Abstract
The sex pheromone of the hibiscus flower borer Rehimena surusalis (Walker) (Lepidoptera: Crambidae) was analyzed by gas chromatography with electroantennographic detection (GC-EAD) and GC-mass spectrometry (GC/MS). Three EAD-active components were found in crude pheromone gland extracts of calling females. GC/MS and GC analyses using synthetic chemicals and derivatization of the extracts identified three components as (10E,12Z)-hexadeca-10,12-dienal (E10,Z12-16:Ald,), (10E,12E)-hexadeca-10,12-dienyl acetate (E10,Z12-16:OAc), and (3Z,6Z,9Z)-tricosa-3,6,9-triene (Z3,Z6,Z9-23:HC). In field tests, male moths were strongly attracted to a ternary blend of E10,Z12-16:Ald, E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC at a ratio of 1:5:14, but single and binary blends showed only weak or no attraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hibiscus flower-bud borer, Rehimena surusalis (Walker) (Lepidoptera: Crambidae), is widely distributed in Africa, Australia, China, India, Indonesia, Taiwan, Korea, and Japan (Ades and Kendrick 2004; Herbison-Evans and Crossley, 2013; Inoue et al. 1982; Shibuya 1929; Shin 2001) and is a pest of Malvaceae garden and street trees including Hibiscus syriacus (rose of Sharon), H. mutabilis (cotton rose), H. rosa-sinensis (Chinese hibiscus), H. tiliaceus and H. glaber (Sea Hibiscus) (Anonymous 1994, 2006). In Japan and Korea, H, syriacus is particularly damaged by R. surusalis. Hibiscus syriacus (mugunghwa in Korean) is authorized as the national flower of Korea, and R. surusalis has been reported to eat the seed of this plant (Bea 2012; Kim et al. 2013; Lee et al. 2005). The larvae bore into the developing flowers and flower buds. Because of the larval feeding habit as a typical borer, it is difficult to control this pest with cover sprays of insecticides. To control insects with a perforative lifestyle in the larval stage, pheromones are advantageous to monitor the flying adults, and disrupt their mating, resulting in a reduction in oviposition (Witzgall et al. 2010).
In this study, we identified components of the female sex pheromone of R. surusalis, and demonstrated sex pheromone activity of the synthetic compounds in the field. We also discuss the occurrence of hybrid-types of sex pheromone in Pyraloidea.
Methods and Materials
Insects
Colonies of R. surusalis were maintained as laboratory cultures. Mated females were allowed to lay eggs in small plastic cylinders that were lined with felt cloth impregnated with methanol extracts of H. syriacus flower buds. Because of heavy cannibalism, larvae of R. surusalis were reared individually on an artificial diet composed of Insecta® F-II (Nosan Corporation, Japan) and dried leaf powder of H. syriacus at a ratio of 8:2. Adults were sexed at the pupal stage and kept separately in cages at 25 ± 2 °C, 60–70 % relative humidity (RH) and a 15L9D photoperiod, and provided with a 10 % sugar solution from cotton pads. A red lamp was used for observations during the scotophase.
Pheromone Extracts
Pheromone extracts were obtained from 2- to 7-d-old calling females, whose abdominal tips were cut with ophthalmology scissors halfway through the scotophase by extraction with redistilled n-hexane for 20 min. Pooled extracts (60 female equivalents, FE) were stored at −20 °C until use for chemical analyses and bioassays. Aliquots of the extracts were subjected to GC analysis for quantitative determination of pheromone candidates in 5 replicates.
Chemicals
The four geometric isomers of 10,12-hexadecadienal (Z10,E12-16:Ald, E10,Z12-16:Ald, Z10,Z12-16:Ald, and E10,E12-16:Ald) and 10,12-hexadecadienyl acetate (Z10,E12-16:OAc, E10,Z12-16:OAc, Z10,Z12-16:OAc, and E10,E12-16:OAc), and (3Z,6Z,9Z)-tricosa-3,6,9-triene (3Z,6Z,9Z-23:CH) were supplied by coauthors T. A. or S. M. The isomeric purities of all compounds were confirmed by GC to be ≥97 %.
Chemical Analysis
Pheromone extracts were subjected to GC-EAD analyses using an HP-5890 series II GS (Agilent Technologies, California, USA) equipped with an HP-5MS capillary column (30 m × 0.32 mm ID, film thickness 0.25 μm; Agilent Technologies, USA) and helium as carrier gas (37 cm/s). Oven temperature was programmed at 130 °C for 2 min, then increased at a rate of 5 °C/min to 250 °C, and held at the final temperature for 10 min. The temperature of the detector and injector was 250 °C, and that of the outlet for the EAD was maintained at 300 °C. Extracts were injected in splitless mode. The GC effluent from the column was split in a 1:1 ratio between the flame ionization detector (FID) and the EAD. The effluent was delivered in humidified air (23 °C) to the antennal preparation connected to an EAG probe (Type PRG-2, Syntech, The Netherlands) via Ag-AgCl electrodes with 0.1.M KCl. EAD responses of male antennae were recorded with GC-EAD 2010 software (Ver. 4.60, Syntech) via a GC-EAD signal acquisition controller (IDAC-2, Syntech).
Analyses of the extracts by GC/MS employed a MS-600 H mass spectrometer (JEOL Ltd., Japan) coupled with an HP-6890 N GC (Agilent), which was equipped with a DB-5MS (25 m × 0.25 mm ID, film thickness 0.25 μm, Agilent) capillary column, and operated in electron impact ionization mode (70 eV). The GC oven temperature was programmed at 100 °C for 1 min, then increased at a rate of 10 °C/min to 320 °C and held at the final temperature for 17 min.
GC analyses were conducted with GC-17A (Shimadzu Co., Ltd., Japan) and GC-6890 N (Agilent) fitted with a nonpolar HP-5MS column and a polar DB-23 column (30 m × 0.25 mm ID, film thickness 0.15 μm; Agilent), respectively. For the nonpolar column, the GC oven temperature was programmed at 130 °C for 2 min, then increased at a rate of 5 °C/min to 250 °C, and held at the final temperature for 10 min. For the polar column, the GC oven temperature was programmed at 80 °C for 2 min, then increased at the rate of 3 °C/min to 250 °C, and held at the final temperature for 5 min.
To determine the positions of conjugated double bonds, pheromone candidates in the extracts were reacted with 4-methyl-1,2,4-triazoline-3,5-dione (MTAD), followed by GC/MS analysis of the resulting derivatives. Kováts retention indices (KRI) (Kováts 1958; Dool and Kratz 1963) of EAD-active components and authentic chemicals were determined by comparison with retention times of n-alkanes. The GC peak area of each component on the HP-5MS column was used to determine the ratio of EAD-active components in the pheromone extracts.
Laboratory and Field Tests
Candidate pheromone components, E10,Z12-16:Ald, E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC and their blends were examined by laboratory and field assays. Laboratory cage tests were conducted in a mesh cage (30 × 25 × 30 cm) with 10 males at the second half of scotophase when the most calling by females was observed. Pheromone extracts or synthetic compounds were applied on a filter paper (1 × 3 cm) in 1 μl hexane as solvent. The filter paper was suspended 10 cm from the ceiling with a wire clip. Amounts of synthetic compounds were adjusted to 1 female equivalent (FE)/μl. Crude extracts were concentrated to 1 FE/μl under a gentle N2 stream. Numbers of males showing orientation flight (OF) by hovering near the pheromone source and source contact (SC) were counted for 3 min with 5 ~ 7 replications, and the cumulative numbers were compared in single, binary, and ternary blends of the candidate compounds.
Field experiments were conducted in fields with H. syriacus plantations on the campus of the University of Tsukuba (36.1°N, 140.1°E) during June and August in 2013. Similar sets of synthetic blends with those used in the laboratory assays were loaded on gray rubber septa (West Corp., Singapore) at 500 μg/trap. In addition to the regular blend, blends with two and five times Z3,Z6,Z9-23:HC (750 μg and 1750 μg/trap) also were tested. Each rubber septum was placed on a sticky board trap with a triangle roof (SE-trap, 30 cm in length × 27 cm in width × 10 cm in height; Sankei Chemical Co., Ltd., Kagoshima, Japan). Traps were hung ca. 1.5 m above the ground on tree branches with at least 10 m between traps, and were set in a completely randomized design. Lures were renewed once a week, and positions of traps were moved on one position every 3 d to avoid positional effects. As a control, empty traps also were tested. Numbers of captured males in each trap were counted and removed every 3 d.
Statistical Analyses
Results of laboratory and field assays were analyzed using one-way analysis of variance (ANOVA), followed by a Tukey-Kramer’s honestly significant difference (HSD) test. Numbers of captured males (x) in field tests were transformed √(x + 0.5) prior to ANOVA. Software package R 3.0.1 (R core team 2013), was used for the statistical analyses.
Results
Chemical Analysis
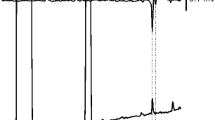
GC-EAD analyses of crude pheromone gland extracts from female Rehimena surusalis showed three active components A (Retention time (Rt) 11.28 min), B (Rt 14.66 min), and C (Rt 18.52 min) on FID chromatogram (Fig. 1). In GC/MS analyses, spectra of the active component A showed a possible molecular ion at m/z 236 (M+, 36 %), and fragment ions at m/z 67 ([C5H7]+, base peak), m/z 95 ([C7H11]+, 41 %), m/z 96 ([C7H12]+, 42 %) and m/z 109 ([C8H13]+, 28 %). The ions spaced by m/z 14 and those at m/z 96 and 109 suggested the double bonds at the 10- and 12- (ω4, ω6) positions in a straight carbon chain (Ando et al. 1988). From these spectral data, the structure of compound A was consistent with a 10, 12-hexadecadienal (C16H28O), the relatively high intensity of the molecular ion at m/z 236 being characteristic of a conjugated, di-unsaturated 16-carbon aldehyde.
GC/MS analysis of component B showed ions at m/z 280 (M+, 38 %), m/z 61 ([CH3COOH + 2 H, 5 %], m/z 67 ([C5H7]+, base peak), m/z 95 ([C7H11]+, 48 %), m/z 96 ([C7H12]+, 58 %), m/z 109 ([C8H13]+, 29 %), and m/z 220 ([M-CH3COOH]+, 16 %). Mass spectra with ions spaced by m/z 14 and two prominent ions at m/z 96 and 109 suggested a straight carbon chain and double bond positions at 10, 12- (ω4, ω6) positions in C16H32O2. Two diagnostic ions at m/z 61 and m/z 220 were consistent with the structure of compound B as 10, 12-hexadecadienyl acetate. The relatively high intensity of the molecular ion at m/z 280 also indicated conjugated double bonds in compound B.
In GC/MS analysis, component C showed ions at m/z 318 (M+, 6 %), m/z 79 ([C6H7]+, 79 %), m/z 93 ([C7H9]+, 33 %), m/z 107 ([C8H11]+, 15 %), m/z 108 ([C8H12]+, base peak), m/z 121 ([C9H13]+, 18 %) and m/z 262 ([M-C4H8]+, 19 %). The fragmentation pattern indicated an unsaturated straight-chain compound, with possible molecular formula of C23H42, consistent with a tricosatriene (3,6,9–23:HC). In addition, three conspicuous diagnostic ion peaks at m/z 79, m/z 108, and m/z 262 indicated three double bonds at 3, 6, and 9-position of compound C (Ando et al. 2004).
The positions of the double bonds in A and B were further confirmed by derivatization with MTAD, which reacts specifically with conjugated dienyl structures. The mass spectra of MTAD reaction products exhibited ions at m/z 349 (M+, [C19H31O3N3]+, 17 %), m/z 208 ([C10H12O2N3]+, base peak), and m/z 306 ([C16H24O3N3]+, 57 %) for compound A, and at m/z 393 (M+, [C21H35O4N3]+, 17 %), m/z 208 ([C10H12O2N3]+, base peak), and m/z 350 ([C18H28O4N3]+ for compound B supporting two conjugated double bonds at either 3- and 5-positions or 10- and 12-positions in hexadecadienal and hexadecadienyl acetate, respectively.
Components A and B had KRIs similar to those of each four isomers of 10,12–16: Ald and 10,12–16: OAc on both nonpolar and polar GC columns (Table 1). The 3,5-dienes would have been expected to elute much earlier than 10,12-dienes on GC (Ando et al. 2004). As shown in Table 1, KRIs of components A and B corresponded well to those of (10E,12Z)- hexadeca-10,12-dien-1-al (E10,Z12-16:Ald,) and (10E,12Z)-hexadeca-10,12-dien-1-yl acetate (E10,Z12-16:OAc), respectively, on both HP-5MS and DB-23 columns. The KRI of component C was compared only with that of Z3,Z6,Z9-23:HC, because 3,6,9-tricosatrienes as insects pheromones are considered to be biosynthesized from (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid with elongation of the carbon chain (Ando et al. 2008). The geometric configuration of component C was confirmed to be 3Z,6Z,9Z–isomer from agreement with the KRI.
The amounts of these three components (A, B, and C) in the extracts were determined to be 0.77 ± 0.08 ng, 3.60 ± 0.56 ng, and 11.1 ± 0.96 ng per female, respectively, at ratio of 1:5:14.
Laboratory and Field Tests
In the laboratory test, pheromone activities of the crude pheromone extract and combinations of synthetic E10,Z12-16:Ald, E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC are summarized in Fig. 2. Three one-component baits and binary blends of E10,Z12-16:Ald and E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC with E10,Z12-16:Ald or E10,Z12-16:OAc showed no pheromone activity in either activity criteria, orientation flight, or source contact by male moths, whereas significantly higher activity in orientation flight was observed with the binary combination of E10,Z12-16:Ald and E10,Z12-16:OAc although it was still lower than that of the extract. Highest activity in orientation flight was observed with the ternary blend of the above synthetics in natural amounts, and it corresponded well to activity of the extract. In source contact by male moths, only the ternary blend showed significantly different activity from that of the crude extract.
Cumulative number of male Rehimena surusalis exhibiting orientation flight (OF) to pheromone source and source contact (SC) in laboratory assays. The amount of the synthetic components in the respective baits are shown under the bars. Bars with the same letters are not significantly different at P < 0.05 by Tukey–Kramer’s HSD test after ANOVA (OF: N = 5, F = 56.75, P < 0.01; SC: F = 21.31, P < 0.01). The number of trapped males was transformed to √(x + 0.5) prior to the test
In the field tests, the ternary blend of E10,Z12-16:Ald, E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC attracted the highest number of male moths in all treatments tested, whereas single and binary blends attracted fewer or no male moths (Fig. 3). Similar to the results of the laboratory tests, the binary blend of E10,Z12-16:Ald and E10,Z12-16:OAc showed also relatively high activity in male attraction. When the amount of Z3,Z6,Z9-23:HC was increased, trap catches somewhat decreased at 700 μg, and significantly decreased at 1750 μg (Figs 3).
Field catches of male Rehimena surusalis in traps baited with synthetic E10,Z12-16:Ald(Ald), E10,Z12-16:OAc(OAC), and Z3,Z6,Z9-23:HC(HC) and their mixtures. Bars with the same letters are not significantly different at P < 0.05 by Tukey–Kramer’s HSD test after ANOVA (N = 9, F = 5.838, P < 0.01). The number of trapped males was transformed to √(x + 0.5) prior to the test
Discussion
Three GC-EAD active components were detected in analyses of crude pheromone gland extracts from female Rehimena surusalis and identified as E10,Z12-16:Ald, E10,Z12-16:OAc, and Z3,Z6,Z9-23:HC by GC retention times and GC/MS analyses. The ternary blend of these compounds in a ratio of 1:5:14 showed pheromone activity to male moths of R. surusalis in laboratory and field bioassays. These results show that the sex pheromone of R. surusalis consists of three components in this ratio. 10,12-Hexadecadienals are widely known as major or minor components of sex pheromones of several moth families including Noctuidae (Cork et al. 1988), Sphingidae (Uehara et al. 2012, 2015), Pyralidae or Crambidae (Honda et al.1994), Saturniidae (Dai et al. 1988; McElfresh and Millar 1999a, 1999b), and also Bombycidae (Daimon et al. 2012). E10,Z12-16:Ac also was identified as a sex pheromone in Bombycidae (Daimon et al. 2012) and Saturniidae (McElfresh and Millar 1999a, 1999b, 1999c, McElfresh et al. 2001).
Sex pheromone components can be categorized into Type I and Type II groups depending on presence or absence of terminal functional groups in the molecules (Ando et al. 2004). Compounds such as E10,Z12-16:Ald, and E10,Z12-16:OAc belong to the Type I group, but polyenyl hydrocarbons such as Z3,Z6,Z9-23:HC belong to the Type II group. Recently, so-called hybrid pheromone systems consisting of Type I and Type II compounds such as that of R. surusalis, have been reported mainly in Crambid and Pyralid species (Cabrera et al. 2001; Gibb et al. 2007; Löfstedt et al. 2012; Leal et al. 2005; Millar et al. 2005; Yan et al. 2014).
Rehimena surusalis male moths showed low but significant orientation flight responses to a binary blend of E10,Z12-16:Ald and E10,Z12-16:OAc, although neither component was active as a single component, in the laboratory cage test or field tests, indicating a crucial synergistic function of E10,Z12-16:Ald and E10,Z12-16:OAc in attraction of males from long distance. Z3,Z6,Z9-23:HC significantly increased male catches in the field traps, indicating a synergistic effect with E10,Z12-16:Ald and E10,Z12-16:OAc. However, trap catches decreased when Z3,Z6,Z9-23:HC was mixed with these dienyl components at 1:5:70 (25, 125, 1750 μg), showing an optimal ratio of the trienyl hydrocarbon component for the pheromone system in this species.
In the laboratory tests, the numbers of source contacts by male moths significantly increased when Z3,Z6,Z9-23:HC was added to the binary blend. In some lepidopteran species, hydrocarbons of body waxes have critical effects, such as a releaser for copulation (Grant et al. 1987) or stimulator for contact to the pheromone source (Schlamp et al. 2005; Xiao and Honda 2010, Xiao et al. 2011, 2012), over short range. Xiao (2011) showed the possibility that although their actual functions are unknown, homologous polyene hydrocarbons including Z3,Z6,Z9-23:HC also exist widely in body wax of moths other than Crambidae, because similar synergistic activity was observed when body wax extracts of some Noctuidae and Sphingidae species were mixed with the two aldehyde sex pheromone components.
The four families, Noctuidae, Arctiidae, Lymantriidae, and Geometridae use Type II compounds in their female sex pheromones (Ando 2014). However, Zahiri et al. (2010) reconstructed Noctuidae sensu lato by molecular phylogeny, and showed traditional Arctiidae and Lymantriidae sensu Miller (1991) were included in Erebidae with various noctuids using Type II pheromone components. This indicated that only Geometroidea and Noctuoidea, which show sister linkages in recent molecular phylogenetic trees (Regier et al. 2009), use Type II sex pheromones, and also that the origin of Type II pheromones may be from a common ancestor of the two taxa. However, recently hybrid type pheromone systems have been reported in several Pyraloidea species (Cabrera et al. 2001; Gibb et al. 2007; Löfstedt et al. 2012; Leal et al. 2005; Millar et al. 2005; Yan et al. 2014). In Pyraustinae sensu lato, R. surusalis is the fourth species that has a hybrid type pheromone system as shown in two Conogethes species (El-Sayed et al. 2013; Xiao and Honda 2010, Xiao et al. 2012) and Omphisa anastomosalis (Yan et al. 2014). These results suggest that the hybrid type pheromone system is at least common in Pyraloidea, and the origin of Type II pheromones may be a common ancestor of Pyraloidea and Geometroidea + Noctuoidea (Fig. 4). However, the Pyraloidea + (Geometroidea + Noctuoidea) clade include some taxa, e.g., Bombycoidea, Lasiocampoidea, or Drepanoidea that have no reports of Type II pheromones to date (Regier et al. 2009). To reveal the origin of Type II pheromones, we must carefully reinvestigate some species that use only Type I compounds for their female sex pheromones, included into the Pyraloidea + (Gemoetridea + Noctuoidea clade), by physiological or molecular methods.
Type of female sex pheromone and molecular phylogenetics in the clade Ditrysia (Lepidoptera). Type II pheromone was identified from 3 taxonomic groups (Geometroidea, Geometridae and Noctuoidea: Erebidae and Pyraloidea). Papilionoidea etc. indicates a clade ((((Nymphalidae + Pieridae) + (Hesperioidea + Hedyloidea)) + Thyridoidea) + (Papilionidae + Calliduloidea)) + (Copromorphoidea + Hyblaeoidea). Alucitoidea, Urodoidea and Choreutoidea were omitted from the phylogenetic tree that was modified from Regier et al. (2009)
Three Crambidae species, Haritalodes derogate, H. basipunctalis, and R. surusalis use E10,Z12-16:Ald as a sex pheromone component, and occur sympatrically in hibiscus plantations. This sympatry is made possible by their species-specific pheromone systems, which consist of binary mixtures of E10,Z12-16:Ald and E10,E12-16:Ald at different ratios in the two Haritalodes (Notracha) species (Honda et al. 1994), and addition of E10,Z12-16:OAc and Z3,Z6,Z9-23:HC in R. surusalis.
References
Ades GWJ, Kendrick RC (Eds) (2004) Hong Kong fauna- a checklist of selected taxa, fauna conservation, Department Kadoorie Farm & Botanic Garden Corporation, 87 pp
Ando T (2014) Internet database: http://www.tuat.ac.jp/~antetsu/LepiPheroList.htm
Ando T, Ogura Y, Uchiyama M (1988) Mass spectra of lepidopterous sex pheromones with a conjugated diene system. Agric Biol Chem 52:1415–1423
Ando T, Inomata S, Yamamoto M (2004) Lepidopteran sex pheromone. Top Curr Chem 239: 51–96
Ando T, Kawai T, Matsuoka K (2008) Epoxyalkenyl sex pheromones produced by female moths in highly evolved groups: biosynthesis and its endocrine regulation. J Pestic Sci 33: 17–20
Anonymous (1994) Check list of insects from Korea. Kon-kuk University Press, Seoul, 744 pp
Anonymous (2006) Major insect and other pests of economic plants in Japan, revised edn. The Japanese Society of Applied Entomology and Zoology, Tokyo, 381 pp
Bea Y. (2012) The final report of researches for publication of Korean Red List: Insects. Nat. Inst. Biol. Res, Biol. Res. Co. Div. 164pp. (in Korean)
Cabrera A, Eiras AE, Gries G, Gries R, Urdaneta N, Mirás B, Badji C, Jaffe K (2001) Sex pheromone of tomato fruit borer, Neoleucinodes elegantalis. J Chem Ecol 27: 2097–2107
Cork A, Chamberlain DJ, Beevor PS, Hall DR, Nesbitt BF, Campion DG, Attique MR (1988) Components of female sex pheromone of spotted bollworm, Earias vittella F. (Lepidoptera: noctuidae): identification and field evaluation in Pakistan. J Chem Ecol 14: 929–945
Dai XJ, Xu SF, Wang MZ, Zhu YX, Tang XH, Zhu JW, Du JW, Dong TX, Du MZ (1988) 10E,12Z-hexadecedienyl acetate - sex pheromone of the mulberry white caterpillar Rondotia menciana Moore (Lepidoptera: bombycidae). Kexue Tongbao 33: 1575–1576
Daimon T. Fujii T, Yago M, Hsu YF, Nakajima Y, Fujii T, Katsuma S, Ishikawa Y, Shimada T (2012) Female sex pheromone and male behavioral responses of the bombycid moth Trilocha varians: comparison with those of the domesticated silkmoth Bombyx mori. Naturwissenshaften 99: 201–215.
Dool HD, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr 11: 463–471
El-Sayed AM, Gibb AR, Mitchell VJ, Manning L-AM, Revell J, Thistleton B (2013) Identification of the sex pheromone of Conogethes pluto: a pest of alpinia. Chemoecology 23:93–101
Gibb AR, Pinese B, Tenakanai D, Kawi AP, Bunn B, Ramankutty P, Suckling DM (2007) (Z)-11-hexadecenal and (3Z,6Z,9Z)-tricosatriene: sex pheromone components of the red banded mango caterpillar Deanolis sublimbalis. J Chem Ecol 33: 579–589
Grant GG, Frech D, MacDonald L, Slessor KN, King GGS (1987) Copulation releaser pheromone in body scales of female whitemarked tussock moth, Orgyia leucostigma (Lepidoptera: lymantriidae): identification and behavioral role. J Chem Ecol 13: 345–356
Herbison-Evans D, Crossley SA (2013) Australian spilomelinae - Lepidoptera larvae of Australia, http://lepidoptera.butterflyhouse.com.au/spil/spilomelinae.html
Honda H, Himeno K, Yoshiyasu Y (1994) Chemotaxonomy of the cotton leaf-roller (Lepidoptera: pyralidae) in Japan with special reference to differences in sex pheromones. Appl Entomol Zool 29: 323–330
Inoue H, Sugie S, Kuroko H, Moriuti S, Kawabe A (1982) Moths of Japan I. Kodansha, Tokyp, Japan
Kim Y, Cho Y, Kang Y-K, Choi M, Nam S-H (2013) A study of the major insect pest communities associated with Hibiscus syriacus (columniferae, malvaceae). J Ecol Environ 36: 125–129
Kováts E (1958) Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv Chim Acta 41:1915–1932
Leal WS, Parra-Pedrazzoli AL, Kaissling KE, Morgan TI, Zalom FG, Pesak DJ, Dundulis EA, Burks CS, Higbee BS (2005) Unusual pheromone chemistry in the navel orangeworm: novel sex attractants and a behavioral antagonist. Naturwissenschaften 92: 139–146
Lee, SY, Nam SH and Seok O (2005) Insect community complex of Hibiscus syriacus. 2005 ESA Annual meeting, http://abstracts.co.allenpress.com/pweb/esa2005/document/49852
Löfstedt C, Svensson GP, Jirle EV, Rosenberg O, Roques A, Millar JG (2012) 3Z,6Z,9Z,12Z,15Z)-pentacosapentaene and (9Z,11E)-tetradecadienyl acetate: sex pheromone of the spruce coneworm Dioryctria abietella (Lepidoptera: pyralidae). J Appl Entomol 136: 70–78
McElfresh JS, Millar JG (1999a) Sex pheromone of the common sheep moth, Hemileuca elganterina, from the San Gabriel mountains of California. J Chem Ecol 25: 687–709
McElfresh JS, Millar JG (1999b) Sex attractant pheromone saturniida moth, Coloradia velda. J Chem Ecol 25: 1067–1078
McElfresh JS, Millar JG (1999c) Geographic variation in sex pheromone blend of Hemileuca electra from southern California. J Chem Ecol 27: 1409–1422
McElfresh JS, Hammond AM, Millar JG (2001) Sex pheromone components of the buck moth Hemileuca maja. J Chem Ecol 25: 2505–2525
Millar JG, Grant GG, McElfresh JS, Strong W, Rudolph C, Stein JD, Moreira JA (2005) Pheromone component of the fir coneworm moth, Dioryctria abietivorella. J Chem Ecol 31: 1229–1234
Miller JS (1991) Cladistics and classification of the notodontidae (Lepidoptera, noctuoidea) based on larval and adult morphology. Bull Am Mus Nat Hist 204:1–230
Regier JC, Zwick A, Cummings MP, Kawahara AY, Cho S, Weller S, Roe A, Baixeras J, Brown JW, Parr C, Davis DR, Epstein M, Hallwachs W, Hausmann A, Janzen DH, Kitching IJ, Solis MA, Yen SH, Bazinet AL, Mitter C (2009) Toward reconstructing the evolution of advanced moths and butterflies (Lepidoptera: Ditrysia): an initial molecular study. BMC Evol Biol 9: 280
Schlamp KK, Gries R, Khaskin G, Brown K, Khaskin E, Judd GTR, Gries G (2005) Pheromone components from body scales of female Anarsia lineatella induce contacts by conspecific males. J Chem Ecol 31:2897–2911
Shibuya J (1929) On the known and unrecorded species of the Japanese pyraustinae (lepid.). J Fac Agric Hokkaido Imp Univ 25:151–242
Shin YH (2001) Coloured Illustrations The moths of Korea. Academy Book Publishing Co., Seoul. (In Korean.)
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2012) Identification and field evaluation of sex pheromones in two hawk moths Deilephila elpenor lewisii and Theretra oldenlandiae oldenlandiae (Lepidoptera: sphingidae). Appl Entomol Zool 47: 227–232
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2015) Sex pheromone of the diurnal hawk moth, Hemaris affinis. J Chem Ecol 41: 9–14
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36: 80–100
R core team (2013) R: A language and environment for statistical com- puting. R Foundation for Statistical Computing, Vienna, URL http://www.R-project.org/
Xiao W (2011) Identification and function of body wax hydrocarbons as a sex pheromone of the yellow peach moth. PhD dissertation of the University of Tsukuba, 97 pp
Xiao W, Honda H (2010) Non-polar body waxes enhance sex pheromone activity in the yellow peach moth, Conogethes punctiferalis (guene’e) (Lepidoptera: crambidae). Appl Entomol Zool 45: 449–456
Xiao W, Honda H, Matsuyama S (2011) Monoenyl hydrocarbons in female body wax of the yellow peach moth as synergists of aldehyde pheromone components. J Chem Ecol 46: 239–246
Xiao W, Matsuyama S, Ando T, Millar JG, Honda H (2012) Unsaturated cuticular hydrocarbons synergize responses to sex attractant pheromone in the yellow peach moth, Conogethes punctiferalis. J Chem Ecol 38: 1143–1150
Yan Q, Vang LV, Khanh CNQ, Naka H, Ando T (2014) Reexamination of the female sex pheromone of the sweet potato vine borer moth: identification of tricosatriene and its field evaluation. J Chem Ecol 40: 590–598
Zahiri R, Kitching IJ, Lafontaine JD, Mutanen M, Kaila L, Holloway JD, Wahlberg N (2010) A new molecular phylogeny offers hope for a stable family level classification of the noctuoidea (Lepidoptera). Zool Scr 40: 158–173
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan [KAKENHI, Grant-in-Aid for Scientific Research (C) No. 25450071]. We thank Dr. DeMar Taylor for improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honda, H., Yamasaki, R., Sumiuchi, Y. et al. Hybrid Sex Pheromones of the Hibiscus Flower-bud Borer, Rehimena surusalis . J Chem Ecol 41, 1043–1049 (2015). https://doi.org/10.1007/s10886-015-0638-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0638-y