Abstract
A library of N-9- and N-7-substituted 1,2,3 triazole analogues were generated on the 2,6-di-substituted purine upon reaction with various substituted aromatic azides. The synthesised analogues were screened for in vitro cytotoxic activity against various human cancer cell lines like (HCT-1 (colon), THP-1 (leukaemia), IMR-32 (neuroblastoma) and A-549 (lung)). From the bioassay results, it was observed that even though most of the synthesized derivatives exhibited a good potency against various screened cancer cell lines, but few of the analogues like 9a, 9b and 9e were found to be the most potent analogues in the series, with compound 9a showing IC50 values of 0.08 and 0.4 μM against THP-1 and A-549 cell lines, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
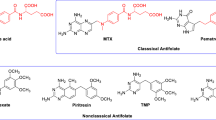
Purine structural framework (7H-Pyrrolo[2,3-d]pyrimidine) is the heterocyclic compound, which is most commonly found in nature and is often found in several molecules with proven biological properties (Jeannette et al. 2015). This heterocyclic structure as such is not known in nature, but exists in the form of substituted purines like adenine and guanine or their derivatives, which play essential roles in a broad variety of functions in living species (Rosemeyer 2004; Legraverend and Grierson 2006). Therefore, this heterocyclic structure received the title of ‘privileged scaffold’ a phrase that has been used in the analysis of multiple molecules of the same scaffold with the same activity (Welsch et al. 2010). The screening of purine analogues against an ample range of biological targets has led to the opening of its new applications as therapeutic agents and has been found effective as chemotherapeutics (antiviral, antibiotic and anticancer agents) (De Clercq 1997; Melroy and Nair 2005; Kay 1981; Nabhan et al. 2004; Marr 1991; Panos et al. 1996; Quan and Peters 2004). They exert their chemotherapeutic property by causing inhibition or acting as substrates of enzymes involved in metabolism of purine like adenosine deaminase, guanase, hypoxanthine-guanine phosphoribosyltransferase (HGPRT), polynucleotide phosphorylase (PNPase), etc. 6-Mercaptopurine, thioguanine are some of the examples of anticancer drugs containing a purine structural framework, and acyclovir, ganciclovir, carbovir, abacavir are few examples of purine-based drugs acting as antiviral agents against herpes or AIDS (Hansen et al. 1985; Munshi et al. 2014; Masson 1983; Wang et al. 2015; Vince 1991). Various substituted purine analogues have been synthesised and screened for their cytotoxic activity over the last decades. Early discoveries of various CDK inhibitors like roscovitine 1, olomoucine 2, purvanalol 3 (Fig. 1), and many other compounds related to them were obtained from the systematic modification of purine skeleton (Havlicek et al. 1997; McClue et al. 2002; MacCallum et al. 2005; Whittaker et al. 2007; Hsieh et al. 2009). Some other purine derivatives like myoseverin are microtubule assembly inhibitors, leading to cytostatic activity by inducing apoptosis (Perez et al. 2002). Most of the purine analogues, which exhibit potent anticancer activity, have been observed to contain a aromatic moiety, such as benzylamine at position 6 that has been found to bind to the hydrophobic pocket near the active site of CDKs (Mary et al. 2015). R-enantiomer of CDK inhibitor (roscovitine) is in clinical trials against cancer in patients diagnosed with non-small lung cancer, or other malignancies (Siegel-Lakhai et al. 2005).
Taking into consideration the toxic side effects of the current convectional chemotherapeutic agents, searching and developing new chemical entities with special characteristics as effective anticancer molecules have been an important driving force for the development of novel anticancer agents, wherein the drug should show cytotoxic effect only on cancer cells without affecting the normal cells (Saman et al. 2018).
Therefore, keeping in view the anticancer potential of purine scaffold and searching for more potent anticancer analogues from purine scaffold, herein we report the synthesis and cytotoxic evaluation of N-9- and N-7-substituted 1,2,3 triazole analogues of 2,6-di-substituted purine.
Results and discussion
Chemistry
2,6,9- and 2,6,7-tri-substituted analogues of purine given in Tables 1 and 2 are synthesised as described in (Scheme 1). 2,6 dichloropurine (4) was subjected for alkylation using propargyle bromide as alkylating agent, potassium carbonate as base and acetone as solvent at room temperature resulting in a mixture of alkylated purines regioisomers (5 and 6) at position N-9 and N-7. N-7-alkylated regioisomer was obtained in minor yield whose structure besides spectral analysis was also confirmed by X-Ray crystal structure (Fig. 2). The SNAr on C6 position was efficiently achieved with complete regiospecifity using primary amine nucleophiles like benzylamine and aminoethanol,1-butanol as solvent and disopropylethylamine as base followed by refluxing for 12 h to form the compound 7 and compound 10, which were, respectively, allowed to undergo 3 + 2 cycloaddition with variously substituted aromatic azides to form N-9- and N-7-substituted 1,2,3 triazoles 8(a–x) and 11(a–x) on the purine scaffold. Finally, the SNAr on C2 of each synthesised analogue was achieved using the cyclic secondary amine bases like pyrolidine and piperidine in the presence of 1-butanol as solvent followed by refluxing for 12 h to form the required compounds 9(a–x) and 12(a–h). All the compounds were identified by 1HNMR, 13C, MS and further verified by X-ray crystal structure. Figure 3 represents the X-ray crystallographic structure of compound 9g.
Biological activity
In vitro anticancer activity of the N-9-substituted 1,2,3 triazole analogues of 2,6-di-substituted purine
N-9-substituted 1,2,3 triazole analogues of 2,6-di-substituted purine (9a–9x) were evaluated for in vitro anticancer activity against a panel of four human cancer cell lines, such as HCT-1 (colon), THP-1 (leukaemia), IMR-32 (neuroblastoma) and A-549 (lung). Table 3 summarises the inhibitory activity (IC50) values of the analogues synthesised and the well-known purine-derived anticancer drug 5-fluorouracil, which was used as standard drug. From the results of screening, it was observed that some of the N-9-substituted 1,2,3 triazole analogues showed very potent anticancer activities in lower μM range against all the human cancer cell lines tested. Further, it was also observed that while most of the synthesised analogues exhibited considerable anticancer activity against leukaemia and lung-derived cancer cell lines. However, compound 9a was found to show potent anticancer activity on all the tested cancer cell lines with IC50 values ranging from 0.08 to 18.9 μM. While compounds 9b and 9e showed potent activity against three tested cancer cell lines, i.e., lung, leukaemia and colon-derived cell lines. Majority of the analogues were also found to show promising activity against the above-mentioned three cancer cell lines.
In vitro anticancer activity of the N-7-substituted 1,2,3 triazole analogues of 2,6-di-substituted purine
N-7-substituted 1,2,3 triazole analogues of 2,6-di-substituted purine (12a–12e) were evaluated for in vitro anticancer activity against a panel of four human cancer cell lines like HCT-1 (colon), THP-1 (leukaemia), IMR-32 (neuroblastoma) and A-549 (lung). Table 4 summarises the inhibitory activity values of analogues synthesised with 5-FU taken as standard drug. From the screening results, it was observed that N-7-substituted compounds 12c and 12d have shown potent cytotoxic activity against all the tested cancer cell lines. While compounds 12a and 12b exhibited significant cytotoxicity on lung-derived cancer cell line.
Conclusion
Commercially available 2,6 dichloropurine was used as a starting substrate for the synthesis of N-9 and N-7-substituted 1,2,3 triazolyl analogues. C6 position of purine scaffold was substituted with amines like benzyl amine and aminoethanol, and C2 position was substituted with cyclic secondary amines like pyrolidine and piperidine. All the analogues were tested for their cytotoxic activity against a panel of four human cancer cell lines. Most of the synthesised derivatives of 2,6-dichloropurine exhibited potent cytotoxicity with three of the compounds 9a, 9b and 9e being most potent within the library of analogues synthesised.

While trying to generate the Structure Activity Relationship (SAR) of the synthesised compounds, it was observed that the activity depended on the substitution on the purine ring. N-9-substituted triazole analogues were found to be more active than N-7-substituted analogues. Further, analogues with benzylamine substitution at C6 position were more active. Activity also varied with the substitution on the aromatic rings attached to 1,2,3 triazole ring at N-9 position. Order of activity observed with the various substituents was H > F > Cl > Br > OCH3. While C2 substitution did not effect the activity to a much extent.
Experimental procedure
Buchi Melting point apparatus D-545 was used to record the melting points of the synthesised compounds. Bruker DPX400 instrument was used to record the Nuclear Magnetic Resonance (NMR) spectra with samples taken in CDCl3. δ (ppm) was used to report chemical shift values with coupling constants taken in Hertz. Electrospray ionization mass spectrometry (ESI MS) was used to record Mass spectra. Monitoring of reactions was done by Thin Layer Chromatography (TLC) on 2–5 cm percolated silica gel 60 F254 plates of thickness 0.25 mm (Merck). UV (254–366 nm) and iodine were used to visualise the chromatograms.
General reaction procedure for synthesis of compounds 5 and 6
Compound 4 (0.2 g, 1 mmol) taken in a two-necked round-bottom flask placed under conditions of inert atmosphere was added K2CO3 (0.2 g, 1.5 mmol) and acetone (10 ml). The resulting mixture was stirred for few minutes. To the mixture was then added propargyl bromide (0.2 ml, 1.5 mmol) and the reaction mixture was allowed to reflux at 60 °C for 2–3 h. The reaction mixture was filtered and concentrated under reduced pressure. Water was added to the reaction mixture and transferred to separatory funnel and extracted with Dichloromethane (DCM). The organic layer was separated, dried with anhydrous MgSO4 and concentrated on rota vapour. Purification of the crude material was done by flash chromatography and eluted with hexane/ethyl acetate. Purified compounds (5) (90%) and (6) with 10% yield were obtained as white crystals; 1H NMR (400 MHz, CDCl3): δ 8.75 (s, 1H8Pu), 5.10 (s, 2HCH2Al), 2.11 (s, 1HCHAl); 13C NMR (100 MHz, CDCl3): 39.5 (CH2), 67.0 (CHAl), 80.3 (CAl), 128.2 (C5Pu), 144.6 (C4Pu), 147.5 (C8Pu), 150.0 (C2Pu), 153.2 (C6Pu); ESI MS: 225.0.
General reaction procedure for synthesis of compound 7
Compound 6 (0.25 g, 1 mmol) was taken in a two-necked round-bottom flask, to which was added n-butanol (10 ml). To the solution was added benzyl amine/ethanolamine (0.13 g, 1.2 mmol) and diisopropylamine (0.19 g, 1.5 mmol), and the reaction mixture was allowed to reflux at 90 °C for 12 h. The reaction mixture was filtered and concentrated under reduced pressure. Water was added to the reaction mixture and transferred to separatory funnel and extracted with DCM. The organic layer was separated, dried with anhydrous MgSO4 and concentrated on rota vapour. Purification of the crude material was done by flash chromatography and eluted with hexane/ethyl acetate. Purified compound (7) was obtained with 95% yield; 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H8Pu), δ 7.30–7.20 (m, 5HBa), 5.20 (s, 2HCH2Ba), 4.80 (s, 2HCH2Al), 2.51 (s, 1HCHAl); 13C NMR (100 MHz, CDCl3): 39.5 (CH2), 67.0 (CHAl), 80.3 (CAl), 58.5(CH2Ba), 126.5 (C4Ba), 127.3 (C3Ba, C5Ba), 127.6 (C2Ba, C6Ba), 128.2 (C5Pu), 142.0 (C1Ba), 144.6 (C4Pu), 147.5 (C8Pu), 150.0 (C2Pu), 153.2 (C6Pu); ESI MS: 297.0.
General reaction procedure for synthesis of compound 8
Compound 7 (0.3, 1 mmol) and substituted aromatic azides (0.1 g, 1.2 mmol) were taken in a 100 ml two-necked round-bottom flask. To the mixture was added CuSO4·5H2O (0.92, 0.5 mmol), sodium ascorbate (0.297, 2 mmol) and tBuOH: H2O 10 ml (1:1) mixture, and allowed to stir for few hours. Water was added to the reaction mixture and transferred to separatory funnel and extracted with ethylacetate. The organic layer was separated, dried with anhydrous MgSO4, and concentrated on rota vapour. Purification of the crude material was done by flash chromatography and eluted with hexane/ethyl acetate. Purified compounds of varying substituent’s on the phenyl ring were obtained with high yields.
General reaction procedure for synthesis of compound 9
Compound 8 (0.416, 1 mmol) taken in a two-necked round-bottom flask placed under inert atmosphere was added nBuOH (10 ml). To the solution was added pyrolidine/piperidine (0.14 ml, 2 mmol) and N,N-Diisopropylethylamine (DIEA) (0.19 ml, 1.5 mmol). The reaction mixture was refluxed for 24 h at 120 °C. The reaction mixture was filtered and concentrated under reduced pressure. Water was added to the reaction mixture and transferred to separatory funnel and extracted with ethylacetate. The organic layer was separated, dried with anhydrous MgSO4, and concentrated on rota vapour. Purification of the crude material was done by flash chromatography and eluted with hexane/ethyl acetate. Purified compounds (9a–x) were obtained with high yields.
General reaction procedure for synthesis of compound 11
Compound 10 (0.416, 1 mmol) taken in a two-necked round-bottom flask placed under inert conditions was added nBuOH (10 ml). To the solution was added pyrole/piperidine (0.14 ml, 2 mmol) and DIEA (0.19 ml, 1.5 mmol). The reaction mixture was subjected to reflux for 24 h at 120 °C. Water was added to the reaction mixture and transferred to separatory funnel and extracted with ethylacetate. The organic layer was separated, dried with anhydrous MgSO4, and concentrated on rota vapour. Purification of the crude material was done by flash chromatography and eluted with hexane/ethyl acetate. Purified compound (11a–e) was obtained with high yields.
Compound characterisation
Benzyl-[9-(1-phenyl-1H-[1,2,3]triazol-4-ylmethyl)-2-pyrrolidin-1-yl-9H-purin-6-yl]-amine (9a)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white crystalline solid with 90% Yield; mp: 156–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H8Pu), 7.75 (s, 1H5T), 7.50 (m, 5HPh), 7.45 (m, 5HBa), 5.40 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.00 (m, 2H1, 2H4(CH2Py)), 1.35 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.7 (C1, C4(CH2py)), 45.2 (C2, C3(CH2py)), 50.3(CH2T)), 58.5(CH2Ba), 126.5 (C4Ba), 127.3 (C3Ba, C5Ba), 127.6 (C2Ba, C6Ba), 128.6 (C3Ph, C4Ph, C5Ph), 128.7 (C1Ph, C2Ph, C6Ph), 138.2 (C5T, C5Pu), 143.6 (C3Pu, C1Ba), 152.0 (C1T), 155.2 (C2Pu), 155.3 (C4Pu, C8Pu), 158.0 (C6Pu); ESI MS: 452 (M+); Anal. calcd. for C25H25N9: C, 66.50; H, 5.58; Found: C, 66.45; H, 5.54.
Benzyl-[9-(1-phenyl-1H-[1,2,3]triazol-4-ylmethyl)-2-piperidin-1-yl-9H-purin-6-yl]-amine (9b)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 80% Yield; mp: 170–172 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H8Pu), 7.75 (s, 1H5T), 7.50 (m, 5HPh), 7.45 (m, 5HBa), 5.40 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.20 (m, 2H1, 2H5(CH2Pi)), 1.50–1.45 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 126.5 (C4Ba), 127.3 (C3Ba, C5Ba), 127.6 (C2Ba, C6Ba),128.6 (C3Ph, C4Ph, C5Ph), 128.7 (C1Ph, C2Ph, C6Ph), 138.2 (C5T, C5Pu), 143.6 (C3Pu, C1Ba), 152.0 (C1T), 155.2 (C2Pu), 155.3 (C4Pu, C8Pu), 158.0 (C6Pu); ESI MS: 466 (M+); Anal. calcd. for C26H27N9: C, 67.08; H, 5.85; Found: C, 66.93; H, 5.04.
Benzyl-{9-[1-(2-chloro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9c)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white crystalline solid with 92% Yield; mp: 150–151 °C; 1H NMR (400 MHz, CDCl3): δ 8.18 (s, 1H8Pu), 7.75 (s, 1H5T), 7.20–7.10 (m, 4HPh), 7.05–7.00 (m, 5HBa), 5.50 (s, HCH2Ba), 4.90 (s, 2HCH2T), 2.7–2.6 (m, 2H1, 2H4(CH2Py)), 1.50–1.45 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 24.6 (C1)(CH2py), 25.9 (C4)(CH2py)), 47.2 (C2, C3(CH2py)), 50.8(CH2T)), 58.5(CH2Ba), 126.5 (C4Ba), 127.3 (C2Ba, C6Ba, C5Ph), 128.0 (C3Ba, C5Ba), 129.0 (C1Ph, C3Ph), 130.7 (C4Ph, C6Ph), 133.0 (C2Ph), 136.2 (C5T, C5Pu), 144.7 (C3Pu, C1Ba), 150.0 (C1T), 154.2 (C2Pu), 157.3 (C4Pu, C8Pu), 158.0 (C6Pu); ESI MS: 486 (M+); Anal. calcd. for C25H24ClN9: C, 61.79; H, 4.85; Found: C, 60.45; H, 5.40.
Benzyl-{9-[1-(2-chloro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9d)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white crystalline solid with 85% Yield; mp: 170–171 °C; 1H NMR (400 MHz, CDCl3): δ 8.68 (s, 1H8Pu), 7.75 (s, 1H5T), 7.20–7.10 (m, 4HPh), 7.05–7.00 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H5(CH2Pi)), 1.60–1.59 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 29.5 (C2, C3), 31.9 (C4)(CH2pi), 44.0 (C1), 46.0 (C5(CH2pi), 50.0(CH2T)), 58.5(CH2Ba), 122.5 (C4Ba), 123.1 (C2Ba), 123.9 (C6Ba, C5Ph), 125.0 (C3Ba), 128.0 (C5Ba), 132.5 (C1Ph, C3Ph), 133.8 (C4Ph, C6Ph), 134.5 (C2Ph), 134.8 (C5T, C5Pu), 149.9 (C3Pu, C1Ba), 151.3 (C1T), 152.6 (C2Pu), 152.8 (C4Pu, C8Pu), 154.7 (C6Pu); ESI MS: 500 (M+); Anal. calcd. for C26H26ClN9: C, 62.46; H, 5.24; Found: C, 61.93; H, 5.04.
Benzyl-{9-[1-(4-chloro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9e)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 95% Yield; mp: 158–159 °C; 1H NMR (400 MHz, CDCl3): δ 8.00 (s, 1H8Pu), 7.85 (s, 1H5T), 7.70 (d, 1H3Ph, 1H5Ph, J = 8.4 Hz), 7.50 (d, 1H2Ph, 1H4Ph, J = 7.5 Hz), 7.30–7.20 (m, 5HBa), 5.40 (s, 2HCH2Ba), 5.30 (s, 2HCH2T), 2.20 (m, 2H1, 2H4(CH2Py)), 1.30 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.7(C1, C4(CH2py)), 45.2 (C2, C3(CH2py)), 50.3(CH2T)), 58.5(CH2Ba), 126.5 (C4Ba), 127.3 (C2Ba, C6Ba, C5Ph), 128.0 (C3Ba, C5Ba), 129.0 (C1Ph, C3Ph), 130.7 (C6Ph, C5T), 134.0 (C6Ph), 138.2 (C1T, C5Pu), 143.6 (C3Pu, C1Ba), 155.2 (C2Pu), 155.3 (C4Pu, C8Pu), 158.0 (C6Pu); ESI MS: 486 (M+); Anal. calcd. for C25H24ClN9: C, 61.79; H, 4.85; Found: C, 60.45; H, 5.40.
Benzyl-{9-[1-(4-chloro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9f)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 80% Yield; mp: 168–169 °C; 1H NMR (400 MHz, CDCl3): δ 8.00 (s, 1H8Pu), 7.85 (s, 1H5T), 7.70 (d, 1H3Ph, 1H5Ph, J = 8.4 Hz), 7.50 (d, 1H2Ph, 1H4Ph, J = 7.5 Hz), 7.30–7.20 (m, 5HBa), 5.40 (s, 2HCH2Ba), 5.30 (s, 2HCH2T), 2.80–2.60 (m, 2H1, 2H5(CH2Pi)), 1.60–1.59 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.3(CH2T)), 58.5(CH2Ba), 126.0 (C4Ba), 127.0 (C2Ba, C6Ba, C5Ph), 128.0 (C3Ba, C5Ba), 129.0 (C1Ph, C3Ph), 130.0 (C6Ph, C5T), 134.0 (C6Ph), 138.0 (C1T, C5Pu), 143.0 (C3Pu, C1Ba), 155.0 (C2Pu), 155.0 (C4Pu, C8Pu), 158.0 (C6Pu); ESI MS: 500 (M+); Anal. calcd. for C26H26ClN9: C, 62.46; H, 5.24; Found: C, 61.93; H, 5.04.
Benzyl-{9-[1-(2-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9g)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 95% Yield; mp: 155–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.75 (s, 1H8Pu), 8.35 (s, 1H5T), 7.70 (m, 4HPh), 7.30–7.20 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.7–2.6 (m, 2H1, 2H4(CH2Py)), 1.60 (m, 2H2, 2H3(CH2Py))(CH2Py)); 13C (100 MHz, CDCl3): 29.6 (C1, C4(CH2py)), 45.6 (C2, C3(CH2py)), 50.2(CH2T)), 60.0(CH2Ba), 110.7 (C1Ph), 126.7 (C4Ba), 127.1 (C2Ba, C6Ba, C5Ph), 128.0 (C3Ba, C5Ba), 128.8 (C1Ph, C3Ph), 137.5 (C5Ph, C5T), 134.0 (C6Ph), 138.2 (C1T, C5Pu), 144.6 (C3Pu, C1Ba, C8Pu), 151.2 (C2Pu), 154.3 (C4Pu), 157.5 (C6Pu); ESI MS: 530 (M+); Anal. calcd. for C25H24BrN9: C, 67.50; H, 5.85; Found: C, 66.45; H, 5.54.
Benzyl-{9-[1-(2-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9h)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 85% Yield; mp: 172–173 °C; 1H NMR (400 MHz, CDCl3): δ 8.75 (s, 1H8Pu), 8.35 (s, 1H5T), 7.70 (m, 4HPh), 7.30–7.20 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H5(CH2Pi)), 1.60–1.59 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 24.6 (C2, C3)(CH2pi), 25.9 (C4)(CH2pi), 50.8 (C1, C5(CH2pi)), 50.3(CH2T)), 58.5(CH2Ba), 124.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C5Ph), 128.0 (C3Ba, C5Ba, C4Ph), 129.0 (C1Ph, C3Ph), 130.7 (C5T), 134.0 (C6Ph), 138.2 (C1T, C5Pu), 143.6 (C3Pu, C1Ba),145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 544 (M+); Anal. calcd. for C26H26BrN9: C, 57.36; H, 4.81; Found: C, 56.80; H, 4.35.
Benzyl-{9-[1-(4-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9i)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 95% Yield; mp: 157–158 °C; 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 1H8Pu), 7.75 (s, 1H5T), 7.60 (d, 1H3Ph, 1H5Ph), 7.25 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 5.40 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.00 (m, 2H1, 2H4(CH2Py)), 1.30 (m, 2H2, 2H3(CH2Py));13C (100 MHz, CDCl3): 23.0 (C1, C4(CH2py)), 45.0 (C2, C3(CH2py)), 50.0(CH2T)), 58.0(CH2Ba), 123.0 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 130.0 (C1Ph, C2Ph, C6Ph), 132.0 (C3Ph, C5Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 530 (M+); Anal. calcd. for C25H24BrN9: C, 67.50; H, 5.85; Found: C, 66.45; H, 5.54.
Benzyl-{9-[1-(4-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9j)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 80% Yield; mp: 172–173 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H8Pu), 7.75 (s, 1H5T), 7.60 (d, 1H3Ph, 1H5Ph), 7.25 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H5(CH2Pi)), 1.80 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.8(CH2T)), 58.4(CH2Ba), 113.4 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 132.9 (C1Ph, C2Ph, C6Ph, C3Ph, C5Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba, C8Pu), 150.2 (C2Pu), 157.3 (C4Pu), 158.0 (C6Pu); ESI MS: 544 (M+); Anal. calcd. for C26H26BrN9: C, 57.36; H, 4.81; Found: C, 56.80; H, 4.35.
Benzyl-{9-[1-(2-fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9k)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 90% Yield; mp: 154–155 °C; 1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H8Pu), 7.85 (s, 1H5T), 7.30–7.20 (m, 4HPh, 5HBa), 5.60 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.50 (m, 2H1, 2H4(CH2Py)), 1.59 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C1, C4(CH2py)), 45.0 (C2, C3(CH2py)), 50.0(CH2T)), 58.0(CH2Ba), 116.0 (C2Ph, C3Ph), 123.0 (C5Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 130.0 (C4Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); 162.0 (C1Ph); ESI MS: 470 (M+); Anal. calcd. for C25H2FN9: C, 63.50; H, 5.15; Found: C, 63.45; H, 5.04.
Benzyl-{9-[1-(2-fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9l)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 80% Yield; mp: 170–171 °C; 1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H8Pu), 7.85 (s, 1H5T), 7.30–7.20 (m, 4HPh, 5HBa), 5.60 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H5(CH2Pi)), 1.60–1.59 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 116.0 (C2Ph, C3Ph), 123.0 (C5Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 130.0 (C4Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); 162.0 (C1Ph); ESI MS: 483 (M+); Anal. calcd. for C26H26FN9: C, 64.58; H, 5.42; Found: C, 64.02; H, 5.40.
Benzyl-{9-[1-(4-fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9m)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 92% Yield; mp: 155–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (s, 1H8Pu), 7.75 (s, H5T), 7.20 (d, 1H2Ph, 1H6Ph, J = 5.4 Hz), 7.15 (d, 1H3Ph, 1H5Ph, J = 6.2 Hz), 7.00 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H4(CH2Py)), 1.60–1.59 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C1, C4(CH2py)), 45.0 (C2, C3(CH2py)), 50.0(CH2T)), 58.0(CH2Ba), 116.0 (C3Ph, C5Ph), 123.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 130.0 (C2Ph, C6Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); 162.0 (C1Ph); ESI MS: 470 (M+); Anal. calcd. for C25H24 FN9: C, 63.50; H, 5.15; Found: C, 63.45; H, 5.04.
Benzyl-{9-[1-(4-fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9n)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’ as white solid with 75% Yield; mp: 165–166 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (s, 1H8Pu), 7.75 (s, H5T), 7.20 (d, 1H2Ph, 1H6Ph, J = 5.4 Hz), 7.15 (d, 1H3Ph, 1H5Ph, J = 6.2 Hz), 7.00 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.90 (s, 2HCH2T), 2.6 (m, 2H1, 2H5(CH2Pi)), 1.39 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 116.0 (C3Ph, C5Ph),123.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba), 130.0 (C2Ph, C6Ph, C5T), 138.2 (C1T, C5Pu), 143.6 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); 162.0 (C1Ph); ESI MS: 483 (M+); Anal. calcd. for C26H26FN9: C, 64.58; H, 5.42; Found: C, 64.02; H, 5.40.
Benzyl-{9-[1-(4-bromo-2-methyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9o)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 160–161 °C; 1H NMR (400 MHz, CDCl3): δ 8.90 (s, 1H8Pu), 8.83 (s, H5T), 7.30–7.20 (m, 2HPh, 5HBa), 7.00 (d, 1H6Ph), 4.70 (s, 2HCH2Ba), 4.30 (s, 2HCH2T), 3.1 (m, 2H1, 2H4(CH2Py)), 2.50 (s, 3HCH3), 1.70 (m, 2H2, 2H3, (CH2Py)); 13C (100 MHz, CDCl3): 12.5 (CH3), 23.0 (C1, C4(CH2py)), 45.0 (C2, C3(CH2py)), 50.0(CH2T)), 58.0(CH2Ba), 123.0 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba, C5Pu), 129.0 (C1Ph, C5Ph), 130.0 (C3Ph, C6Ph, C5T), 133.0 (C1T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 544 (M+); Anal. calcd. for C26H26BrN9: C, 57.36; H, 4.81; Found: C, 55.45; H, 4.04.
Benzyl-{9-[1-(4-bromo-2-methyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9p)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 165–166 °C; 1H NMR (400 MHz, CDCl3): 8.90 (s, 1H8Pu), 8.83 (s, H5T), 7.30–7.20 (m, 2HPh, 5HBa), 7.00 (d, 1H6Ph), 4.70 (s, 2HCH2Ba), 4.30 (s, 2HCH2T), 2.8–2.6 (m, 2H1, 2H5(CH2Pi)), 2.50 (s, 3HCH3), 1.60–1.59 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 12.5(CH3), 25.1(C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 123.0 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba), 128.0 (C3Ba, C5Ba, C5Pu), 129.0 (C1Ph, C5Ph), 130.0 (C3Ph, C6Ph, C5T), 133.0(C1T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 558 (M+); Anal. calcd. for C27H28BrN9: C, 58.08; H, 5.02; Found: C, 58.02; H, 5.40.
Benzyl-{9-[1-(3-bromo-4-methyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9q)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 92% Yield; mp: 155–157 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H8Pu), 8.30 (s, 1H5T), 8.20 (s, 1H2Ph), 7.80 (d, 1H2Ph, J = 4.5 Hz), 7.50 (d, 1H6Ph, J = 5.5 Hz), 7.30–7.20 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 3.30(m, 2H1, 2H4(CH2Py), 2.50 (s, 3HCH3), 2.30 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 13.5 (CH3), 23.5 (C1, C4(CH2py)), 45.0 (C2, C3(CH2py)), 50.0(CH2T)), 58.0(CH2Ba), 124.0 (C3Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5Ph, C5T), 140.2 (C1Ba, C4Ph), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 544 (M+); Anal. calcd. for C26H26BrN9: C, 57.36; H, 4.81; Found: C, 55.45; H, 4.04.
Benzyl-{9-[1-(3-bromo-4-methyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9r)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 160–161 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H8Pu), 8.30 (s, 1H5T), 8.20 (s, 1H2Ph), 7.80 (d, 1H2Ph, J = 4.5 Hz), 7.50 (d, 1H6Ph, J = 5.5 Hz), 7.30–7.20 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.80 (m, 2H1, 2H5(CH2Pi), 2.50 (s, 3HCH3), 1.80 (m, 2H2, 2H3(CH2Pi)); 13C (100 MHz, CDCl3): 13.5(CH3), 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 124.0 (C3Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5Ph, C5T), 140.2 (C1Ba, C4Ph), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 558 (M+); Anal. calcd. for C27H28BrN9: C, 58.08; H, 5.02; Found: C, 58.02; H, 5.40.
Benzyl-{9-[1-(4-methoxy-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-yl}-amine (9s)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 87% Yield; mp: 150–152 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.70 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 6.80 (d, 1H3Ph, 1H5Ph), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 3.50 (s, 3HOCH3), 2.50 (m, 2H1, 2H4(CH2Py), 1.80 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 56.0(OCH3), 58.5(CH2Ba), 110.0 (C3Ph, C5Ph), 120.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 544 (M+); Anal. calcd. for C26H27N9O: C, 64.85; H, 5.65; Found: C, 64.45; H, 5.06.
Benzyl-{9-[1-(4-methoxy-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-yl}-amine (9t)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 160–162 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.70 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 6.80 (d, 1H3Ph, 1H5Ph), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 3.50 (s, 3HOCH3), 3.30 (m, 2H1, 2H5(CH2Pi)), 2.30 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.1 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 56.0(OCH3), 58.5(CH2Ba), 110.0 (C3Ph, C5Ph), 120.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 496 (M+); Anal. calcd. for C27H29N9O: C, 65.48; H, 5.92; Found: C, 64.02; H, 5.69.
2-{9-[1-(4-Fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-ylamino}-ethanol (9u)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 160–162 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.75 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10 (d, 1H3Ph, 1H5Ph), 5.00 (s, 2HCH2Ba), 3.76 (t, 3HCH2Ea), 3.50 (t, 3HCH2Ea), 2.80 (m, 2H1, 2H4(CH2Py)), 1.80 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2py)), 45.3 (C1, C4(CH2py)), 50.5(CH2T)), 54.5(CH2Ea), 60.0(CH2Ea), 115.0(C3Ph, C5Ph), 124.0 (C1Ph), 128.0 (C5Pu), 130.0 (C2Ph, C6Ph), 132.0 (C5T), 145.0 (C8Pu, C1T), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 423 (M+); Anal. calcd. for C20H22 FN9O: C, 56.73; H, 5.25; Found: C, 56.45; H, 5.04.
2-{9-[1-(4-Fluoro-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-ylamino}-ethanol (9v)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 76% Yield; mp: 148–149 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.75 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10 (d, 1H3Ph, 1H5Ph), 5.00 (s, 2HCH2Ba), 3.76 (t, 3HCH2Ea), 3.50(t, 3HCH2Ea), 3.00 (m, 2H1, 2H5(CH2Pi)), 1.50 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 25.0 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 54.5(CH2Ea), 60.0(CH2Ea), 115.0 (C3Ph, C5Ph), 124.0 (C1Ph), 128.0 (C5Pu), 130.0 (C2Ph, C6Ph), 132.0 (C5T), 145.0 (C8Pu, C1T), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 438 (M+); Anal. calcd. for C21H24 FN9O: C, 57.65; H, 5.53; Found: C, 57.45; H, 5.14.
2-{9-[1-(2-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-9H-purin-6-ylamino}-ethanol (9w)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 75% Yield; mp: 157–158 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.75 (s, 1H5T), 7.40 (d, 1H3Ph), 7.20 (m, 1H4Ph, 1H5Ph,1H6Ph), 5.00 (s, 2HCH2Ba), 3.76 (t, 3HCH2Ea), 3.50 (t, 3HCH2Ea), 2.80 (m, 2H1, 2H4(CH2Py)), 1.80 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2py)), 45.3 (C1, C4(CH2py)), 50.5(CH2T)), 54.5(CH2Ea), 60.0(CH2Ea), 123.0 (C2Ph), 128.0 (C5Pu, C5Ph), 131.0 (C4Ph, C6Ph), 132.0 (C5T, C1Ph, C3Ph), 145.0 (C8Pu, C1T), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 487 (M+); Anal. calcd. for C20H22BrN9O: C, 49.50; H, 4.55; Found: C, 46.55; H, 4.54.
2-{9-[1-(4-Bromo-2-methyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-9H-purin-6-ylamino}-ethanol (9x)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 9’. It was obtained as a white solid with 76% Yield; mp: 148–150 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.75 (s, 1H5T), 7.20 (m, 1H3Ph, 1H5Ph), 7.10 (d, 1H6Ph), 5.00 (s, 2HCH2Ba), 3.76 (t, 3HCH2Ea), 3.50 (t, 3HCH2Ea), 3.00 (m, 2H1, 2H5(CH2Pi)), 2.30 (s, 3HCH3), 1.80 (m, 2H2, 2H3, 2H4(CH2Pi)); 13C (100 MHz, CDCl3): 10.5 (CH3), 25.0 (C2, C3, C4(CH2pi)), 45.3 (C1, C5(CH2pi)), 50.5 (CH2T)), 54.5 (CH2Ea), 60.0 (CH2Ea), 123.0 (C4Ph), 128.0 (C5Pu, C1Ph), 129.0 (C5Ph), 130.0 (C6Ph), 132.0 (C5T, C3Ph), 145.0 (C8Pu, C1T), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 512 (M+); Anal. calcd. for C22H26 BrN9O: C, 51.57; H, 5.11; Found: C, 51.45; H, 5.10.
Benzyl-{7-[1-(4-methoxy-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-7H-purin-6-yl}-amine (12a)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 11’. It was obtained as a white solid with 78% Yield; mp: 157–158 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.70 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 6.80 (d, 1H3Ph, 1H5Ph), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 3.50 (s, 3HOCH3), 2.75 (m, 2H2CH2py), 2H3CH2py), 1.80 (m, H2, H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2pi)), 50.3 (C1, C4(CH2pi)), 50.5(CH2T)), 56.0(OCH3), 58.5(CH2Ba), 110.0 (C3Ph, C5Ph), 120.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 482 (M+); Anal. calcd. for C26H27N9O: C, 64.80; H, 5.65; Found: C, 64.55; H, 5.54.
Benzyl-{7-[1-(4-methoxy-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-piperidine-1-yl-7H-purin-6-yl}-amine (12b)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 11’. It was obtained as a white solid with 80% Yield; mp: 148–149 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.70 (s, 1H5T), 7.20 (d, 1H2Ph, 1H6Ph), 7.10–7.00 (m, 5HBa), 6.80 (d, 1H3Ph, 1H5Ph), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 3.50 (s, 3HOCH3), 2.65 (m, 2H2CH2pi), 2H3CH2pi, 2H4CH2pi), 1.80 (m, 2H1, 2H5(CH2Pi)); 13C (100 MHz, CDCl3): 25.0 (C2, C3, C4(CH2pi)), 50.3 (C1, C5(CH2pi)), 50.5(CH2T)), 56.0(OCH3), 58.5(CH2Ba), 110.0 (C3Ph, C5Ph), 120.0 (C1Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 132.0 (C2Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu), 162.0 (C4Ph); ESI MS: 469 (M+); Anal. calcd. for C25H26N9O: C, 64.08; H, 5.59; Found: C, 63.93; H, 5.14.
Benzyl-{2-pyrrolidin-1-yl-7-[1-(4-trifluoromethyl-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-7H-purin-6-yl}-amine (12c)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 11’. It was obtained as a white solid with 75% Yield; mp: 155–156 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (s, 1H8Pu), 7.70 (s, 1H5T), 7.40 (d, 1H3Ph, 1H5Ph, J= 7.8 Hz), 7.20 (d, 1H2Ph, 1H6Ph, J= 6.8 Hz), 7.10–7.00 (m, 5HBa), 5.50 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.75(m, 2H2CH2py), 2H3CH2py), 1.80 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2pi)), 50.3 (C1, C4(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 114.0 (CF3), 125.0 (C3Ph, C5Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C6Ph), 128.0 (C3Ba, C5Ba, C5Pu), 129.0 (C6Ph, C2Ph),132.0 (C1Ph, C4Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 520 (M+); Anal. calcd. for C26H24F3N9: C, 60.11; H, 4.66; Found: C, 60.05; H, 4.54.
Benzyl-{7-[1-(4-bromo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-7H-purin-6-yl}-amine (12d)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 11’. It was obtained as a white solid with 80% Yield; mp: 152–153 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H8Pu), 8.50 (s, 1H5T), 7.40 (d, 1H3Ph, 1H5Ph, J= 7.8 Hz), 7.30 (d, 1H2Ph, 1H6Ph, J= 6.8 Hz), 7.10–7.00 (m, 5HBa), 5.90 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.75 (m, 2H2CH2py), 2H3CH2py), 1.80 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2pi)), 50.3 (C1, C4(CH2pi)), 50.5(CH2T)), 58.5(CH2Ba), 123.0 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C1Ph), 128.0 (C3Ba, C5Ba, C5Pu, C1Ph), 131.0 (C3Ph, C5Ph), 132.0 (C2Ph, C6Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 530 (M+); Anal. calcd. for C25H24BrN9: C, 56.61; H, 4.56; Found: C, 55.05; H, 4.50.
Benzyl-{7-[1-(4-iodo-phenyl)-1H-[1,2,3]triazol-4-ylmethyl]-2-pyrrolidin-1-yl-7H-purin-6-yl}-amine (12e)
This compound was prepared according to the procedure given in the section ‘General reaction procedure for synthesis of compound 11’. It was obtained as a white solid with 77% Yield; mp: 150.0–153 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H8Pu), 8.50 (s, 1H5T), 7.65 (d, 1H3Ph, 1H5Ph, J= 6.4 Hz), 7.30 (d, 1H2Ph, 1H6Ph, J= 4.8 Hz), 7.10–7.00 (m, 5HBa), 5.90 (s, 2HCH2Ba), 4.60 (s, 2HCH2T), 2.80 (m, 2H2CH2py, 2H3CH2py), 2.00 (m, 2H2, 2H3(CH2Py)); 13C (100 MHz, CDCl3): 23.0 (C2, C3(CH2py)), 50.3 (C1, C4(CH2py)), 50.5(CH2T)), 58.5(CH2Ba), 100 (C4Ph), 126.5 (C4Ba), 127.0 (C2Ba, C6Ba, C1Ph), 128.0 (C3Ba, C5Ba, C5Pu), 130.0 (C3Ph, C5Ph), 132.0 (C2Ph, C6Ph, C5T), 140.2 (C1Ba), 145.0 (C8Pu), 155.2 (C2Pu), 155.3 (C4Pu), 158.0 (C6Pu); ESI MS: 578 (M+); Anal. calcd. for C25H24IN9: C, 52.00; H, 4.19; Found: C, 51.65; H, 4.00.
Cell culture and growth conditions
A panel of human cancer cell lines—HCT-116 (colon), HT-29 (colon), MCF-7 (breast), prostate (PC-3), SF-268 (CNS)—were procured from U.S. National Cancer Institute (NCI). Cell lines were grown in tissue culture flasks in complete growth medium (RPMI-1640) supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin and 100 units/ml penicillin in carbon dioxide incubator (New Brunswick, Galaxy 170 R, Eppendorf) at 37 °C, 5% CO2 and 98% RH.
Method for sulforhodamine B assay (SRB assay)
For cytotoxicity evaluation, cell suspension of optimum cell density was seeded in 96-well flat-bottom plates (NUNC). Cell lines were seeded at respective inoculation densities per well as HCT-116 (7500), HT-29 (7500), MCF-7 (8000), Prostate (7500), SF-268 (10,000). One-hundred microlitres of cell suspension was plated. The cells were then exposed to different concentrations of test materials containing complete growth medium for 24 h. The plates were again incubated under the same conditions for another 48 h at 37 °C. Further, cells were fixed with ice cold TCA (trichloroacetic acid) for 1 h at 4 °C. After 1 h, the plates were rinsed three times with water and allowed to air dry. After drying, 100 μl of 0.4% SRB dye was added for half an hour at room temperature. Plates were then washed three times with 1% vol/vol acetic acid to remove the unbound SRB. After drying at room temperature, the bound dye was solublized by adding 100 μl of 10 mM TRIS (tris (hydroxymethyl)aminomethane) buffer (pH-10.4) to each well. The plates were kept on the shaker for 5 min to solublize the protein bound dye. Finally, optical density (OD) was taken at 540 nm in a microplate reader (Thermo Scientific). IC50 was determined by plotting OD against concentration using graph pad prism software.
Abbreviations
- Ph:
-
Phenyl ring
- Ba:
-
Benzyl amine
- T:
-
Triazole ring
- Pu:
-
Purine ring
- Ea:
-
Ethanol amine
- Py:
-
Pyrolidine
- Pi:
-
Piperidine
- CH2Ba:
-
Methylene group attached to benzene ring of benzyl amine
- CH2T:
-
Methylene group attached to Triazole ring
References
De Clercq E (1997) Current and potential therapies for the treatment of herpes-virus infections. Clin Microbiol Rev 10:674–669
Hansen SW, Skovsgaard T, Sorensen JB (1985) Treatment of small cell lung cancer with 6-mercaptopurine: A phase II study. Cancer Treat Rep 69:555
Havlicek L, Hanus J, Vesely J, LeClerc S, Meijer L, Shaw G, Strnad M (1997) 8-Azapurines as new inhibitors of cyclin-dependent kinases. J Med Chem 40:408–412
Jeannette CA, Christian EP, Álvaro CM, Ricardo AT, Mario F, Maria JT, Adam A, Margot P, Cristian OS (2015) Synthesis and pharmacophore modelling of 2,6,9-trisubstituted purine derivatives and their potential role as apoptosis-inducing agents in cancer cell lines. Molecules 20(4):6808–6826
Kay NE (1981) Abnormal T cell subpopulation function in CLL: excessive suppressor and deficient helper activity with respect to B cell proliferation. Blood 57:418–420
Legraverend M, Grierson DS (2006) The purines: potent and versatile small molecule inhibitors and modulators of key biological targets. Med Chem 14:3987–4006
MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, Lane DP, Green SR (2005) Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res 65:5399–5407
Marr JJ (1991) Purine analogs as chemotherapeutic agents in leishmaniasis and American trypanosomiasis. J Lab Clin Med 118:111–119
Mary EL, Patrick EC, Satya N, Brian KL (2015) Cyclin-dependent kinase inhibitors as anticancer therapeutics. Mol Pharmacol 88:846–852
Masson C (1983) Treatment of herpes with acyclovir. Presse Med 12:1399–1400
McClue SJ, Blake D, Clarke R, Cowan A, Cummings L, Fischer PM, MacKenzie M, Melville J, Stewart K, Wang S, Zhelev N, Zheleva D, Lane DP (2002) Int J Cancer 102:463–468
Melroy J, Nair V (2005) The antiviral activity, mechanism of action, clinical significance and resistance of abacavir in the treatment of pediatric AIDS. Curr Pharm Des 11:3847–3852
Munshi PN, Lubin M, Bertino JR (2014) 6-Thioguanine: a drug with unrealized potential for cancer therapy. Oncologist 19:760–765
Nabhan C, Gartenhaus RB, Tallman MS (2004) Purine nucleoside analogues and combination therapies in B-cell chronic lymphocytic leukaemia: dawn of a new era. Leuk Res 28(5):429–442
Panos F, Bruce CA, Michael LG (1996) Purine analogs for the treatment of low-grade lymph proliferative disorder. Oncologist 1(3):125–139
Perez OD, Chang YT, Rosania G, Sutherlin D, Schultz PG (2002) Inhibition and reversal of myogenic differentiation by purine-based microtubule assembly inhibitors. Chem Biol 9(4):475–483
Quan DJ, Peters MG (2004) Antiviral therapy: nucleotide and nucleoside analogs. Clin Liver Dis 8(2):371–385
Rosemeyer H (2004) The chemo diversity of purine as a constituent of natural products. Chem Biodivers 1:361–401
Saman MV, Salim KY, Danter WR, Koropatnick J (2018) PLoS ONE 13(1):e0191766
Siegel-Lakhai WS, Rodenstein DO, Beijnen JH, Gianella-Borradori A, Schellens JHM, Talbot DC (2005) Clinical trial designs. J Clin Oncol 23:2060–2060
Vince R (1991) Synthesis and anti-HIV activity of carbovir and related carbocyclic nucleosides. Nucl Acids Symp Ser 25:193–194
Wang X, Wang L, Wu N, Ma X, Xu J (2015) Clinical efficacy of oral ganciclovir for prophylaxis and treatment of recurrent herpes simplex keratitis: corrigendum. Chin Med J 128:46–50
WS Hsieh, R Soo, BK Peh, T Loh, D Dong, D Soh, LS Wong, S Green,J Chiao, CY Cui, YF Lai, SC Lee, B Mow, R Soong, M Salto-Tellez, BC Goh (2009) Pharmacodynamic effects of seliciclib, an orally administered cell cycle modulator, in undifferentiated nasopharyngeal cancer. Clin Cancer Res 15:1435–1442
Welsch ME, Snyder SA, Stockwell BR (2010) Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 2010(14):347–361
Whittaker SR, Te Poele RH, Chan F, Linardopoulos S, Walton MI, Garrett MD, Workman P (2007) The cyclin-dependent kinase inhibitor seliciclib (R-roscovitine; CYC202) decreases the expression of mitotic control genes and prevents entry into mitosis. Cell Cycle 6:3114–3131
Acknowledgements
We thank University of Pretoria for providing postdoctoral fellowship to JK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Khazir, J., Mir, B.A., Chashoo, G. et al. Synthesis and anticancer activity of N-9- and N-7- substituted 1,2,3 triazole analogues of 2,6-di-substituted purine. Med Chem Res 29, 33–45 (2020). https://doi.org/10.1007/s00044-019-02456-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02456-9