10024–10035. https://doi.org/10.1039/C8NJ01120F
Engel-Andreasen J, Shimpukade B, Ulven T (2013) Selective copper catalysed aromatic N-arylation in water. Green Chem 15:336–340. https://doi.org/10.1039/C2GC36589H
Vabre R, Legraverend M, Piguel S (2014) Synthesis and evaluation of spectroscopic properties of newly synthesized push–pull 6-amino-8-styryl purines. Dyes Pigm 105:145–151. https://doi.org/10.1016/j.dyepig.2014.01.025
Toche RB, Chavan SN (2014) Synthesis and study the effect of donor-acceptor substituent on fluorescence behavior of thieno[3, 2-c]pyridine derivatives. J Fluoresc 24:285–293. https://doi.org/10.1007/s10895-013-1313-8
Pigulski B, Męcik P, Cichos J, Szafert S (2017) Use of stable amine-capped polyynes in the regioselective synthesis of push-pull thiophenes. J Org Chem 82:1487–1498. https://doi.org/10.1021/acs.joc.6b02685
Nagarasu P, Kundu A, Pitchaimani J et al (2020) Structure controlled solvatochromism and halochromic fluorescence switching of 2,2′-bipyridine based donor–acceptor derivatives. New J Chem 44:14421–14428. https://doi.org/10.1039/D0NJ02560G
Chen J, Shao C, Wang X et al (2020) Imaging of formaldehyde fluxes in epileptic brains with a two-photon fluorescence probe. Chem Commun 56:3871–3874. https://doi.org/10.1039/D0CC00676A
Sharma DK, Adams ST, Liebmann KL, Miller SC (2017) Rapid access to a broad range of 6′-substituted firefly luciferin analogues reveals surprising emitters and inhibitors. Org Lett 19:5836–5839. https://doi.org/10.1021/acs.orglett.7b02806
Heravi MM, Kheilkordi Z, Zadsirjan V et al (2018) Buchwald-Hartwig reaction: An overview. J Organomet Chem 861:17–104. https://doi.org/10.1016/j.jorganchem.2018.02.023
Dorel R, Grugel CP, Haydl AM (2019) The Buchwald-Hartwig amination after 25 years. Angew Chem Int Ed 58:17118–17129. https://doi.org/10.1002/anie.201904795
Wang D, Zhang E, Xu T et al (2015) Sequential C-C, C–O, and C–N bond-forming reaction of methyl (–)-3-dehydroshikimate, malononitrile, and bromoalkanes: simple synthesis of 2-(alkylamino)-3-cyanobenzofurans from a biomass-derived substrate. Synlett 27:287–293. https://doi.org/10.1055/s-0035-1560582
Thomas S, Roberts S, Pasumansky L et al (2003) Aminoborohydrides 15. The first mild and efficient method for generating 2-(dialkylamino)-pyridines from 2-fluoropyridine. Org Lett 5:3867–3870. https://doi.org/10.1021/ol035430j
Lee M, Rucil T, Hesek D et al (2015) Regioselective control of the S N Ar amination of 5-substituted-2,4-dichloropyrimidines using tertiary amine nucleophiles. J Org Chem 80:7757–7763. https://doi.org/10.1021/acs.joc.5b01044
Khazir J, Mir BA, Chashoo G et al (2020) Synthesis and anticancer activity of N-9- and N-7- substituted 1,2,3 triazole analogues of 2,6-di-substituted purine. Med Chem Res 29:33–45. https://doi.org/10.1007/s00044-019-02456-9
Pang JH, Kaga A, Chiba S (2018) Nucleophilic amination of methoxypyridines by a sodium hydride–iodide composite. Chem Commun 54:10324–10327. https://doi.org/10.1039/C8CC05979A
Zhao X, Webster CE (2015) Novel metal complex catalysts and uses thereof
Pang JH, Kaga A, Roediger S et al (2019) Revisiting the Chichibabin reaction: C2 amination of pyridines with a NaH−iodide composite. Asian J Org Chem 8:1058–1060. https://doi.org/10.1002/ajoc.201900094
Smith AJ, Kalkman ED, Gilbert ZW, Tonks IA (2016) ZnCl 2 capture promotes ethylene polymerization by a salicylaldiminato Ni complex bearing a pendent 2,2′-bipyridine group. Organometallics 35:2429–2432. https://doi.org/10.1021/acs.organomet.6b00485
Gavlik KD, Sukhorukova ES, Shafran YM et al (2017) 2-Aryl-5-amino-1,2,3-triazoles: New effective blue-emitting fluorophores. Dyes Pigm 136:229–242. https://doi.org/10.1016/j.dyepig.2016.08.015
Prokhorov AM, Kozhevnikov DN (2012) Reactions of triazines and tetrazines with dienophiles (Review). Chem Heterocycl Compd (N Y) 48:1153–1176. https://doi.org/10.1007/s10593-012-1117-9
Pabst GR, Sauer J (1999) The new and simple ‘LEGO’ system: Its application to the synthesis of 4-stannyl-, 4-bromo- and branched oligopyridines. Tetrahedron 55:5067–5088. https://doi.org/10.1016/S0040-4020(99)00179-9
Foster RAA, Willis MC (2013) Tandem inverse-electron-demand hetero-/retro-Diels–Alder reactions for aromatic nitrogen heterocycle synthesis. Chem Soc Rev 42:63–76. https://doi.org/10.1039/C2CS35316D
Kozhevnikov DN, Kozhevnikov VN, Prokhorov AM et al (2006) Consecutive nucleophilic substitution and aza Diels-Alder reaction—an efficient strategy to functionalized 2,2′-bipyridines. Tetrahedron Lett 47:869–872. https://doi.org/10.1016/j.tetlet.2005.12.006
Savchuk MI, Khasanov AF, Kopchuk DS et al (2019) New Push-Pull fluorophores on the basis of 6-Alkoxy-2,2’-Bipyridines: Rational synthetic approach and photophysical properties. Chem Heterocycl Compd (N Y) 55:554–559. https://doi.org/10.1007/s10593-019-02495-5
Kopchuk DS, Krinochkin AP, Starnovskaya ES et al (2018) 6-Arylamino-2,2′-bipyridine “push-pull” fluorophores: solvent-free synthesis and photophysical studies. ChemistrySelect 3:4141–4146. https://doi.org/10.1002/slct.201800220
Savchuk MI, Starnovskaya ES, Shtaitz YK et al (2018) Synthesis of 5-Phenyl-2,2’-bipyridines 6-Substituted with Donor Groups by aza-Diels–Alder Reactions of 5-R-1,2,4-Triazines under High Pressure Conditions. Russ J Gen Chem 88:2213–2215. https://doi.org/10.1134/S1070363218100316
Frisch MJ, Trucks GW, Schlegel HB et al (2010) Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Kozhevnikov VN, Kozhevnikov DN, Nikitina TV et al (2003) A versatile strategy for the synthesis of functionalized 2,2‘-Bi- and 2,2‘:6‘,2‘ ‘-Terpyridines via Their 1,2,4-Triazine Analogues. J Org Chem 68:2882–2888. https://doi.org/10.1021/jo0267955
Kozhevnikov VN, Shabunina OV, Kopchuk DS et al (2008) Facile synthesis of 6-aryl-3-pyridyl-1,2,4-triazines as a key step toward highly fluorescent 5-substituted bipyridines and their Zn(II) and Ru(II) complexes. Tetrahedron 64:8963–8973. https://doi.org/10.1016/j.tet.2008.06.040
Reichardt C (2006) Solvents and solvent effects in organic chemistry, 3rd, updated and enlarged edition, 3rd edn. WILEY-VCH Verlag GmbH & Co, KGaA
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94:2319–2358. https://doi.org/10.1021/cr00032a005
Kosower EM (1958) The effect of solvent on spectra. I. A new empirical measure of solvent polarity: Z-values. J Am Chem Soc 80:3253–3260. https://doi.org/10.1021/ja01546a020
Kosower EM (1968) Introduction to physical organic chemistry hardcover. John Wiley & Sons
Lakowicz JR (2006) Principles of Fluorescence Spectroscopy, 3rd edn. Springer, US, Boston, MA
Mataga N, Kaifu Y, Koizumi M (1956) Solvent effects upon fluorescence spectra and the dipolemoments of excited molecules. Bull Chem Soc Jpn 29:465–470. https://doi.org/10.1246/bcsj.29.465
Lippert E (1957) Spektroskopische bestimmung des dipolmomentes aromatischer verbindungen im ersten angeregten singulettzustand. Electro chem 61:962–975
Zhao YH, Abraham MH, Zissimos AM (2003) Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J Org Chem 68:7368–7373. https://doi.org/10.1021/jo034808o
Le Bahers T, Adamo C, Ciofini I (2011) A qualitative index of spatial extent in charge-transfer excitations. J Chem Theory Comput 7:2498–2506. https://doi.org/10.1021/ct200308m
Funding
This work was supported by the Russian Science Foundation grant # 19–73-10144-P (synthesis and primar photophysical studies), by the Russian Science Foundation grant # 21–13-00304 (fluorosolvatochromic studies), and by the RUDN University Strategic Academic Leadership Program (quantum chemical calculations).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and photophysical studies were performed by M.R.Guda, M.I.Valieva, R.Aluru, A.F.Khasanov, quantum chemical calculations were performed by A.S.Novikov. Data interpretation was performed by A.F.Khasanov, D.S.Kopchuk, O.S.Taniya. Writing—original draft preparation was performed by A.F.Khasanov. Writing—review and editing were performed by D.S.Kopchuk and G.V.Zyryanov. Supervising by B.C.Ranu. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflicts of Interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guda, M.R., Valieva, M.I., Kopchuk, D.S. et al. One-pot Synthesis and Photophysical Studies of Α-cycloamino-substituted 5-aryl-2,2'-bipyridines. J Fluoresc 34, 579–586 (2024). https://doi.org/10.1007/s10895-023-03304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03304-1