Abstract
Nesidiocoris tenuis Reuter (Het.: Miridae) is widely used as a biological control agent of whiteflies and other pests in greenhouse-grown tomatoes. It is typically released augmentatively some weeks after transplanting and needs several weeks to establish. Releasing N. tenuis prior to transplanting could accelerate its establishment. However, timing for releases could affect biological control and require changes in release rates of the predator. Because N. tenuis is also phytophagous it must be released at a rate which provides the best equilibrium between adequate biological control of Bemisia tabaci Genn. and acceptable injury to the crop. The objective of this study was therefore to evaluate different release rates for releasing N. tenuis prior to transplanting for maximizing control capacity and minimizing injury to crop. The study was carried out in two subsequent trials in which different release rates were evaluated under a worst case scenario of rapid immigration of the pest into a tomato greenhouse. In the first experiment (winter experiment), four treatments were compared: (1) B. tabaci (0 N. tenuis/plant), (2) B. tabaci + 0.5 N. tenuis/plant, (3) B. tabaci + 1 N. tenuis/plant and (4) B. tabaci + 2 N. tenuis/plant. In the second experiment (summer experiment), the treatments were: (1) B. tabaci (0 N. tenuis/plant), (2) B. tabaci + 0.5 N. tenuis/plant and (3) B. tabaci + 1 N. tenuis/plant. All the evaluated rates significantly reduced the population of whitefly and gave adequate control of the pest. However, only 0.5 N. tenuis/plant did not increase crop damage compared to the treatment with no N. tenuis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mirid bug Nesidiocoris tenuis Reuter (Het.: Miridae) commonly appears in tomato and other agricultural crops and on natural vegetation in several places in the Mediterranean region and the Canary Islands (Malausa and Henao 1988; Goula and Alomar 1994; Tavella and Goula 2001; Sánchez et al. 2003). It has been reported as an effective natural enemy for reducing populations of whitefly and other pests (Urbaneja et al. 2003, 2009; Sánchez and Lacasa 2008; Calvo et al. 2009) and has been used for whitefly control in tomato crops since 2002 (Calvo and Urbaneja 2004).
In tomato greenhouses N. tenuis is commonly released augmentatively three or four weeks after transplanting at a rate of 1–2 individuals per m2 (Calvo and Urbaneja 2004) and this approach usually results in a successful biocontrol of whitefly (Calvo et al. 2009). However, the predator normally requires five to eight weeks after release to reach a sufficiently high population density to keep the pest under control and consequently, other control measures such as insecticide applications are frequently needed to keep the pest under control during this period.
Establishing the predator earlier in the crop could decrease the investment in other control measures and would improve biocontrol. Lenfant et al. (2000) developed an alternative method for the release of the mirid bug, Macrolophus caliginosus Wagner (Het.: Miridae) which resulted in a successful and sooner establishment of the predator in tomato. This method consisted in the release of the predator adults on the seedlings before transplanting at the plant propagator facilities. The predators are fed with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs when released and kept for several days for egg laying. Nymphs start to hatch from eggs afterwards, once the crop has been transplanted inside the greenhouse. N. tenuis which is biologically comparable to M. caliginosus, could possibly be released in the same way.
Timing, rate and frequency of release, synchronization between prey and predator and abiotic factors (humidity, photoperiod and temperature) can modify control capacity (Crowder 2007). Release strategies must therefore be adapted to implement augmentative biological control successfully. Among these factors, release rate may determine the ultimate effectiveness and subsequent economic benefits and can be most easily modified.
The objective of the present study was to evaluate different release rates for a plant propagator application of N. tenuis. This was carried out in two subsequent trials in which different release rates were evaluated under a worst case scenario of rapid immigration of the pest into a tomato greenhouse. However, this strategy has to take into account that N. tenuis is an omnivore and therefore it feeds both on insects and plants (Sánchez 2008; Calvo et al. 2009; Arnó et al. 2010). It can cause damage to the crop which is characterized by the appearance of necrotic rings on leaves, shoots, sprouts and flower petioles, flower abortion and punctures in tomato fruits (El-Dessouki et al. 1976). Therefore, the release rate for N. tenuis must provide equilibrium between adequate biological control of B. tabaci under typical commercial greenhouse conditions and acceptable injury to the crop.
Materials and methods
Greenhouse
The experiments were conducted in a multi-tunnel greenhouse located in Vicar (Almeria, Andalusia, Spain). Walk-in cages were constructed inside the greenhouse to accommodate the plants and maintain treatments. Each walk-in cage (5 L × 3.5 W × 4 H m) was constructed of ‘anti-thrips’ polyethylene screening with 220 × 331 μm interstices and supported by heavy wires. Floors were covered with woven 2-mm-thick polyethylene cloth and access to each cage was through a zippered doorway. Sixteen and twelve of these compartments were used for the first (winter experiment) and second experiment (summer experiment), respectively. The greenhouse was equipped with Climatec™ system (Novedades Agrícolas, Murcia, Spain) for temperature and relative humidity (RH) control. Temperature and RH were monitored in four randomly selected walk-in cages with HOBO H8 RH/Temp Loggers (Onset Computer, Bourne, MA, USA).
Experimental design
Four treatments were compared in the winter and three in the summer experiment in a completely randomized block design with four replicates. The treatments in the winter experiment were: (1) B. tabaci only (0 N. tenuis/plant), (2) B. tabaci + 0.5 N. tenuis/plant (0.5 N. tenuis/plant), (3) B. tabaci + 1 N. tenuis/plant (1 N. tenuis/plant) and (4) B. tabaci + 2 N. tenuis/plant (2 N. tenuis/plant). In the summer experiment, the treatments were: (1) B. tabaci only (0 N. tenuis/plant), (2) B. tabaci + 0.5 N. tenuis/plant (0.5 N. tenuis/plant) and (3) B. tabaci + 1 N. tenuis/plant (1 N. tenuis/plant). These rates were chosen based on results of earlier experiments (Calvo et al. 2009).
Whiteflies, predators and supplemental food
Bemisia tabaci adults to infest the tomato plants were collected from a mass-rearing colony maintained on tobacco plants (Nicotiana tabacum L.; Solanaceae) and originally obtained locally and identified with polymerase chain reaction (PCR) as biotype ‘Q’. Nesidiocoris tenuis was provided by Koppert Biological Systems in bottles containing 500 adults (NESIBUG™, Koppert Biological Systems, The Netherlands). Eggs of E. kuehniella used as supplemental food during the experiment were supplied by Koppert Biological Systems in bottles containing 10 g of eggs (ENTOFOOD™, Koppert Biological Systems, The Netherlands).
Experimental procedure
Seeds of tomato, Solanum lycopersicum L. (Solanaceae) cv. Boludo (Seminis Vegetable Seeds, Enkhuizen, The Netherlands), were first sown into 5.4 cm2 peat moss root cubes. When seedlings reached the five-leaves stage, they were moved to ‘inoculation’ cages (1 × 1 × 1.5 m). Each inoculation cage contained 40 seedlings, all of which were destined for one treatment. Adult N. tenuis were then cooled briefly in a cold room at 8 °C for counting before being released into designated inoculation cages at a sex ratio of 1:1 and at the established release rate for each treatment (20 adults for 0.5 N. tenuis/plant, 40 adults for 1 N. tenuis/plant and 80 adults for 2 N. tenuis/plant). Four paper strips (3 × 1 cm) with eggs of E. kuehniella glued to one side were also placed inside the inoculation cages to serve as a food source for the mirids. Plants were maintained inside the inoculation cages for five days, after which adult N. tenuis were removed. Seedlings were then transplanted on 27 February 2009 and 23 June 2009 for the winter and summer experiments, respectively, into 25 l coco peat fibre bags placed inside the designated walk-in cage, at ten seedlings per cage providing a plant density of 2 plants m−2. Eggs of E. kuehniella were sprinkled on all plants at a rate of 0.01 g in each walk-in cage with N. tenuis, beginning at transplanting and for four and two weeks thereafter in the winter and summer experiment, respectively. Availability of whitefly nymphs was lowest during this period and supplementary food was added to increase the likelihood of establishment due to the incapability of N. tenuis nymphs to reach maturity in the absence of prey (Urbaneja et al. 2005).
Crop cultivation techniques typical for greenhouse tomato cultivation were followed: plants were trained by the main stem to a black polyethylene string tied to a stainless steel overhead wire. Secondary shoots were removed and water and fertilizers were supplied as required through a drip irrigation system.
For both experiments, whitefly adults for infestation were cooled briefly in a cold room at 8 °C for counting and then released in all the cages at a rate of 10 whiteflies/plant over three consecutive weeks for a total of 30 whiteflies/plant. First whitefly release was carried out just after transplanting. This release schedule was used to simulate gradual but heavy immigration of the pest into the greenhouse. Adult whiteflies to be released into walk-in cages were collected each week from a single colony cohort to assure homogeneity in age and sex ratio.
Sampling
Plants were monitored weekly for 13 weeks in the winter experiment and six weeks in the summer experiment, beginning on 5 March 2009 and 30 June 2009, respectively. For both experiments, five plants were randomly selected in each experimental cage. Whitefly nymphs were counted on three leaves from each of the five selected plants. One leaf was selected at random from the upper, middle, and bottom third of the plant. Nymphs and adults of N. tenuis as well as the number of necrotic rings were counted on five leaves from each of these five plants. In this case, three of the latter leaves were selected at random from the upper third, one from the middle third, and one from the bottom third of the plant. In each case, leaves were turned carefully to count first whitefly and N. tenuis adults and then the other insect stages using a 15× hand lens. Finally, five fully flowering trusses per cage were randomly selected and then the number of non injured, injured (viable but showing necrotic rings) and aborted flowers were counted. Selected leaves or trusses were not removed after counting.
Ambient conditions
The mean weekly temperature during the winter experiment ranged from 17.2 to 23.1 °C. During the summer experiment it varied between 24.4 to 28.1 °C. Mean weekly RH fluctuated from 60.1 to 70.4 and 53.8 to 67.9 % in the winter and summer experiment, respectively.
Analysis
Treatment effects on whitefly, N. tenuis and injury to crop (necrotic rings and percentage of flower abortion) in both experiments were analysed with linear mixed effects models, with time (weeks) as random factor nested in blocks to correct for pseudoreplication due to repeated measures as in previous experiments with repeated measurements (see Messelink et al. 2008; Calvo et al. 2009). Thereafter, treatments were compared through model simplification by combining treatments (Crawley 2002). The number of whiteflies (nymphs and adults), N. tenuis and necrotic rings were log (x + 1) transformed prior to analysis. The proportion of flower abortion was arcsin √x + 1, transformed prior to analysis. In both cases this was done to stabilize error variance. Untransformed values are given in the figures. Temperature and humidity were initially included in the analysis, but they proved not to be significant and were therefore removed from further analysis. Abbott’s formula 100 × [(1 − (treated/control)] (Abbott 1925) was used to represent the degree of nymphal whitefly suppression obtained by the release of N. tenuis during both experiments.
Results
Winter experiment
Whitefly
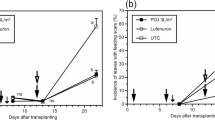
Number of whitefly adults was different among treatments (F 3, 153 = 20.471, P < 0.001). However, the mean whitefly adult density per leaf was similar in all the treatments until eight weeks after transplanting (Fig. 1a). Thereafter it increased strongly and was greatest in the treatment receiving only B. tabaci (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 51 = 22.622, P < 0.001; 0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 15.647, P < 0.001; 0 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 29.229, P < 0.001). In contrast, in the treatment receiving the highest rate of N. tenuis the number of whitefly adults per leaf remained nearly constant until the end of the experimental period and was lowest among the four treatments (2 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 51 = 8.616, P = 0.005; 2 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 21.165, P < 0.001). Population density of whitefly adults in the other two treatments receiving N. tenuis also increased after the eighth week, but decreased rapidly until the end of the experiment. However, the abundance of whitefly adults was higher in these two treatments compared to the treatment receiving the highest rate but did not differ from each other (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 0.718, P = 0.404).
The number of whitefly nymphs per leaf was similar in all treatments during the first ten weeks. Thereafter, the whitefly nymphs were significantly (F 3, 153 = 27.063, P < 0.001) suppressed by N. tenuis by 91.8, 96.2 and 98.5 % in the treatments receiving 0.5, 1 and 2 N. tenuis/plant compared to an exponential increase to more than 160 nymphs per leaf in the absence of N. tenuis (Fig. 1b). Consequently, the highest density was found in the treatment with only B. tabaci (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 51 = 17.483, P < 0.001; 0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 21.201, P < 0.001; 0 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 39.866, P < 0.001), the lowest in the treatment receiving the highest release rate of N. tenuis (2 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 51 = 31.115, P < 0.001; 2 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 22.718, P < 0.001) and intermediate in the treatments receiving 0.5 and 1 N. tenuis per plant (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 0.541, P = 0.412).
Nesidiocoris tenuis
The first N. tenuis individuals were observed one week after transplanting in all treatments (Fig. 2). Two generations were observed during the experiment and the highest number of N. tenuis per leaf was always observed in the treatment with the highest release rate. During the first generation the population density was similar in treatments receiving 0.5 and 1 N. tenuis/plant, but during the second generation densities were higher in the treatment with the intermediate rate. Thus, the abundance of N. tenuis was different among treatments (F 2, 102 = 83.544, P < 0.001), with progressively higher abundance of the predators at higher release rates (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 26.518, P < 0.001; 0.5 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 119.534, P < 0.001; 1 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 77.892, P < 0.001).
Injury to crop
Necrotic rings appeared soon after the transplanting in the crop in all treatments where N. tenuis was released and the number of necrotic rings per leaf was related to the abundance of N. tenuis per leaf (Fig. 3a). Consequently, the number of necrotic rings was increasingly higher with the higher release rate (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 51 = 15.261, P < 0.001; 0.5 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 43.323, P < 0.001; 1 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 51 = 23.212, P < 0.001).
Incidence of flower abortion was also different between treatments (Fig. 3b) (F 3, 102 = 17.314, P < 0.001), but the lowest rate did not increase flower losses compared to the treatment without N. tenuis (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 107 = 1.462, P = 0.229), whereas the two highest rates, which were not different (1 N. tenuis/plant vs. 2 N. tenuis/plant: F 1, 107 = 1.675, P = 0.198), increased the flower abortion in respect to the treatment with no predators (0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 107 = 22.706, P < 0.001; B. tabaci vs. 2 N. tenuis/plant: F 1, 107 = 36.429, P < 0.001).
Summer experiment
Whitefly
The number of whitefly adults remained constant over the experimental period and was not different in both treatments receiving N. tenuis (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 0.630, P = 0.438). In the treatment with whitefly only the population density of whitefly adults started to increase three weeks after transplanting and decreased three weeks later, but it was higher compared to the treatments with N. tenuis (Fig. 4a) (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 23 = 29.964, P < 0.001; 0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 25.745, P < 0.001).
The number of whitefly nymphs per leaf increased constantly during the experiment and was highest in the whitefly only treatment (Fig. 4b) (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 23 = 32.747, P < 0.001; 0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 30.735, P < 0.001), whereas in the treatments with N. tenuis, which were not different (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 2.444, P = 0.136), it increased only during the first week after transplanting and decreased progressively afterwards until the end of the experiment, when the degree of whitefly nymph suppression was more than 98 % in both treatments.
Nesidiocoris tenuis
In both treatments receiving N. tenuis, the first individuals of the predator were observed one week after transplanting. The density decreased slowly until the third week when it started to increase again until the end of the experiment (Fig. 5). However, the predator density differed between treatments with N. tenuis (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 5.785, P = 0.028).
Crop damage
In both treatments with N. tenuis the first necrotic rings were observed one week after transplanting and the number per leaf remained constant until the fourth week when it started to increase until the end of the experiment (Fig. 6a). Therefore, changes in the number of necrotic rings per leaf were similar in both treatments, but their abundance was higher with 1 N. tenuis/plant (0.5 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 23 = 6.638, P = 0.017).
Flower abortion was higher in the treatment with 1 N. tenuis per plant (0 N. tenuis/plant vs. 1 N. tenuis/plant: F 1, 107 = 21.551, P < 0.001; 0.5 N. tenuis/plant vs. 1 N. tenuis/plant : F 1, 107 = 13.968, P < 0.001) and the lower rate did not increase the percentage of aborted flowers in respect to the treatment with no predators (Fig. 6b) (0 N. tenuis/plant vs. 0.5 N. tenuis/plant: F 1, 107 = 2.379, P = 0.126).
Discussion
Relatively high numbers of N. tenuis per leaf were observed very soon after transplanting, reflecting that the pre-plant release application allowed a quick establishment of the predator. Comparison of these findings with earlier studies releasing the predator after transplanting (Calvo et al. 2009) confirms that establishment of the predator is accelerated when N. tenuis is released before transplanting. This also agrees with the observation of Lenfant et al. (2000) on M. caliginosus, who reported that M. caliginosus established earlier in the season when it was released before transplanting. In addition, Calvo (unpublished data), who conducted a later experiment evaluating pre- and post-planting releases of N. tenuis, also observed that the predator established and colonized the crop more rapidly and reached higher population densities when released before transplanting. Nesidiocoris tenuis females need to mate periodically in order to maintain a sufficient sperm supply to fertilize their eggs (Franco et al. 2011) and predator populations are more concentrated when released before planting. This may result in a higher encounter rate, giving more opportunities for adults to mate and to fertilize maturing eggs. This would increase the progeny, and therefore abundance of insect in the subsequent generation, which would accelerate the establishment. Moreover, fecundity and longevity and preimaginal survival of N. tenuis increase when the predator is fed with E. kuehniella eggs (Urbaneja et al. 2005). Thus, addition of E. kuehniella eggs during the first weeks of the experiment, which is a normal procedure in practice, should have also a positive effect enhancing establishment of N. tenuis.
During the first experiment significantly fewer whitefly nymphs and adults were seen where N. tenuis was released, with the highest release rate (2 N. tenuis/plant) providing more rapid suppression of B. tabaci than the two other rates, which also controlled the pest very effectively. The second experiment confirmed that 0.5 and 1 N. tenuis/plant provide similar and effective control of the pest. This capability of N. tenuis to control whitefly in tomato has been reported earlier by Calvo et al. (2009) and Sánchez and Lacasa (2008). However, in our study 0.5 and 1 N. tenuis/plant were equally effective, whereas 2 N. tenuis/plant was more effective than the lower rates. This is contrary to the observation from Calvo et al. (2009) who reported similar control of an initial infestation of approximately 5 whitefly adults per plant with 1 and 4 N. tenuis per plant. Crowder (2007) identified eight different factors that limit the relative impact of release rates on the effectiveness of augmentative biological control: prey availability, initial settlement rates, fecundity, dispersal, cannibalism, the method of release, the timing of releases, and insecticides. Among these factors, differences in initial settlement rates could explain why Calvo et al. (2009) did not find such differences, whereas they were observed in the present experiments.
In the first (winter) experiment the two higher rates increased the percentage of aborted flowers compared to the treatment with B. tabaci only. Therefore, the highest release rate was not considered for the second (summer) experiment. The results of the second experiment confirmed that 1 N. tenuis/plant increased the flower abortion in respect to the lower rate and the treatment receiving B. tabaci only. These results show that plant feeding by N. tenuis can be managed to maximize its benefits as a predator and minimize its disadvantages as an herbivore by modifying the release rate and indicate that optimal rate for releasing N. tenuis before transplanting should be between 0.5 and 1 N. tenuis/plant.
Damage caused by N. tenuis to plants has been related to prey availability (Sánchez and Lacasa 2008; Calvo et al. 2009; Sánchez 2009), which agrees with the results of the present study, where abundance of necrotic rings was higher when prey availability was lower. Plant feeding in dicyphines greatly varies depending on the mirid, ambiental conditions and host plant species (Alomar and Albajes 1996; Castañé et al. 2003, 2011; Gillespie et al. 2007; Sánchez 2008). In this sense, Gillespie and McGregor (2000) proposed three simple models for feeding behaviour in omnivorous Heteroptera: (1) the amount of plant feeding decreases with increased prey feeding (facultative), (2) the amount of plant feeding increases with increased prey feeding (essential) and (3) the amount of plant feeding is independent of the amount of prey feeding. N. tenuis would therefore appear to follow the first model. However, necrotic rings were observed from the start, when E. kuehniella eggs and first whitefly nymphs were available, suggesting that feeding behaviour of N. tenuis could also fit under certain circumstances to the second model. This can be explained by the fact that predaceous Heteroptera need substantial amounts of water enabling them to feed on their prey based on extra-oral digestion and to maintain their physiological balance. Water is mainly obtained by feeding on plant tissues. Likewise, Castañé et al. (2011) concluded that these models are appropriate for explaining the behavior of individual predators and are not mutually exclusive: individuals could behave according to one model or another depending on crop circumstances and plant feeding is essential rather than facultative. In addition, N. tenuis can develop by preying upon other pests like the western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), the two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) or the tomato borer, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) (Urbaneja et al. 2003, 2009). Thus, the presence of these other pests could potentially increase the prey availability and therefore the tolerance level for N. tenuis per plant without significant increase in plant damage. The artificial addition of a supplementary food source such as E. kuehniella eggs could have the same effect. Managing the availability of alternative food sources may therefore offer an extra tool for managing the level of plant feeding by N. tenuis.
The pre-plant application has an additional technical advantage. When N. tenuis is released after transplanting it is distributed in release spots placed regularly throughout the crop (Calvo and Urbaneja 2004). Thus, N. tenuis needs some weeks after the release to colonize the crop. Contrary, when releasing before transplanting, most of the plants contain eggs when they are transplanted, resulting in a faster and more regular distribution of the predator throughout the crop. In addition, releasing the predator in the nursery reduces application costs. Growers releasing the predator after planting need to walk around the entire greenhouse to distribute the insects, which is time consuming. Less time is required when releases are done in the nursery given the higher concentration of plants. In conclusion, the present experiment provides practical guidelines for successful control of high initial pest populations of whitefly in tomato based exclusively on augmentative biological control with N. tenuis released before transplanting and demonstrates the great potential of this new approach.
References
Abbott WA (1925) A method to computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Alomar O, Albajes R (1996) Greenhouse whitefly (Homoptera: Aleyrodidae) predation and tomato fruit injury by the zoophytophagous predator Dicyphus tamaninii (Heteroptera: Miridae). In: Alomar O, Wiedenmann RN (eds) Zoophytophagous Heteroptera: implications for life history and integrated pest management. Entomological Society of America, Lanham, USA, pp 155–177
Arnó J, Castañé C, Riudavets J, Gabarra R (2010) Risk of damage to tomato crops by generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull Entomol Res 100:105–115
Calvo J, Urbaneja A (2004) Nesidiocoris tenuis un aliado para el control biológico de mosca blanca. Horticultura Internacional 44:20–25
Calvo FJ, Bolckmans K, Belda JE (2009) Development of a biological control-based IPM method for Bemisia tabaci for protected sweet pepper crops. Entomol Exp Appl 133:9–18
Castañé C, Alomar O, Riudavets J (2003) Potential risk of damage to zucchinis caused by mirid bugs. IOBC/WPRS Bull 26:135–138
Castañé C, Arnó J, Gabarra R, Alomar O (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29
Crawley MJ (2002) Statistical computing. An introduction to data analysis using S-plus. Wiley & Sons Press, Chichester, UK
Crowder D (2007) Impact of release rates on the effectiveness of augmentative biological control agents. J Insect Sci 7:1–11
El-Dessouki SA, El-Kifl AH, Helal HA (1976) Life cycle, host plants and symptoms of damage of the tomato bug, Nesidiocoris tenuis Reut. (Heteroptera: Miridae), in Egypt. J Plant Dis Prot 83(4):204–220
Franco K, Jauset A, Castañé C (2011) Monogamy and polygamy in two species of mirid bugs: a functional-based approach. J Insect Physiol 57:307–315
Gillespie DR, McGregor RR (2000) The functions of plant feeding in the omnivorous predator Dicyphus hesperus: water places limits on predation. Ecol Entomol 25:380–386
Gillespie DR, McGregor RR, Sánchez JA (2007) Dicyphus hesperus (Hemiptera: Miridae) as a success story in development of endemic natural enemies as biological control agents. In: Vincent CM, Goettel M, Lazarovits G (eds) Case studies in biological control: a global perspective. CABI Publishing, Wallingford, UK, pp 128–135
Goula M, Alomar O (1994) Míridos (Heteroptera: Miridae) de interés en el control integrado de plagas en tomate. Guía para su identificación. Bol Sanid Veg Plagas 20:131–143
Lenfant C, Ridray G, Schoen L (2000) Biopropagation of Macrolophus caliginosus (Wagner) for a quicker establishment in southern tomato greenhouses. IOBC/WPRS Bull 23(1):247–252
Malausa JC, Henao B (1988) First observations in France of Cyrtopeltis (Nesidiocoris) tenuis Reuter, 1895 (Het. Miridae). Nouv Rev Entomol 5:180
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Sánchez JA (2008) Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric For Entomol 10:75–80
Sánchez JA (2009) Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol Control 51:493–498
Sánchez JA, Lacasa A (2008) Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J Econ Entomol 101(6):1864–1870
Sánchez JA, Martinez-Cascales JI, Lacasa A (2003) Abundance and wild host plants of predator mirids (Heteroptera: Miridae) in horticultural crops in the Southeast of Spain. IOBC/WPRS Bull 26:147–151
Tavella L, Goula M (2001) Dicyphini collected in horticultural areas of northwestern Italy (Heteroptera Miridae). Bollettino di Zoologia Agraria e di Bachicoltura 33:93–102
Urbaneja A, Tapia G, Fernández E, Sánchez E, Contreras J, Gallego A, Bielza P (2003) Influence of the prey on the biology of Nesidiocoris tenuis (Hem.: Miridae). IOBC/WPRS Bull 26:159
Urbaneja A, Tapia G, Stansly PA (2005) Influence of host plant and prey availability on the developmental time and survival of Nesidiocoris tenuis Reuter (Het.: Miridae). Biocontrol Sci Technol 15:513–518
Urbaneja A, Montón H, Mollá O (2009) Suitability of the tomato borer Tuta absoluta as prey for Macrolophus caliginosus and Nesidiocoris tenuis. J Appl Entomol 133:292–296
Acknowledgments
The authors thank David A. Gillespie (Pacific Agri-Food Research Centre, Agriculture and Agri-food Canada) and Markus Knapp (R&D Koppert BV, The Netherlands) for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq
Rights and permissions
About this article
Cite this article
Calvo, F.J., Bolckmans, K. & Belda, J.E. Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 57, 809–817 (2012). https://doi.org/10.1007/s10526-012-9455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9455-1