Abstract
Knowledge about the orientation mechanisms used by two important predaceous mirids (Macrolophus pygmaeus Rambour and Nesidiocoris tenuis (Reuter)) in finding their prey (whitefly Bemisia tabaci (Gennadius) and the tomato borer Tuta absoluta (Meyrick)) is limited. In a Y-tube olfactometer, we tested the behavioral responses of naïve and experienced predators to uninfested plants, herbivore-induced plant volatiles (HIPVs) from plants infested with T. absoluta and/or B. tabaci, the sex pheromone of T. absoluta, and volatiles produced by plants injured by the predators. Nesidiocoris tenuis responds to volatiles produced by uninfested plants only after experience with the plant, whereas naïve and experienced M. pygmaeus show positive chemotaxis. Both predators are attracted to volatiles from prey-infested plants, and we provide the first evidence that experience affects this response in M. pygmaeus. Infestation of the same plant by both prey species elicited similar responses by the two predators as plants infested by either herbivore singly. Neither predator responded to sex pheromones of T. absoluta. Macrolophus pygmaeus avoided plants injured by conspecifics, while N. tenuis females were attracted by such plants. The implications of these results for augmentative biological control are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), native to South America (Guedes and Picanço 2012), recently invaded Europe, Africa and Asia, where it is a devastating pest of tomato crops (Desneux et al. 2010, 2011). Another serious worldwide tomato pest, often occurring simultaneously with the tomato borer, is the silverleaf whitefly Bemisa tabaci (Gennadius) (Hemiptera: Aleyrodidae) (Calvo et al. 2012).
Two generalist predators, Macrolophus pygmaeus (=M. caliginosus) Rambour and Nesidiocoris tenuis (Reuter) (both Hemiptera: Miridae) are successfully used in Europe for control of these pests (Urbaneja et al. 2012). Use of a natural enemy feeding on more than one pest reduces the complexity and costs of biological control (Calvo et al. 2012). Polyphagous predators may increase the levels of biocontrol, as their populations can be maintained by alternative prey and their efficacy may be improved by mixed diet effects (Bompard et al. 2013; Messelink et al. 2008). Mirid predators are polyphagous and considered important candidate biological control agents (e.g. Bueno and van Lenteren 2012). Mirids also feed on plants. This zoophytophagous behavior may at times enhance biological control (predators can survive without prey), but also has disadvantages (predators may injure plants). Plant feeding by M. pygmaeus does usually not cause problems (Castañé et al. 2011), but N. tenuis often causes injury to tomato, visible as necrotic brown rings around stems and shoots caused by repeated feeding at the same point (Arnó et al. 2010). This injury may cause yield reduction (Sanchez and Lacasa 2008), and injury is inversely proportional to prey availability (Sanchez 2009). According to Raman et al. (1984), plant feeding of N. tenuis on tomato causes biochemical changes in wounded tissue, leading to increased levels of oxidative enzymes and phenolic compounds which may result in production of volatile compounds. We hypothesize that repeated feeding at the same location by N. tenuis is the result of positive chemotaxis to volatiles produced by wounded tissue.
Predators may use volatiles produced by uninfested plants and by plants attacked by herbivores as cues to locate their prey (Dicke 1999; Vet and Dicke 1992). Use of info-chemicals such as these herbivore-induced plant volatiles (HIPVs) occurs among many predaceous arthropods regardless of their diet specialization (Dicke and Sabelis 1988), but learning to respond to info-chemicals is more common in generalists than in specialists (Steidle and van Loon 2003). The ability to learn may increase searching efficiency, because generalists exploit a range of resources that vary in quality and quantity both in time and space (Glinwood et al. 2011). Plants under multiple attack by different species of herbivores show differences in blends of volatiles they produce (Dicke et al. 2009; Moayeri et al. 2007b; Zhang et al. 2009), thus predators face highly variable information when exposed to HIPVs (Vet and Dicke 1992). This becomes even more complex when predators are also plant feeders (Eubanks and Styrsky 2005).
Mirid predators from the tribe Dicyphini are attracted by prey-infested plants (Ingegno et al. 2011, 2013; McGregor and Gillespie 2004; Moayeri et al. 2006a, b, 2007b; Mollá 2013). Macrolophus pygmaeus and N. tenuis react to volatiles from prey-infested plants, but not to volatiles emitted directly by the prey (Ingegno et al. 2011; Moayeri et al. 2006a, b; Mollá 2013). The two mirids’ wide commercial use together with the limited knowledge of their chemical communication motivated our study of their responses to volatiles produced from the interaction among plants, prey and predators in the context of single and multiple attack by different herbivores. We also decided to test their response to pheromones of T. absoluta. Several species of natural enemies are known to be attracted to or arrested by pheromones produced by pest species (e.g. Erbilgin and Raffa 2001; Fatouros et al. 2008); we expect that predators reacting to prey pheromones would be able to locate sites with prey quicker than predators not responsive to pheromones.
We used two herbivores belonging to different feeding guilds as prey. Bemisia tabaci is a very generalist phloem-feeding herbivore that feeds on >1,000 plant species (EFSA 2013), while T. absoluta is an oligophagous leaf miner that feeds on 17 plant species, particularly Solanaceae (Desneux et al. 2010). Mouttet et al. (2013) showed that previous feeding by B. tabaci negatively affected performance of T. absoluta on tomato, but effects of the interaction between the two herbivores on the mirid predators have not yet been investigated. Based on their different feeding habits, we expect significant differences in the HIPV blends of attacked plants, because chewing herbivores mainly activate the jasmonic acid (JA) signaling pathway, while phloem-feeding insects generally activate the salicylic acid (SA) signaling pathway (Walling 2000). Cross-talk between these pathways can mold the final defense response of the plant (Kunkel and Brooks 2002; Thaler et al. 2012), including volatile production that functions as indirect defense by attracting natural enemies. Thus, we studied whether HIPVs from plants attacked by these two herbivores affected the capacity of the mirid predators to use the info-chemicals.
In Y-tube olfactometer experiments, we tested responses of naïve and experienced N. tenuis and M. pygmaeus to uninfested plants, and to HIPVs from plants infested with various stages of T. absoluta and/or B. tabaci. We also studied how experience with the prey affects the response of predators in the context of single and multiple herbivory. Next, we investigated whether the predators responded to the natural and synthetic pheromone of T. absoluta. Finally, we assessed how the predators respond to injury as a result of plant feeding by the predators in the absence of prey.
Materials and methods
Plants and insects
Tomato plants, Solanum lycopersicon L. cv. Moneymaker grown in a greenhouse (25 ± 2 °C, 70 ± 10 % RH, L16:D8) were used in all experiments when they were 30–35 days old, 20–25 cm in height, with 5–6 leaves. Tuta absoluta adults were kept in mesh cages (60 × 40 × 40 cm) with a potted tomato plant in a greenhouse (25 ± 2 °C, 60 ± 10 % RH, L16:D8). After egg hatching, tomato leaves were excised from stems, placed in glass tubes with water, and kept in plastic cages (30 × 30 × 30 cm) until larval development. Uninfested tomato leaves were introduced into the cages when necessary to ensure ad libitum feeding. Pupae were collected from these cages and introduced in the cages with adults.
Bemisia tabaci was reared under the same conditions in another greenhouse. Adults were kept inside mesh cages on potted tomato plants. Once per week new plants and adults were introduced in the cages.
Nymphs of the predators were supplied by Koppert Biological Systems (The Netherlands), where mass rearing used eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) as prey, without exposure to plants or tomato plant material. After arrival at our laboratory, predators were kept in climate cabinets (25 ± 1 °C, 70 ± 5 % RH, L16:D8) inside cages (60 × 40 × 40 cm) containing a potted tomato plant and eggs of E. kuehniella as food.
Y-tube olfactometer
Responses of predator females to volatiles were observed in a two-choice Y-shaped Pirex tube (3.5 cm inside diameter) formed by an entry arm (20 cm in length) and two side arms (23 cm in length, 70° angle). The tube was positioned vertically as in other studies with Dicyphini (Ingegno et al. 2011, 2013; Moayeri et al. 2006a, b). The two side arms were each connected to a glass container (35 l in volume) harboring the volatile source. The airflow was provided by an air pump adjusted with a flow meter to 2.5 l min−1. Before reaching the glass container the air passed through an activated charcoal filter. The glass containers with volatile sources were kept behind a black panel, preventing insects from visually detecting the plants. In tests where plants were used, a single plant was introduced in each glass container. Single female predators of 1–7 days old were introduced at the downwind end of the entry arm and observed until they walked at least 10 cm up one of the side arms. Females not choosing a side arm within 10 min were considered as having made no choice and were excluded from data analysis. Each female was tested only once and then discarded. For each pair of volatile sources, 50 females were tested during five different experimental days, ten on each day. After testing a batch of five females, the volatile sources were switched between left and right sides of the arms to minimize positional bias. After testing ten females, the Y-tube and glass containers were washed with neutral soap and alcohol (70 %). Y-tube bioassays were carried out in a climate room at 22 ± 1 °C, 70 ± 10 % RH.
Volatile sources tested
The following experiments were conducted:
-
i.
Response to volatiles emitted by tomato plants infested either by T. absoluta (eggs or larvae) or by B. tabaci (mixed stages). Tomato plants were covered with organza bags, then five males and five females of 1–3 days old T. absoluta were released into each bag. Females were allowed to lay eggs for 48 h, then the adults were removed, and the plants infested with eggs were used in the experiments. For larval infestation, the same process was repeated. Eggs hatched 4–5 days after oviposition, and larvae were allowed to feed for 72 h before the tests. Fifty adult B. tabaci were released in a cage (60 × 30 × 40 cm) with tomato plants. Ten days after infestation, the plants with adults, eggs and nymphs were used in the tests.
-
ii.
Response to volatiles emitted by tomato plants infested simultaneously by both pest species. Because injury produced by T. absoluta larvae is more severe than injury produced by whiteflies, plants were first infested with B. tabaci as described above. After seven days, the plants were infested with 30 first and second instar T. absoluta larvae. After ten days, plants were used in the experiments.
-
iii.
Response to natural and synthetic sex pheromone of T. absoluta. Pupae of T. absoluta were individually put into glass tubes (5.5 × 1.3 cm) covered with Parafilm® and kept in a climate cabinet (25 ± 1 °C, 70 ± 5 % RH, L16:D8) until adult emergence when the sex could easily be determined. Female calling behavior was checked to determine sex pheromone release (Hickel and Vilela 1991). During calling behavior, females extrude the ovipositor, exposing the pheromone gland. Ten 1–3 days old calling females were released in a glass container (volume 1 l) connected to one of the arms of the olfactometer. Synthetic sex pheromone of T. absoluta was provided by Pherobank B.V. (Wageningen, The Netherlands). This commercial solution is used for monitoring and mass trapping T. absoluta and contains the main and minor component of the sex pheromone [(3E,8Z,11Z)-tetradecatrien-1-yl acetate and (3E,8Z)-tetradecadien-1-yl acetate, respectively] in a ratio of 95:5. One ml of solution (containing 1,000 ng) was impregnated in filter paper discs (8.4 cm in diameter). After complete evaporation of the solvent (hexane), the filter paper disc was placed inside a glass container of 1 l connected to the Y-tube. One ml of evaporated pure hexane solution was used as a control treatment.
-
iv.
Response to volatiles emitted by tomato plants injured by the predators in the absence of the prey. Tomato plants without prey were covered with organza bags and 20 newly emerged adults of either predator species were released into a bag. The plants were kept in climate cabinets at 25 ± 1 °C, 70 ± 10 % RH, L16:D8. After four and ten days the predators were removed from the cages and the plants were used in experiments. The number of feeding lesions (i.e. necrotic rings) caused by predators on the plants was counted.
Naïve and experienced predators
Predators that did not have contact with T. absoluta or B. tabaci nor with HIPVs before the tests were considered naïve. Predators that were kept together with prey-infested plants for 24 h preceding the tests and had the opportunity to feed and be exposed to HIPVs were considered experienced. In single infestation experiments experienced predators had the opportunity to feed on B. tabaci or T. absoluta (larvae or eggs), while in double infestation experiments experienced predators had the opportunity to feed on both pests simultaneously. All predators were kept on tomato plants except the naïve ones used in the test of uninfested tomato vs. clean air. Those predators not in contact with tomato plants before the tests were kept on tobacco, Nicotiana tabacum L. Ten naïve and ten experienced females of each predator species were tested for each pair of volatile sources on one day.
Statistical analysis
Responses of the predators were analyzed by Generalized Linear Models with a binomial distribution and a logit-link function. The response variable was the proportion of insects responding to one of the volatile sources. In experiments of single infestation and double infestation, the effects of day and experience were included in the GLM model. For all experiments, we fitted a separate binomial GLM to estimate the proportional response of each predator to test whether their choice was significantly different from a 50 % distribution. The significance of the response was tested using a χ2 test. The Mann–Whitney test was used to compare the differences in the number of feeding lesions caused by N. tenuis. All statistical procedures were performed using SPSS version 21.0 (SPSS Inc., Chicago, USA).
Results
The Y-tube olfactometer set-up used in this study worked well for both mirid predators: 76.8 % of M. pygmaeus females and 93.1 % of N. tenuis females responded. No influence of experimental day was found on the response of either predator in any of the treatments (GLM, P > 0.005).
Single infestation experiments
Experienced N. tenuis females preferred the odor of uninfested tomato plants over clean air (χ 2experienced = 4.01; df = 1; P = 0.042) and naïve females did not (χ2 = 0.84; df = 1; P = 0.359), while both naïve and experienced M. pygmaeus females had a significant preference for volatiles from uninfested tomato plants over clean air (χ 2naïve = 5.46; df = 1; P = 0.019; χ 2experienced = 5.67; df = 1; P = 0.017) (Fig. 1).
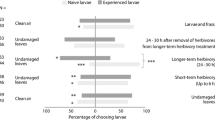
Responses of naïve and experienced females of Nesidiocoris tenuis (a) and Macrolophus pygmaeus (b) to volatiles from tomato plants infested with eggs or larvae of Tuta absoluta in a Y-tube olfactometer. Numbers in bars represent individual predators that moved towards the volatile sources indicated. NC indicates the number of tested individuals that did not make a choice. ***P < 0.01, **P ≤ 0.05, ns P > 0.05 (GLM, χ2 test)
Both naïve and experienced predators of both species preferred volatiles from egg-infested plants over clean air (N. tenuis: χ 2naïve = 5.68; df = 1; P = 0.017; χ 2experienced = 4.36; df = 1; P = 0.037; M. pygmaeus: χ 2naïve = 8.52; df = 1; P = 0.004; χ 2experienced = 5.8; df = 1; P = 0.016), but did not prefer volatiles from egg-infested plants over volatiles from uninfested plants (N. tenuis: χ 2naïve = 0.55; df = 1; P = 0.455; χ 2experienced = 1.12; df = 1; P = 0.289; M. pygmaeus: χ 2naïve = 2.24; df = 1; P = 0.134; χ 2experienced = 0.41; df = 1; P = 0.520) (Fig. 1).
Naïve and experienced N. tenuis preferred volatiles from plants infested with larvae of T. absoluta over volatiles from uninfested plants (χ 2naïve = 10.95; df = 1; P < 0.001; χ 2experienced = 4.09; df = 1; P = 0.043) and also over clean air (χ 2naïve = 6.46; df = 1; P = 0.011; χ 2experienced = 8.93; df = 1; P = 0.003) (Fig. 1a). Naïve M. pygmaeus did not show preference for volatiles from plants infested with larvae over volatiles from uninfested plants (χ2 = 0.62; df = 1; P = 0.43), but after experience with the prey, females preferred volatiles from infested plants (χ2 = 7.86; df = 1; P = 0.005) (Fig. 1b).
Naïve N. tenuis did not prefer volatiles released by whitefly-infested plants over volatiles from uninfested plants (χ2 = 3.6; df = 1; P = 0.057) whereas experienced females did (χ2 = 5.39; df = 1; P = 0.02). Naïve and experienced females were attracted by volatiles from plants infested by whiteflies over clean air (χ 2naïve = 13.5; df = 1; P < 0.001; χ 2experienced = 7.71; df = 1; P = 0.005) (Fig. 2a). Naïve M. pygmaeus showed no preference for volatiles from whitefly-infested plants over volatiles from uninfested plants (χ2 = 1.74; df = 1; P = 0.18), while experienced females preferred volatiles from whitefly-infested plants (χ2 = 10.27; df = 1; P = 0.001) (Fig. 2b).
Responses of naïve and experienced females of Nesidiocoris tenuis (a) and Macrolophus pygmaeus (b) to volatiles from tomato plants infested with Bemisia tabaci. Numbers in bars represent individual predators that moved towards the volatile sources indicated. NC indicates the number of tested individuals that did not make a choice. ***P < 0.01, **P ≤ 0.05, ns P > 0.05 (GLM, χ2 test)
Double infestation experiment
Naïve and experienced females of both predators preferred volatiles from plants infested with whiteflies and larvae of T. absoluta over clean air (N. tenuis: χ 2naïve = 9.63; df = 1; P = 0.002; χ 2experienced = 12.55; df = 1; P < 0.001; M. pygmaeus: χ 2naïve = 7.75; df = 1; P < 0.005; χ 2experienced = 9.17; df = 1; P = 0.002) (Fig 3). Neither naïve nor experienced N. tenuis preferred either of the volatile sources when choosing between plants with both whiteflies and T. absoluta vs. plants with whiteflies only (χ 2naïve = 0.06; df = 1; P = 0.795; χ 2experienced = 2.29; df = 1; P = 0.13) or T. absoluta larvae only (χ 2naïve = 0.008; df = 1; P = 0.93; χ 2experienced = 0.009; df = 1; P = 0.928) (Fig. 3a). When offered a choice between volatiles from plants with whiteflies only vs. plants with whiteflies and T. absoluta larvae, naïve M. pygmaeus preferred the latter (χ2 = 12.01; df = 1; P < 0.001), but lost this preference after experience (χ2 = 3.49; df = 1; P = 0.061) (Fig. 3b). Neither naïve nor experienced M. pygmaeus showed a preference when choosing between volatiles from plants with both whiteflies and T. absoluta vs. volatiles from plants infested with T. absoluta only (χ 2naïve = 3.43; df = 1; P = 0.06; χ 2experienced = 1.14; df = 1; P = 0.28).
Responses of naïve and experienced females of Nesidiocoris tenuis (a) and Macrolophus pygmaeus (b) to volatiles from tomato plants double-infested with Bemisia tabaci (Bt) and Tuta absoluta (Ta) compared to odors from plants single-infested with B. tabaci or T. absoluta. Numbers in bars represent individual predators that moved towards the volatile sources indicated. NC indicates the number of tested individuals that did not make a choice. ***P < 0.01, **P ≤ 0.05, ns P > 0.05 (GLM, χ2 test)
Experiment with natural and synthetic sex pheromone of T. absoluta
Neither N. tenuis nor M. pygmaeus were attracted by natural pheromones from calling virgin female T. absoluta (N. tenuis: χ2 = 0.15; df = 1; P = 0.69; M. pygmaeus: χ2 = 0.04; df = 1; P = 0.85), nor by its synthetic pheromone (N. tenuis: χ2 = 0.28; df = 1; P = 0.59; M. pygmaeus: χ2 = 1.69; df = 1; P = 0.19) (Fig. 4).
Responses of Macrolophus pygmaeus and Nesidiocoris tenuis females to virgin calling females and to synthetic sex pheromone of Tuta absoluta. Numbers in bars represent individual predators that moved towards the volatile sources indicated. NC indicates the number of tested individuals that did not make a choice, ns P > 0.05 (GLM, χ2 test)
Mirid plant feeding experiment
The number of feeding lesions (i.e., necrotic rings) caused by N. tenuis was higher on tomato plants exposed to ten days of feeding (17.2 ± 1.3 lesions) than on plants exposed for four days (10.0 ± 3.9 lesions) (N = 5, Mann–Whitney test: Z = −2,562; P = 0.008). No feeding lesions were found on plants exposed to M. pygmaeus.
Macrolophus pygmaeus preferred volatiles from plants previously exposed to feeding by conspecifics during four days over clean air (χ2 = 6.22; df = 1; P = 0.013), but not over volatiles from clean plants (χ2 = 0.09; df = 1; P = 0.76). Also, they were no longer attracted after ten days of plant feeding (χ2 = 1.53; df = 1; P = 0.21) (Fig. 5a), and these plants even became repellent (χ2 = 5.06; df = 1; P = 0.02). In contrast, N. tenuis were also attracted by plants previously exposed to conspecific feeding for four days over clean air (χ2 = 11.81; df = 1; P = 0.001), but in this case the attraction increased over time. After ten days of feeding, volatiles from injured plants were still preferred over clean air (χ2 = 13.18; df = 1; P < 0.001) and over volatiles from plants not exposed to predators (χ2 = 5.89; df = 1; P = 0.015) by N. tenuis (Fig. 5b).
Responses of Macrolophus pygmaeus and Nesidiocoris tenuis females to odors from tomato plants previously exposed to four and ten days of feeding by conspecifics in the absence of any prey. Numbers in bars represent individual predators that moved towards the volatile sources indicated. NC indicates the number of tested individuals that did not make a choice. ***P < 0.01, **P ≤ 0.05, ns P > 0.05 (GLM, χ2 test)
Discussion
Both M. pygmaeus and N. tenuis are attracted to plants infested with either T. absoluta or B. tabaci. We provide the first evidence that (1) a mirid predator (M. pygmaeus) can learn to respond to volatiles emitted by prey-infested plants, and (2) a mirid predator (N. tenuis) can be attracted to plants injured by conspecifics.
Plants infested simultaneously with two prey species were as attractive to the predators as plants infested with one prey species, which is promising for augmentative release programmes because the pests often occur together on tomato. Two species of herbivores sharing a common plant may alter the production of HIPVs with effects on tritrophic interactions (Dicke et al. 2009). Herbivores such as B. tabaci that induce the SA-pathway can modify or attenuate the defensive response of a plant subsequently attacked by a JA-pathway-inducer like T. absoluta, and vice versa (Ponzio et al. 2013). Zhang et al. (2009) showed that Lima bean plants infested with both whiteflies and spider mites were less attractive to a predatory mite than single-infested plants. However, synergistic interactions between the two pathways are also known to result in stronger attraction of some predators (de Boer et al. 2008). Moayeri et al. (2007b) found that M. pygmaeus responded more to volatiles emitted by plants with both aphids and spider mites than those emitted from single-infested plants. Bompard et al. (2013) showed that shortly after introduction of M. pygmaeus, the presence of T. absoluta caused a disruption of the mirid’s control of B. tabaci, resulting in increased densities of the whitefly. However, after ten weeks the whitefly density was 7.3-fold lower in the presence T. absoluta, which the authors attribute to an increased number of predators in response to higher prey availability (i.e., numerical response). The learning ability of M. pygmaeus, as we demonstrated, may provide another explanation for their results, as learning shortens the predator’s foraging time (i.e., functional response). Interestingly, Jaworski et al. (2013) recently showed that in a patch with T. absoluta and B. tabaci co-occurring, M. pygmaeus shows switching behavior and attacks the more abundant prey.
In our study, N. tenuis showed an innate preference for plants infested by T. absoluta larvae and all stages of B. tabaci whereas M. pygmaeus acquired this preference only as a result of experience. Learning may increase foraging efficiency, while innate use of info-chemical cues by generalist predators avoids exclusive reliance on, and may result in a higher prey finding efficiency than, random searching (Steidle and van Loon 2003). In augmentative biological control it might be better to use predators with innate responses to volatiles than those that have to learn to respond, because learning may have costs in terms of foraging time, as responses are not immediate (Vet and Dicke 1992). Foraging costs might be avoided by subjecting predators during mass rearing to essential HIPVs or to prey, prior to release in the environment (Carvalho et al. 2011). Olfactory learning has been documented well in parasitoids (Vet and Dicke 1992), but is as yet poorly documented for arthropod predators (De Boer et al. 2005; Drukker et al. 2000; Glinwood et al. 2011), and we provide the first evidence of olfactory learning in a predatory mirid bug.
Egg deposition by T. absoluta did not render tomato plants more attractive to naïve or experienced predators than uninfested plants. Egg deposition by herbivores can modify a plant’s surface chemistry and change the emission of plant volatiles, favoring production of oviposition-induced plant volatile (OIPVs) that are used by some natural enemies to locate their hosts (Fatouros et al. 2012). There is no information about the induction of OIPVs by T. absoluta eggs, but if such compounds are induced in tomato plants, either the level of infestation was not high enough to induce them, the amount of OIPVs was too low to elicit a response in the predators, or these predators do not respond to OIPVs from tomatoes. Another predaceous mirid, Dicyphus errans Knight, was similarly unable to distinguish between volatiles from tomato plants infested with T. absoluta eggs vs. uninfested plants (Ingegno et al. 2013). However, Mollá (2013) showed that N. tenuis responded to volatiles from tomato plants that were four times more heavily infested with T. absoluta eggs than in our test, suggesting that the amount of putative volatiles produced in our tests might have been too low.
As previously discussed, prey-infested plants were more attractive to both predators than were control treatments in all trials, except when naïve N. tenuis females chose between volatiles from egg-infested plants vs. uninfested plants. Our findings are in accordance with other Y-tube olfactometer studies with mirid bugs (Ingegno et al. 2011, 2013; McGregor and Gillespie 2004; Moayeri et al. 2006a, b, 2007b; Mollá 2013). Macrolophus pygmaeus does not prefer volatiles emitted by prey themselves (e.g., aphids, spider mites or whiteflies) (Ingegno et al. 2011; Moayeri et al. 2006a, b). There is as yet very limited information about olfactory responses of N. tenuis to prey-infested plants (Mollá 2013), but our results clearly show that they do respond.
Neither predator responded to natural or synthetic sex pheromones of T. absoluta, so contrary to several other natural enemies (Erbilgin and Raffa 2001; Fatouros et al. 2008), these mirids apparently do not use T. absoluta pheromones to locate prey patches. Additional support for this conclusion comes from the observation neither predator has ever been found in T. absoluta pheromone traps in tomato crops, even when large-scale predator releases were made (F. Griepink, Pherobank B.V., 2013, personal communication). This is beneficial for biological control, as it indicates that synthetic pheromones and predators can safely be used together in IPM programmes.
Concerning plant injury caused by mirids, we found no visible feeding lesions caused by M. pygmaeus. According to Castañé et al. (2011) this predator is considered safe for pest control, only causing economic damage when its density is very high (50–300 individuals per plant) and prey are scarce (Sampson and Jacobson 1999). Moayeri et al. (2007a) showed that feeding by M. pygmaeus for four days on green bean plants induced production of 11 additional compounds besides those volatiles emitted by clean plants. Still, predators were unable to distinguish between volatiles from conspecifically injured and clean plants (Moayeri et al. 2007a). We found similar results for M. pygmaeus after four days of feeding, but after ten days of feeding they preferred volatiles from clean plants more than from injured plants. We hypothesize that M. pygmaeus avoids habitats occupied by conspecifics to minimize intraspecific competition for resources. This may explain why this predator usually does not cause economic damage.
Nesidiocoris tenuis often causes visible feeding lesions and economic damage in the absence of prey (Castañé et al. 2011). When prey were unavailable, the number of necrotic brown rings was higher in plants exposed to ten days of predator feeding than in plants exposed for four days. In greenhouse and field experiments, it has been shown that the abundance of necrotic rings and flower abortion caused by N. tenuis is inversely proportional to prey availability (Sanchez 2009). Repeated feeding by N. tenuis at the same site of the plant (Arnó et al. 2010) causes biochemical changes in wounded tissue leading to increased levels of oxidative enzymes and phenolic compounds (Raman et al. 1984). After four days of feeding by conspecifics, N. tenuis did not distinguish between volatiles from injured and clean plants, but after ten days of feeding, predators preferred the volatiles from injured plants, suggesting increased production of volatiles with extended duration of plant feeding. Attraction of predators to conspecifically injured plants supports the observation of Arnó et al. (2010).
In summary, we found that HIPVs play an important but slightly different role in prey finding by two generalist mirid predator species. Based on these findings and knowledge that the predation capacity of the two species is similar (Urbaneja et al. 2009), we speculate that N. tenuis may be the better natural enemy for control of tomato pests because it does not need experience, it responds faster to HIPVs, and a substantially larger percentage N. tenuis respond to volatiles than M. pygmaeus. A drawback of N. tenuis might be its phytophagy during periods of low prey density, which will require management to achieve the optimal balance between predator and prey densities by the grower (Calvo et al. 2012). A more definitive answer regarding which mirid is the better choice for augmentative biological control can only be provided after testing in commercial greenhouses.
References
Arnó J, Castañé C, Riudavets J, Gabarra R (2010) Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull Entomol Res 100:105–115
Bompard A, Jaworski CC, Bearez P, Desneux N (2013) Sharing a predator: can an invasive alien pest affect the predation on a local pest? Popul Ecol 55:433–440
Bueno VHP, van Lenteren JC (2012) Predatory bugs (Heteroptera). In: Panizzi AR, Parra JRP (eds) Insect bioecology and nutrition for integrated pest management. CRC Press, Boca Raton, USA, pp 539–569
Calvo FJ, Lorente MJ, Stansly PA, Belda JE (2012) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp Appl 143:111–119
Carvalho LM, Bueno VHP, Castañé C (2011) Olfactory response towards its prey Frankliniella occidentalis of wild and laboratory-reared Orius insidiosus and Orius laevigatus. J Appl Entomol 135:177–183
Castañé C, Arnó J, Gabarra R, Alomar O (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29
de Boer JG, Snoeren T, Dicke M (2005) Predatory mites learn to discriminate between plant volatiles induced by prey and non-prey herbivores. Anim Behav 69:869–879
de Boer JG, Hordijk CA, Posthumus MA, Dicke M (2008) Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J Chem Ecol 34:281–290
Desneux N, Wajnberg E, Wyckhuys KA, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Ruescas DC, Tabone E, Frandon J (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84:403–408
Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl 91:131–142
Dicke M, Sabelis MW (1988) Infochemical terminology: based on cost-benefit analysis rather than origin of compounds? Funct Ecol 2:131–139
Dicke M, van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol 5:317–324
Drukker B, Bruin J, Sabelis MW (2000) Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol Entomol 25:260–265
EFSA Panel on Plant Health (PLH) (2013) Scientific opinion on the risks to plant health posed by Bemisia tabaci species complex and viruses it transmits for the EU territory. EFSA J 11:3162–3464
Erbilgin N, Raffa KF (2001) Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia 127:444–453
Eubanks MD, Styrsky JD (2005) Effects of plant feeding on the performance of omnivorous “predators”. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, UK, pp 148–177
Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M (2008) Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol 19:677–689
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607
Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V (2011) Olfactory learning of plant genotypes by a polyphagous insect predator. Oecologia 166:637–647
Guedes R, Picanço M (2012) The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. EPPO Bull 42(2):211–216
Hickel ER, Vilela EF (1991) Comportamento de chamamento e aspectos do comportamento de acasalamento de Scrobipalpula absoluta (Lep., Gelechiidae), sob condições de campo. Ann Soc Entomol Bras 20:173–182
Ingegno BL, Pansa MG, Tavella L (2011) Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol Control 58:174–181
Ingegno BL, Ferracini C, Gallinotti D, Alma A, Tavella L (2013) Evaluation of the effectiveness of Dicyphus errans (Wolff) as predator of Tuta absoluta (Meyrick). Biol Control 67:246–252
Jaworski CC, Bompard A, Genies L, Amiens-Desneux E, Desneux N (2013) Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS ONE 8:e82231
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
McGregor RR, Gillespie DR (2004) Olfactory responses of the omnivorous generalist predator Dicyphus hesperus to plant and prey odours. Entomol Exp Appl 112:201–205
Messelink GJ, van Maanen R, van Steenpaal SE, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Moayeri HRS, Ashouri A, Brodsgaard HF, Enkegaard A (2006a) Odour-mediated preference and prey preference of Macrolophus caliginosus between spider mites and green peach aphids. J Appl Entomol 130:504–508
Moayeri HRS, Ashouri A, Brodsgaard HF, Enkegaard A (2006b) Odour-mediated responses of a predatory mirid bug and its prey, the twospotted spider mite. Exp Appl Acarol 40:27–36
Moayeri HRS, Ashouri A, Brodsgaard HF, Enkegaard A (2007a) Males of the predatory mirid bug Macrolophus caliginosus exploit plant volatiles induced by conspecifics as a sexual synomone. Entomol Exp Appl 123:49–55
Moayeri HRS, Ashouri A, Poll L, Enkegaard A (2007b) Olfactory response of a predatory mirid to herbivore induced plant volatiles: multiple herbivory vs. single herbivory. J Appl Entomol 131:326–332
Mollá Ó (2013) Control biológico de la polilla del tomate Tuta absoluta (Lepidoptera: Gelechiidae) mediante la gestión de míridos depredadores. Ph.D. Thesis, Faculty of Biological Sciences, University of Valencia, Spain
Mouttet R, Kaplan I, Bearez P, Amiens-Desneux E, Desneux N (2013) Spatiotemporal patterns of induced resistance and susceptibility linking diverse plant parasites. Oecologia 173:1379–1386
Ponzio C, Gols R, Pieterse CM, Dicke M (2013) Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct Ecol 27:587–598
Raman K, Sanjayan K, Suresh G (1984) Impact of feeding injury of Cyrtopeltis tenuis Reut. (Hemiptera: Miridae) on some biochemical changes in Lycopersicon esculentum Mill. (Solanaceae). Curr Sci 53:1092–1093
Sampson C, Jacobson R (1999) Macrolophus caliginosus Wagner (Heteroptera: Miridae): a predator causing damage in UK tomatoes. IOBC/WPRS Bull 22:249–256
Sanchez JA (2009) Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol Control 51:493–498
Sanchez J, Lacasa A (2008) Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J Econ Entomol 101:1864–1870
Steidle JLM, van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol Exp Appl 108:133–148
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270
Urbaneja A, Montón H, Mollá O (2009) Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J Appl Entomol 133:292–296
Urbaneja A, González-Cabrera J, Arnó J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Zhang PJ, Zheng SJ, van Loon JJA, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. P Natl Acad Sci USA 106:21202–21207
Acknowledgments
We thank Dr. Russell Messing and the two reviewers for helping us to improve the manuscript, the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) for a research grant to Lins Jr within the CAPES/Nuffic Programme Project 044/12, the Foundation for Support of Research of the State of Minas Gerais (FAPEMIG) and the Laboratory of Entomology of Wageningen University for financial support of the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stefano Colazza.
Rights and permissions
About this article
Cite this article
Lins, J.C., van Loon, J.J.A., Bueno, V.H.P. et al. Response of the zoophytophagous predators Macrolophus pygmaeus and Nesidiocoris tenuis to volatiles of uninfested plants and to plants infested by prey or conspecifics. BioControl 59, 707–718 (2014). https://doi.org/10.1007/s10526-014-9602-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-014-9602-y