Abstract
The ability of zoophytophagous predators to produce defensive plant responses due to their phytophagous behavior has been recently demonstrated. In the case of tomatoes, the mirids Nesidiocoris tenuis and Macrolophus pygmaeus are able to attract or repel pests and/or natural enemies in different ways. Nevertheless, the herbivore-induced plant volatiles (HIPVs) released by the phytophagy of both mirids, which are responsible for these behaviors, are unknown. In this work, the HIPVs produced by the plant feeding of N. tenuis and M. pygmaeus were characterized. In addition, the role of each HIPV in the repellence or attraction of two tomato pests, Bemisia tabaci and Tuta absoluta, and of the natural enemy Encarsia formosa was evaluated. Six green leaf volatiles (GLVs) plus methyl salicylate and octyl acetate clearly stood out as major differential peaks on the chromatogram in a directed analysis. The six GLV and methyl salicylate were repellent for B. tabaci and attractive to E. formosa, whereas they showed no effect on T. absoluta. Octyl acetate, which was significantly present only in the M. pygmaeus-punctured plants, was significantly attractive to T. absoluta, repellent to E. formosa and indifferent to B. tabaci. Unlike the remaining HIPVs, octyl acetate was emitted directly by M. pygmaeus and not by the plant. Our results showed that mirid herbivory could modulate the pest and natural plant enemy locations, since tomato plants release a blend of volatiles in response to this activity. These results could serve as a basis for future development of plant protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the large insect family Miridae, members of the Dicyphini tribe are generalist predators well-known for their polyphagy on herbivorous pests such as whiteflies, leafminers, aphids, thrips, mites and lepidopterans (Barnadas et al. 1998; Urbaneja et al. 2009, 2012; Abbas et al. 2014). Dicyphini are also characterized by their zoophytophagous behavior, which means that they can feed on plants and prey during the same developmental stage (Castañé et al. 2011). Zoophytophagy is a positive feature for natural enemies because these predators can survive in a crop even when prey is scarce or totally absent (Eubanks and Denno 1999; Sanchez et al. 2004; Urbaneja et al. 2005), since the plant can provide them with water (essential for predation) and nutrients (Gillespie and McGregor 2000; Sinia et al. 2004). Herbivory of some mirid species may result in injuries to the vegetative and reproductive parts of the plant, causing even yield loss (Calvo et al. 2009; Sanchez 2009; Castañé et al. 2011; Biondi et al. 2016). Nevertheless, the capacity of mirids to induce plant damage varies among species (Castañé et al. 2011). During recent decades, zoophytophagous mirid bugs received special attention due to their increasing role in the biological control of important agricultural pests (Arnó et al. 2010; Perdikis et al. 2011; Pérez-Hedo and Urbaneja 2015; Messelink et al. 2015; Naselli et al. 2016). Among them, Macrolophus pygmaeus (Rambur) and Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) are probably the mirid species that are most used in biological control programs since both are mass-reared and recommended to be released and/or conserved in several successful integrated pest management (IPM) programs (Calvo et al. 2012; Urbaneja et al. 2012; Zappalà et al. 2012; Zappalà et al. 2013; Pérez-Hedo and Urbaneja 2015; Pérez-Hedo and Urbaneja 2016; Pérez-Hedo et al. 2017; van Lenteren et al. 2017).
It is widely known that the attack of a plant by herbivorous arthropods induces the release of semiochemicals called herbivore-induced plant volatiles (HIPVs) (Turlings et al. 1990; Paré and Tumlinson 1999), most of which are terpenoids, fatty acid derivatives, phenylpropanoids and benzenoids (Dudareva et al. 2004). These volatiles are qualitatively and quantitatively different among different herbivore species (Dicke 2009), which can attract natural enemies, repel herbivores and alert neighboring plants (priming) (Sabelis et al. 1999; Paré and Tumlinson 1999; Frost 2008). Tritrophic interactions in plant defense (plants, herbivores and natural enemies) are important to understanding both the evolution of such interactions and improving biological control (Sabelis et al. 1999). Previous works demonstrated that the plant-feeding activities of N. tenuis and M. pygmaeus could differentially induce plant responses in tomato (Pérez-Hedo et al. 2015a, b; Naselli et al. 2016). While the phytophagy activity of N. tenuis resulted in a non-preference effect on two key tomato pests, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), plants punctured by M. pygmaeus did not repel B. tabaci and curiously resulted in an attraction to T. absoluta, although both mirid predators induced the attraction of the whitefly parasitoid Encarsia formosa (Gahan) (Hymenoptera: Aphelinidae) (Pérez-Hedo et al. 2015b). Pappas et al. (2015) obtained similar results for M. pygmaeus, which induced defensive responses in tomato plants against the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). These plants, when previously exposed to M. pygmaeus, reduced the performance of a subsequently infesting herbivore. Such defensive responses activated by mirid plant feeding seemed to be activated through the upregulation of the phytohormone pathways of abscisic acid (ABA) and jasmonic acid (JA) (Pérez-Hedo et al. 2015a, b; Naselli et al. 2016). However, which volatiles are elicited through these pathways’ upregulation and their roles in the repellence or attraction of herbivores or natural enemies remains unknown. The response of plants to herbivory is very specific and makes the relation between HIPV blends and specific plant-herbivore systems, more complicate.
In this work, we characterized the volatile emissions from intact and M. pygmaeus- and N. tenuis-punctured tomato plants using a solid phase microextraction technique combined with gas chromatography-mass spectrometry, assuming that the volatile blend released is specific for a particular insect-plant system. When identified, the role of each HIPV in the repellence and/or attraction of two key tomato pests, B. tabaci and T. absoluta, and to one parasitoid, E. formosa, were evaluated. Because one of the identified compounds (octyl acetate) could not be assigned as HIPV, whether this compound was emitted directly by both mirid species was also studied.
Materials and methods
Plants and insects
Solanum lycopersicum (cv. Optima) plants were germinated in soil, and two weeks after germination, the seedlings were individually transferred to pots and maintained at 25 ± 2 °C with high RH (> 60%) and a 16:8 h L:D photoperiod. Six-week-old plants with seven to eight fully expanded leaves were used for the experiments.
To induce the plants, we enclosed one tomato plant exposed to 20 N. tenuis or M. pygmaeus adults in a plastic cage of 60 × 60 × 60 cm (BugDorm-2; MegaView Science Co., Ltd.; Taichung, Taiwan) for 24 h prior to the assay. All mirid individuals were removed from the punctured plants before volatile collection. The intact plants were left undisturbed and isolated from arthropods until use.
B. tabaci, E. formosa, N. tenuis and M. pygmaeus individuals were obtained directly from the mass rearings of Koppert Biological Systems, S.L. (Águilas, Murcia, Spain), and T. absoluta individuals were obtained from colonies maintained at IVIA (Abbas et al. 2014). Once received at IVIA, the two mirids and the whitefly remained on tomato plants for 24 h before their use. The parasitoids used in the assays had no previous contact with any plant or host and were referred to as naïve. Newly emerged adult females of the six species of insects (1–5 days old) were used in all experiments. All females were presumably mated except the parasitoid E. formosa which is an uniparental species.
Headspace collection of volatiles
The HIPVs of the tomato plants involved in the responses to the two zoophytophagous plant bugs were collected using an olfactometer, described below, in a static condition for 3 h. Thus, the volatile compounds were captured by means of solid phase microextraction (SPME) and were separated and detected by means of gas chromatography coupled to mass spectrometry (GC/MS). Volatile compounds were adsorbed in a 65-µm PDMS/DVB SPME fiber (polydimethylsiloxane/divinylbenzene; Supelco, Bellefonte, PA, USA). The adsorbent-coated fiber was mounted on an SPME fiber holder and injected through the first septum of the sample container (glass jars of 5 l volume). Agitation of the atmosphere inside the container was achieved by pumping at a rate of 5 ml min−1 using an injecting syringe through the second septum of the sample container. In total, 11 biological replicates were sampled per treatment (intact plants, M. pygmaeus-punctured plants and N. tenuis-punctured plants), each replicate consisting in one plant.
Desorption was performed using a CombiPAL autosampler (CTC Analytics) at 250 °C over 1 min in splitless mode in the injection port of a 6890 N gas chromatograph coupled to a 5975B mass spectrometer (Agilent Technologies). To prevent cross-contamination, fibers were cleaned after desorption in an SPME fiber conditioning station (CTC Analytics) at 250 °C for 5 min under helium flow. Chromatography was performed on a DB-5 ms (60 m, 0.25 mm, 1.00 µm) column with helium as carrier gas at a constant flow of 1.2 ml min−1. The GC interface and MS source temperatures were 260 °C and 230 °C, respectively. The oven programming conditions were 40 °C for 2 min, 5 °C min−1 ramp to 250 °C, and a final hold at 250 °C for 6 min. Data were recorded in the 35–300 m/z range at 5 scans s−1, with the electronic impact ionization set at 70 eV. Untargeted analysis of the chromatograms was performed using MetAlign software (WUR, http://www.metalign.nl).
Kovats retention indexes were calculated for all the compounds. Differentially emitted compounds were first tentatively identified based on the comparison of their mass spectra with those in the NIST 05 Mass Spectral Library. When available, identity was confirmed by co-elution with pure standards (Sigma-Aldrich). For relative quantitation of the selected compounds, one specific ion was selected for each, and the corresponding peak area from the extracted ion chromatogram was integrated by means of the ChemStation E.02.02 software (Agilent Technologies). The criteria for ion selection were the highest signal-to-noise ratio and sufficient specificity in that particular region of the chromatogram to provide good peak integration.
Olfactory response to HIPV
Once the HIPVs involved in the plant response were identified, the olfactory responses to them were evaluated on two herbivore pests (B. tabaci and T. absoluta) and a parasitoid (E. formosa) in a Y-tube olfactometer (Analytical Research Systems, Gainesville, FL, USA) consisting of a Y-shaped glass tube connected via plastic tubes to two identical 5 l glass jars, each of which contained a tested odor source and was connected to an air pump that produced a unidirectional airflow. The environmental conditions in the Y-tube experiments were 23 ± 2 °C and 60 ± 10% RH (Pérez-Hedo and Urbaneja 2015). Each female was observed until she had walked at least 3 cm up one of the side arms or until 15 min had elapsed. Females that had not walked up one of the side arms after 15 min were considered to be ‘non-responders’ and were excluded from the subsequent data analysis. There was no Y-tube experiment in which the number of non-responder was higher than six. Each individual was tested only once. After testing five individuals, odor sources were interchanged to avoid any spatial effect on choices. All synthetic standards of the tomato volatile compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The volatiles released into the jar consisted of a piece of paper with 10 µl to 1:10,000 methanol:water (control) or 1:10,000 with the volatile to test. The dilutions of 1:10,000 of pure compounds were also collected on SPME fibers and analyzed using the GC/MS. The peak areas in the chromatograms were very similar to those observed in the biological samples, they did not differ from those emitted by tomato plants, and in all cases, they were of the same order of magnitude.
Octyl acetate volatile
The octyl acetate volatile was emitted only by M. pygmaeus-punctured plants, and traces were not detected either on intact or N. tenuis-punctured plants. For this reason, we wondered whether this compound was released by the tomato plant as a response to M. pygmaeus activity or by the mirid itself. To unveil this question, the octyl acetate was collected in SPME fibers using an olfactometer in static condition for 3 h and analyzed using the GC/MS as explained above in the following treatments: ten couples of M. pygmaeus, ten couples of N. tenuis, tomato plant with the presence of ten couples of either N. tenuis or M. pygmaeus and two controls, one of them consisting of an empty jar (5 l in volume) and the other of an intact tomato plant. For the treatments with plant and mirid, the mirids were put in contact with the plant 24 h before the volatile collection was done. To analyze the octyl acetate volatile, three biological replicates were conducted when the volatile was collected on both mirids without plant, whereas four replicates were conducted for the rest of the treatments.
Data analysis
Differences in volatile compounds were subjected to one-way analysis of variance, and the Tukey test was used for mean separation at p < 0.05. The results are expressed as the mean ± SE. The data for the octyl acetate volatile were analyzed using the one-tailed Student’s t test (p < 0.05). χ 2 tests were used to test the hypothesis that the distribution of side-arm choices between pairs of odors deviated from the null model of odor sources being chosen with equal frequency.
Results
Composition of volatile blends
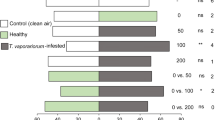
When identifying the HIPVs involved in the tomato plant responses induced by M. pygmaeus and N. tenuis, six green leaf volatiles (GLV), methyl salicylate and octyl acetate clearly stood out as major differential peaks on the chromatogram in a directed analysis (Fig. 1). In general, N. tenuis-punctured plants emitted more volatiles than M. pygmaeus-punctured plants, and the latter emitted more volatiles than did the intact plants. When analyzing the relative peak areas of the eight representative compounds, four of the volatiles were significantly emitted in the largest amount by N. tenuis-punctured plants, compared to M. pygmaeus-punctured or intact tomato plants: (Z)-3-hexenol (F 2,32 = 4.57; p = 0.0184), (Z)-3-hexenyl propanoate (F 2,32 = 4.059; p = 0.0269), (Z)-3-hexenyl butanoate (F 2,32 = 4.022; p = 0.0277) and methyl salicylate (F 2,32 = 10.17; p = 0.0004). Surprisingly, octyl acetate was present only in the M. pygmaeus-punctured plants. The amounts of 1-hexanol (F 2,32 = 0.2552; p = 0.7764), (Z)-3-hexenyl acetate (F 2,32 = 2.103; p = 0.1397) and hexyl butanoate (F 2,32 = 0.7096; p = 0.4999) were not significantly different among treatments.
Total emission of volatiles (mean peak area of all mass fragments + SE) emitted by intact plants, M. pygmaeus-punctured plants and N. tenuis-punctured plants. Different letters over the bars indicate significant differences (ANOVA and Tukey comparisons; p < 0.05) which must be read for each graph separately
Biological activities of HIPVs induced by zoophytophagous predatory mirids
The response of the whitefly B. tabaci in a Y-tube olfactometer when exposed to the control and the eight synthetic HIPVs identified in the previous section is shown in Fig. 2. The whitefly showed a strong preference for the control over the jars with the volatiles 1-hexanol (χ 21 = 20.0, p < 0.0001), (Z)-3-hexenol (χ 21 = 57.8, p < 0.0001), (Z)-3-hexenyl acetate (χ 21 = 9.8, p = 0.0017), (Z)-3-hexenyl propanoate (χ 21 = 16.2, p < 0.0001), (Z)-3-hexenyl butanoate (χ 21 = 9.8, p = 0.0017), hexyl butanoate (χ 21 = 51.2, p < 0.0001) and methyl salicylate (χ 21 = 12.8, p = 0.0003). No preference was observed between the control and octyl acetate volatile, which was detected only in plants punctured by M. pygmaeus.
The parasitoid E. formosa behaved contrary to the whitefly (Fig. 3). Octyl acetate had a clear repellent effect to the parasitoid (χ 21 = 33.8, p < 0.0001), whereas the remaining compounds, 1-hexanol (χ 21 = 33.8, p < 0.0001), (Z)-3-hexenol (χ 21 = 7.2, p = 0.0073), (Z)-3-hexenyl acetate (χ 21 = 20.0, p < 0.0001), (Z)-3-hexenyl propanoate (χ 21 = 39.2, p < 0.0001), (Z)-3-hexenyl butanoate (χ 21 = 16.2, p < 0.0001), hexyl butanoate (χ 21 = 20.0, p < 0.0001) and methyl salicylate (χ 21 = 12.8, p = 0.0003) significantly attracted this parasitoid.
Response (% + SE) of T. absoluta females in a Y-tube olfactometer when exposed to control (1:10,000 methanol:water) and the eight synthetic HIPVs identified (1:10,000 with the volatile to test). Asterisks indicate significant differences in the distribution of side-arm choices (χ 21 tests; p < 0.05)
The lepidopteran T. absoluta was significantly more attracted by octyl acetate over the control jar in the Y-tube olfactometer (χ 21 = 16.69, p < 0.0001), whereas a lack of preference was observed for the remaining volatiles tested, 1-hexanol (χ 21 = 0.2667, p = 0.6056), (Z)-3-hexenol (χ 21 = 0.00, p = 1.00), (Z)-3-hexenyl acetate (χ 21 = 3.200, p = 0.0736), (Z)-3-hexenyl propanoate (χ 21 = 0.2667, p = 0.6056), (Z)-3-hexenyl butanoate (χ 21 = 0.00, p = 1.00), hexyl butanoate (χ 21 = 2.632, p = 0.1080) and methyl salicylate (χ 21 = 1.800, p = 0.1797) (Fig. 4).
Response (% + SE) of E. formosa females in a Y-tube olfactometer when exposed to control (1:10,000 methanol:water) and the eight synthetic HIPVs identified (1:10,000 with the volatile to test). The asterisks indicates a significant difference in the distribution of side-arm choices (χ 21 tests; p < 0.05)
Octyl acetate volatile released by M. pygmaeus
As shown above, the octyl acetate volatile was emitted only by M. pygmaeus-punctured plants. To know whether this compound was released by the tomato plant as a response to M. pygmaeus activity or by the mirid itself, the octyl acetate emitted by the tomato plant with or without both mirid species or by an empty jar with or without the mirids was collected and analyzed. A strong significant difference was observed for the emission of octyl acetate between M. pygmaeus and N. tenuis individuals (t 7 = 5.935, p = 0.020), and was 77 times higher for M. pygmaeus than for N. tenuis (Fig. 5a). When octyl acetate was collected and analyzed in the treatments that had both tomato plant and mirid, this compound could be detected only in the treatment with M. pygmaeus (Fig. 5b).
Total emission of volatiles (mean peak area of all mass fragments + SE) emitted by a control (empty jar), ten pairs of M. pygmaeus, and ten pairs of N. tenuis and b tomato intact plant, tomato plant with the presence of ten couples of either N. tenuis or M. pygmaeus that were put in contact with the plant 24 h before. The asterisk indicates a significant difference for the total emission of octyl acetate between M. pygmeus and N. tenuis when placed alone into the jars (t test; p < 0.05)
Discussion
Plants have different strategies to protect themselves against insect attack, ranging from production of physical and chemical defenses to changes in the plant’s primary and/or specialized metabolism (War et al. 2012; Zhou et al. 2015). In this complex metabolic network that determines specific responses, HIPVs play an important role in tritrophic interactions, which are crucial to understand the evolution of plant-predator mutualisms. We identified six green leaf volatiles and methyl salicylate involved in the tomato plant responses induced by M. pygmaeus and N. tenuis. The herbivory of insects always induce the production of plant volatiles (Paré and Tumlinson 1999; Leitner et al. 2005; Shiojiri et al. 2006; Dicke 2009). One of the novelties of our work is that the insects, which are considered beneficial and widely used and are released as biocontrol agents in many agricultural crops, can also be responsible to induce these HIPVs.
Our results confirm that HIPVs induced by zoophytophagous predators are species-dependent and have a differential response on pest and natural enemies, although this fact had previously been reported for different herbivores on the same plant species (Turlings et al. 1998; Delphia et al. 2007). In particular, when analyzing the emission levels of the eight dominant compounds in a directed analysis, we observed that five compounds [(Z)-3-hexenol, (Z)-3-hexenyl propanoate, (Z)-3-hexenyl butanoate, methyl salicylate and octyl acetate] were the discriminating compounds that resulted in the difference in the volatile profile between the intact tomato plants and N. tenuis- and M. pygmaeus-punctured plants. Our work also confirms that both herbivores and natural enemies can perceive a wide range of plant volatiles that are herbivore-induced (Ardanuy et al. 2016), in our case by the plant feeding of two zoophytophagous mirid bugs and that a single synthetic HIPV can have an effect of attraction or repellence by itself on pests and/or natural enemies (James 2005; Rodriguez-Saona et al. 2011; Ozawa et al. 2008; Giunti et al. 2017).
Attraction of insect parasitoids by volatiles emitted from damaged plants has been well documented, as plants can defend themselves against herbivores by attracting natural enemies of the herbivores (Bukovinszky et al. 2005; Ozawa et al. 2008). However, little is known about the effects of these chemicals on the herbivores as an alternative function of HIPV, which could repel herbivores (Bernasconi et al. 1998; Kessler and Baldwin 2001; Ulland et al. 2008). Our results showed that E. formosa may use methyl salicylate and the six GLVs tested as an olfactory cue for host location, showing a clear attraction for the parasitoid. Contrarily, the biological assays conducted in the Y-tube olfactometer demonstrated that these HIPVs, which were attractive for E. formosa, had a repellent effect on the whitefly B. tabaci. These results should be seen in the context of different selection pressures on a plant’s emission of volatiles in a multitrophic context. Understanding these selection pressures will provide insight into the roles of induced volatiles in the biology of plants (Dicke and Baldwin 2010).
Interestingly, the lepidopteran T. absoluta did not respond to any of the above-mentioned HIPVs. However, T. absoluta was attracted to octyl acetate, a compound significantly identified only in M. pygmaeus punctured plants and in M. pygmaeus alone treatment. Taking these results together with those obtained by Pérez-Hedo et al. (2015b) who showed that T. absoluta was also attracted by tomato plants previously infested by M. pygmaeus, prompted the hypothesis that octyl acetate is a volatile emitted directly by M. pygmaeus and not by the M. pygmaeus-punctured tomato plant, which results in attraction due to the traces that M. pygmaeus leaves on the plant. Octyl acetate is known as a specific compound of some species of the Miridae family as some Phytocoris spp. (Millar and Rice 1998; Millar et al. 1997; Zhang and Aldrich 2008). Octyl acetate was also detected as a pheromone alert in both males and females of the hemipteran Leptocorisa chinensis Dallas (Hemiptera: Alydidae), although in significantly higher amounts in females (Yamashita et al. 2016), and in the composition of the natural sting alarm pheromone components in bees (Wager and Breed 2000; Wang et al. 2016), which cause other bees to behave defensively when alarm pheromones are released at the moment that a bee stings another animal. Interestingly this compound was also detected as part of the sex pheromone of the moth Batrachedra amydraula Meyrick (Lepidoptera: Batrachedridae) (Levi-Zada et al. 2013). Thus, to study ecological functions of the octyl acetate volatile, we investigated its responses on two key tomato pests, T. absoluta and B. tabaci, and on the parasitoid E. formosa in the olfactometer. The lack of response to this volatile by the whitefly and the parasitoid and the strong attraction of the lepidopteran to this volatile could be attributed to the specificity of this compound for T. absoluta. We could hypothesize from these results that this compound or one very close could be or be part of a pheromone for this lepidopteran. To date the (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate has been identified as the major sex pheromone component of T. absoluta (Attygalle et al. 1996). Therefore, further experiments should be conducted to clarify the role of this potential compound on T. absoluta management.
The obtained results might open the door to the exploitation of these volatiles in new strategies for pest management. As an example, using new mesoporous dispensers, which could emit regular concentrations of one or a mix of these volatiles, could result in saturated repellent and attractant environments for B. tabaci and E. formosa, respectively. Plant breeding programs could also be focused on obtaining plants with higher rates of emission of one of the most active HIPVs, as in the work of Kappers et al. (2005), who manipulated HIPVs through genetic engineering, which resulted in attracting the phytoseiid predator Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). Further research will address the potential benefits of inducing plant volatile emissions under field conditions as a potential tool for enhancing populations of beneficial insects as a component of conservation biological control (James 2005; Rodriguez-Saona et al. 2011). In this respect, the possible trade-offs that the continuous exposure to these volatiles could induce on plants might be evaluated.
In summary, our results suggest that the effectiveness attributed to predatory mirids in pest management is due not only to their zoophagy but also to their herbivory which, as demonstrated in our work, could modulate pest and natural plant enemy locations, since tomato plants release a blend of volatile compounds in response to their activity. These results could partially explain the great success achieved by the predatory mirids in recent years in tomato crops.
References
Abbas S, Pérez-Hedo M, Colazza S, Urbaneja A (2014) The predatory mirid Dicyphus maroccanus as a new potential biological control agent in tomato crops. BioControl 59:565–574
Ardanuy A, Albajes R, Turlings TC (2016) Innate and learned prey-searching behavior in a generalist predator. J Chem Ecol 42:497–507
Arnó J, Gabarra R, Liu TX, Simmons AM, Gerling D (2010) Natural enemies of Bemisia tabaci: predators and parasitoids. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Dordrecht, pp 385–421
Attygalle AB, Jham GN, Svatos A, Frighetto RTS, Ferrara FA, Vilela EF, Uchôa-Fernandes MA, Meinwald J (1996) 3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, major sex pheromone component of the tomato pest Scrobipalpuloides absoluta (Lepidoptera: Gelechiidae. Bioorg Med Chem 4:305–314
Barnadas I, Gabarra R, Albajes R (1998) Predatory capacity of two mirid bugs preying on Bemisia tabaci. Entomol Exp Appl 86:215–219
Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S (1998) Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol Exp Appl 87:133–142
Biondi A, Zappalà L, Di Mauro A, Tropea Garzia G, Russo A, Desneux N, Siscaro G (2016) Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 61:79–90
Bukovinszky T, Gols R, Posthumus MA, Vet LE, van Lenteren JC (2005) Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellén). J Chem Ecol 31:461–480
Calvo FJ, Bolckmans K, Stansly PA, Urbaneja A (2009) Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 54:237–246
Calvo FJ, Soriano J, Bolckmans K, Belda JE (2012) A successful method for whitefly and Tuta absoluta control in tomato. Evaluation after two years of application in practice. IOBC/WPRS Bull 80:237–244
Castañé C, Arnó J, Gabarra R, Alomar O (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29
Delphia CM, Mescher MC, De Moraes CM (2007) Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J Chem Ecol 33:997–1012
Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl 91:131–142
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Eubanks MD, Denno RF (1999) The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 80:1253–1266
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824
Gillespie DR, Mcgregor RR (2000) The functions of plant feeding in the omnivorous predator Dicyphus hesperus: water places limits on predation. Ecol Entomol 25:380–386
Giunti G, Benelli G, Palmeri V, Canale A (2017) Bactrocera oleae-induced olive VOCs routing mate searching in Psyttalia concolor males: impact of associative learning. Bull Entomol Res. https://doi.org/10.1017/S0007485317000451
James DG (2005) Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. J Chem Ecol 31:481–495
Kappers IF, Aharoni A, van Herpen TW, Luckerhoff LL, Dicke M, Bouwmeester HJ (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309:2070–2072
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Leitner M, Boland W, Mithöfer A (2005) Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol 167:597–606
Levi-Zada A, Sadowsky A, Dobrinin S, David M, Ticuchinski T, Fefer D, Greenberg A, Blumberg D (2013) Reevaluation of the sex pheromone of the lesser date moth, Batrachedra amydraula, using autosampling SPME-GC/MS and field bioassays. Chemoecology 23:13–24
Messelink GJ, Bloemhard CMJ, Hoogerbrugge H, van Schelt J, Ingegno BL, Tavella L (2015) Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J Appl Entomol 139:333–341
Millar JG, Rice RE (1998) Sex pheromone of the plant bug Phytocoris californicus (Heteroptera: Miridae). J Econ Entomol 91:132–137
Millar JG, Rice RE, Wang Q (1997) Sex pheromone of the mirid bug Phytocoris relativus. J Chem Ecol 23:1743–1754
Naselli M, Urbaneja A, Siscaro G, Jaques JA, Zappalà L, Flors V, Pérez-Hedo M (2016a) Stage-related defense response induction in tomato plants by Nesidiocoris tenuis. Int J Mol Sci 17:1210–1223
Naselli M, Zappalà L, Gugliuzzo A, Tropea Garzia G, Biondi A, Rapisarda C, Cincotta F, Condurso C, Verzera A, Siscaro G (2016b) Olfactory response of the zoophytophagous mirid Nesidiocoris tenuis to tomato and alternative host plants. Arthropod-Plant Interact 11:121–131
Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J (2008) Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl 129:189–199
Pappas ML, Steppuhn A, Geuss D, Topalidou N, Zografou A, Sabelis MW, Broufas GD (2015) Beyond predation: the zoophytophagous predator Macrolophus pygmaeus induces tomato resistance against spider mites. PLoS ONE 10(5):e0127251
Paré PW, Tumlinson JH (1999) Plant volatiles as a defence against insect herbivores. Plant Physiol 121:325–331
Perdikis D, Fantinou A, Lykouressis D (2011) Enhancing pest control in annual crops by conservation of predatory Heteroptera. Biol Control 59:13–21
Pérez-Hedo M, Urbaneja A (2015) Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J Pest Sci 88:65–73
Pérez-Hedo M, Urbaneja A (2016) The zoophytophagous predator Nesidiocoris tenuis: a successful but controversial biocontrol agent in tomato crops. In: Horowitz AR, Ishaaya I (eds) Advances in insect control and resistance management. Springer International Publishing, Cham, pp 121–138
Pérez-Hedo M, Suay R, Alonso M, Ruocco M, Giorgini M, Poncet C, Urbaneja A (2017) Resilience and robustness of IPM in protected horticulture in the face of potential invasive pests. Crop Prot 97:119–127
Pérez-Hedo M, Urbaneja-Bernat P, Jaques JA, Flors V, Urbaneja A (2015a) Defensive plant responses induced by Nesidiocoris tenuis (Hemiptera: Miridae) on tomato plants. J Pest Sci 88:543–554
Pérez-Hedo M, Bouagga S, Jaques JA, Flors V, Urbaneja A (2015b) Tomato plant responses to feeding behavior of three zoophytophagous predators (Hemiptera: Miridae). Biol Control 86:46–51
Rodriguez-Saona C, Kaplan I, Braasch J, Chinnasamy D, Williams L (2011) Field responses of predaceous arthropods to methyl salicylate: a meta-analysis and case study in cranberries. Biol Control 59:294–303
Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanuu P (1999) Behavioural responses of predatory and herbivorous arthropods to induced plant volatiles: From evolutionary ecology to agricultural applications. In: Agrawal A, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. American Phytopathological Society Press, St. Paul, pp 269–296
Sanchez JA (2009) Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol Control 51:493–498
Sanchez JA, Gillespie DR, McGregor RR (2004) Plant preference in relation to life history traits in the zoophytophagous predator Dicyphus hesperus. Entomol Exp Appl 112:7–19
Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J (2006) Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proc Natl Acad Sci USA 103:16672–16676
Sinia A, Roitberg B, McGregor RR, Gillespie DR (2004) Prey feeding increases water stress in the omnivorous predator Dicyphus hesperus. Entomol Exp Appl 110:243–248
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1998) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11:122–129
Ulland S, Ian E, Mozuraitis R, Borg-Karlson AK, Meadow R, Mustaparta H (2008) Methyl salicylate, identified as primary odorant of a specific receptor neuron type, inhibits oviposition by the moth Mamestra brassicae L. (Lepidoptera, Noctuidae). Chem Senses 33:35–46
Urbaneja A, Tapia G, Stansly P (2005) Influence of host plant and prey availability on developmental time and survivorship of Nesidiocoris tenuis (Het.: Miridae). Biocontrol Sci Techn 15:513–518
Urbaneja A, Montón H, Mollá O (2009) Suitability of the tomato borer Tuta absoluta as prey for Macrolophus caliginosus and Nesidiocoris tenuis. J Appl Entomol 133:292–296
Urbaneja A, González-Cabrera J, Arnó J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222
van Lenteren J, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2017) Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl. https://doi.org/10.1007/s10526-017-9801-4
Wager BR, Breed MD (2000) Does honey bee sting alarm pheromone give orientation information to defensive bees? Ann Entomol Soc Am 93:1329–1332
Wang Z, Wen P, Qu Y, Dong S, Li J, Tan K, Nieh JC (2016) Bees eavesdrop upon informative and persistent signal compounds in alarm pheromones. Sci Rep-UK 6:25693
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
Yamashita KI, Isayama S, Ozawa R, Uefune M, Takabayashi J, Miura K (2016) A pecky rice-causing stink bug Leptocorisa chinensis escapes from volatiles emitted by excited conspecifics. J Ethol 34:1–7
Zappala L, Biondi A, Alma A, Al-Jboory IJ, Arno J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Aznar RV, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86:635–647
Zappalà L, Siscaro G, Biondi A, Mollá O, González-Cabrera J, Urbaneja A (2012) Efficacy of sulphur on Tuta absoluta and its side effects on the predator Nesidiocoris tenuis. J App Entomol 136:401–409
Zhang QH, Aldrich JR (2008) Sex pheromone of the plant bug, Phytocoris calli Knight. J Chem Ecol 34:719–724
Zhou S, Lou YR, Tzin V, Jander G (2015) Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol 169:1488–1498
Acknowledgements
The research leading to these results was funded by the Spanish Ministry of Economy and Competitiveness (AGL2014-55616-C3). The authors thank Javier Calvo (KOPPERT BS) for the supply of insects, and Sandra Fresquet and Virginia Pedroche for their technical assistance. MP-H was the recipient of a research fellowship from the INIA Spain (Subprogram DOC-INIA-CCAA). Analyses of volatile compounds were performed in the Metabolomics service facilities at IBMCP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Pérez-Hedo, M., Rambla, J.L., Granell, A. et al. Biological activity and specificity of Miridae-induced plant volatiles. BioControl 63, 203–213 (2018). https://doi.org/10.1007/s10526-017-9854-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9854-4