Abstract
Pheromonal communication of adult peach twig borers, Anarsia lineatella Zeller (Lepidoptera: Gelechiidae), was reinvestigated based on recent findings that virgin female-baited traps were more attractive to mate-seeking males than a two-component synthetic sex pheromone consisting of (E)-5-decen-1-yl acetate (1000 μg) and (E)-5-decen-1-ol (100 μg), suggesting that females use additional pheromone components. Hypothesizing that these additional components may be released from body parts other than abdominal sex pheromone glands, we extracted female body scales and analyzed aliquots by coupled gas chromatographic–electroantennographic detection (GC-EAD) and GC–mass spectrometry. Eight straight-chain and four methylated aliphatic hydrocarbons, as well as two acetates, all elicited responses from excised male antennae. In laboratory experiments with synthetic candidate pheromone components, a combination of octadecyl acetate, (R)-11-methyltricosane, and (S)-11-methyltricosane in the presence of gland-derived sex pheromone components were shown to elicit contact of female decoys by males. However, body pheromone components did not enhance attractiveness of sex pheromone components in field trapping experiments, suggesting that they are effective only at close range and that other stimuli are responsible for superior attractiveness of female-baited traps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Roelofs et al. (1975) identified a two-component sex pheromone consisting of (E)-5-decen-1-yl acetate (E5-10:OAc; 87%) and (E)-5-decen-1-ol (E5-10:OH; 13%) in pheromone gland extracts of female Anarsia lineatella Zeller (Lepidoptera: Gelechiidae) that attracted conspecific males. Synthetic pheromones released from various dispensers and traps were tested as tools for monitoring populations of A. lineatella in commercial fruit orchards (Rice and Jones, 1975; Hathaway, 1981; Kehat et al., 1994). Deployment of synthetic pheromone for control of A. lineatella by pheromone-based mating disruption yielded unsatisfactory results (Rice, personal observation, cited in Millar and Rice, 1992). Re-analysis of the pheromone of A. lineatella led to the identification of several candidate pheromone components [decyl acetate, (E)- and (Z)-4-decenyl acetate, and (E,E)-3,5- and (Z,E)-3,5-decadienyl acetates; Millar and Rice, 1992], but none enhanced long-range attractiveness of the previously identified two-component blend (Roelofs et al., 1975). Traps baited with the two-component blend of E5-10:OAc (1000 μg) and E5-10:OH (100 μg) remained significantly less attractive than those baited with virgin female A. lineatella (Schlamp, 2005). These results suggested that if additional pheromonal communication signals existed, they probably were present in, or released from, body parts other than abdominal pheromone glands.
Contact- or copulation-inducing pheromones are typically present on the body surface of (signaling) insects. Although they appear effective only at short range, they often complement attractiveness of long-range sex or aggregation pheromones. Close-range pheromones have been noted and/or identified, in several orders of the Insecta, including Diptera (Stoffolano et al., 1997), Hymenoptera (Kimani and Overholt, 1995), Coleoptera (Ginzel et al., 2003), Isoptera (Clement, 1982), and Lepidoptera (Grant et al., 1987). Conceivably, similar pheromone components may exist in A. lineatella and play a role in short-range communication among males and females.
Our objective was to test the hypothesis that pheromone components derived from body scales of females are part of the sexual communication system in A. lineatella.
Methods and Materials
Rearing of Insects
Insects were collected from peach orchards in Keremeos, British Columbia, and reared according to protocols developed and modified, respectively, by McElfresh and Millar (1993) and Sidney (2005).
Body Extraction of Moths
Separate groups of 10, 3- to 6-d-old males or females were submerged in pentane. After 5 min, the supernatant was withdrawn and pipetted into a new vial. This procedure was repeated twice with the same group of moths, and extracts were then combined and concentrated such that 12.5 μl equaled one body-extract equivalent. All extracts were prepared during the photophase, well separated from the (pre)dawn calling period of females, thus minimizing potential extraction of sex pheromone components from abdominal pheromone glands.
Analyses of Extracts
Aliquots of body extracts were analyzed by coupled gas chromatographic–electroantennographic detection (GC-EAD; Arn et al., 1975; Gries et al., 2002), employing a Hewlett-Packard 5890 gas chromatograph fitted with a GC column (30 m × 0.25 or 0.32 mm ID) coated with DB-5, DB-23, DB-210 (J&W Scientific, Folsom, CA, USA), or SP-1000 (Supelco, Bellefonte, PA, USA). For GC-EAD recordings, an antenna was gently pulled from an insect's head, the distal segment removed, and then suspended between glass capillary electrodes filled with Ringer's solution [NaCl (6.5 g/l), KCl (1.4 g/l), CaCl2 (0.12 g/l), Na2CO3 (0.1 g/l), Na2HPO4 (0.01 g/l)] in distilled water. Coupled GC–mass spectrometric (MS) analyses of pheromone extract [300 female equivalents (FE)] and of synthetic standards employed a Varian Saturn 2000 Ion Trap GC-MS fitted with the above-referenced DB-5 column.
General Instrumentation and Syntheses
Nuclear magnetic resonance (NMR) spectroscopy of synthetic compounds was conducted on a Varian AS500 (at 499.77 MHz for 1H and 125.68 MHz for 13C) spectrometer with chemical shifts reported in ppm relative to TMS (1H, δ = 0.00) and CDCl3 (13C, δ = 77.00). Elemental analyses were performed using a Carlo-Erba model 1106 elemental analyzer. Optical rotations were measured with a Perkin-Elmer 341 polarimeter.
(Z)-11-Eicosenyl acetate (Sugawara et al., 1978) was obtained by reduction of (Z)-11-eicosanoic acid (Aldrich) with lithium aluminum hydride in tetrahydrofuran (THF) to (Z)-11-eicosen-1-ol and acetylation of this alcohol (Pederson et al., 2003) with acetic anhydride in the presence of pyridine.
Previously reported methylated hydrocarbons 11-methyltricosane (11me-23Hy), 2-methyltetracosane (2me-24Hy), 11-methylpentadecane (11me-25Hy), and 13-methylheptacosane (13me-27Hy) (Jackson, 1970; Tarvita and Jackson, 1970; Howard et al., 1978; Tsuda et al., 1981; Lange, 1993; Szafranek et al., 1994; Finidori-Logli et al., 1996; Wagner et al., 1998; Haverty et al., 2000) were synthesized from corresponding carbonyl precursors and ylids by Wittig reactions and by subsequent hydrogenation of the resulting olefins in the presence of platinum oxide.
(S)-and (R)-11-Methyltricosanes (8 and 12, Figure 1).
tert-Butyldimethylsilylchloride (3.50 g; 1.1 equiv.) and 1.60 g (1.1 equiv.) of imidazole were added to 2.50 g of methyl (R)-3-hydroxy-2-methyl propanoate 1 (21.2 mmol; Aldrich) dissolved in 10 ml dimethylformamide. After stirring overnight at room temperature (RT), methyl (R)-3-tert-butyldimethylsilyloxy-2-methyl-propanoate 2 was obtained in quantitative yield. Borane reduction of silyl ether 2 with 45 ml of a 1.0 M solution of BH3 in a THF matrix under argon yielded known (S)-2-methyl-3-tert-butyldimethylsilyloxy-1-propanol (3) (King et al., 1995) after 48 hr. Ether 3 (quantitative yield) was isolated by quenching the reaction mixture with concentrated aq. NaHCO3. The product was extracted with a 1:1 mixture of ether/hexane (3 × 50 ml), dried (MgSO4), and the solvent was removed in vacuo.
All of monosilyl ether 3 (>99% pure, GC) was converted to (R)-mesylate 4 (King et al., 1995) at 0°C in dichloromethane with 1.1 equiv. of methanesulfonyl chloride and 1.5 equivalent of triethylamine. After 30 min of vigorous stirring at 0°C, the mixture was allowed to warm to RT and quenched with water. The organic layer was extracted with hexane, washed with 0.5 M HCl, concentrated aq. NaHCO3 and brine, and dried (MgSO4). After removal of excess solvents at 15 mm Hg, 10 ml of dry THF were added to the sulfonate. The mixture was transferred slowly via cannula under argon pressure to a stirred suspension of Grignard reagent [freshly prepared from 10.5 ml (55 mmol) of n-nonyl bromide and 2.7 g (111 mmol) of Mg] and CuI (0.84 g, 4.4 mmol) in 100 ml of THF at −23°C. After 1 hr, the reaction mixture was warmed to RT and quenched with a concentrated aq. NH4Cl solution. The organic layer was extracted with hexane (2 × 75 ml), washed with water and brine, and dried (Na2SO4). The product was concentrated in vacuo and filtered through 10 g of silica to yield crude (S)-2-methyl-1-(tert-butyldimethylsilyloxy)-dodecane (5). Without any further purification, the silyl protective group was removed by stirring 5 with an excess of tetrabutylammonium fluoride in THF/H2O overnight. Alcohol 6 was extracted from the reaction mixture with 100 ml of ether/hexane (1:1) and washed with water and brine. The organic layer was then dried (MgSO4) and concentrated in vacuo. Flash column chromatography [50 g of silica, hexane/ether as eluent with gradual increase (5–15%) of the ether content] afforded 2.30 g (11.5 mmol, 54% yield based on propanoate 1) of 96% pure (S)-2-methyl-1-dodecanol (6), [α]D 23 = −8.4°C (c 1.0; CHCl3). Anal. calculated for C13H28O (%): C 77.93, H 14.09; found: C 77.80, H 14.01. 1H NMR (CDCl3), δ (ppm): 0.87 (t, 3H, J = 7.0 Hz), 0.90 (d, 3H, J = 6.7 Hz), 1.22–1.40 (m, 17H), 1.59 (m, 2H), 3.39 (dd, 1H, J = 6.2, 10.5 Hz), 3.49 (dd, 1H, J = 6.2, 10.5 Hz). 13C NMR (CDCl3) δ (ppm): 14.08, 16.54, 22.66, 26.96, 29.32, 29.62, 29.63, 29.65, 29.93, 31.90, 33.13, 35.73, 68.35.
Mesylation of alcohol 6 (2.00 g, 10.0 mmol; conditions, reagent ratio, and workup as described for conversion of alcohol 3 to mesylate 4) and immediate Grignard coupling of methanesulfonate 7 with 10-undecen-1-ylmagnesium bromide in the presence of CuI [7.60 ml (35.0 mmol) of 11-bromo-undec-1-ene (Aldrich), 1.70 g (70 mmol) of Mg, and 0.57 g (3.0 mmol) of CuI; reaction conditions and workup as described for the synthesis of ether 5] yielded (S)-13-methyl-1-tridecene 9 (28%) with the following impurities in the mixture: 1,9-undecadiene (7%), 1-undecene (52%), 10-undecen-1-ol (2%), 1,21-docosadiene (7%), alcohol 6 (2%), and mesylate 7 (1%). Polar impurities were removed by filtering the mixture through 10 g of silica with hexane. Filtrates containing hydrocarbons were concentrated in vacuo and added to a cold solution of 11.2 g (77% pure, 50 mmol) of m-chloroperbenzoic acid (Aldrich) in 20 ml of CH2Cl2. The mixture was stirred for 3 hr at 0°C, allowed to warm to RT, and then quenched with 100 ml of 1 N NaOH. The organic layer was extracted with ether (2 × 50 ml), washed twice with water and brine, dried (MgSO4), and concentrated in vacuo, yielding a mixture of mono- and di-epoxides. Flash column purification (50 g of silica, 2% ether in hexane as eluent) of this mixture gave 3.10 g of epoxide 10 (61% pure by GC) with 1,2-epoxyundecane as the main impurity (30%). No di-epoxides were present as impurities. De-epoxidation of the mixture containing 10 was carried out with freshly prepared triphenylphosphonium selenide [obtained by stirring 8.26 g (31.5 mmol) of TPP and 2.49 g (31.5 mmol) of Se for 30 min] in 50 ml of CH2Cl2 with 1 ml of trifluoroacetic acid (Clive, 1978). After 1 hr of stirring at RT, solvents were removed in vacuo. The mixture was filtered through 20 g of silica with 150 ml of hexane. Olefin 9 (65% pure by GC) was then hydrogenated in hexane with 10% Pd/C (3 hr). The catalyst was eliminated by filtering through 5 g of silica, and the solvent was removed in vacuo at 15 mm Hg. Undecane and other low-boiling impurities were removed at 2–3 mm Hg (70°C, 2 hr), yielding >98% pure (S)-11-methyltricosane (8) (1.68 g, 4.96 mmol, 50% yield based on alcohol 6, overall yield 26.5%). Anal. calculated for C24H50 (%): C 85.12, H 14.88; found: C 85.06, H 15.08. 1H NMR (in CDCl3), δ (ppm): 0.83 (d, 3H, J = 6.6 Hz), 0.88 (t, 6H, J = 6.9 Hz), 1.18–1.37 (m, 41 H); 13C NMR (in CDCl3), δ (ppm): 14.09, 19.70, 22.67, 27.06, 29.34, 29.63–29.68 (several unresolved peaks), 30.00, 31.90, 32.72, 37.07.
Coupling of the mesylate 7 with 1-undecylmagnesium bromide leads directly to hydrocarbon 8, which was impossible to separate from by-product docosane. In the reaction mixture, 8 comprised 20%; after the removal of low-boiling and polar impurities, it was ∼60% pure.
(R)-11-Methyltricosane (12) was synthesized through the same route, starting with methyl (S)-3-hydroxy-2-methylpropanoate (11; overall yield 20%). GC retention times and NMR data matched those of (S)-11-methyltricosane (8). Optical rotation for intermediate (R)-2-methyl-1-dodecanol: [α]23 D = +6.1°C (c 7.7; CHCl3).
Laboratory Experiments with Pheromone Components
Candidate body pheromone (BP) components were tested in laboratory bioassays, employing a mesh (200 μm) cage (90 × 90 × 100 cm; BioEquip Products, Inc., Rancho Dominguez, CA, USA), with one of the two test stimuli randomly assigned to opposite corners of the cage. A test stimulus consisted of a white Teflon® decoy (0.25 × 0.75 cm) pinned to the center of an inverted Petri dish (10 × 2 cm) and impregnated with gland pheromone (GP) components or GP plus synthetic candidate BP components at 10 FE. For each replicate, 10 3- to 6-d-old males were introduced into each cage and acclimatized for 12 hr to environmental conditions (23°C; >70% RH; 16-hr light–8-hr dark) prior to testing. Bioassays were initiated by introducing test stimuli, starting a custom-designed computer program (Raymond G. Holland, Electronic Supervisor, Science Technical Centre, SFU, unpublished data) that increased the intensity of the light source (60-W Phillips incandescent light bulb) from 0 to 600 lx within 15 min, and by manually turning on a desk swing fan (Windmere, Miramar, FL, USA) behind the bioassay cage, which delivered intermittent pulses of air (0.3 m/sec). For each bioassay, numbers of contacts with test stimuli were recorded for 15 min. Repeated contacts by the same male were recorded, if that male was more than one body length apart from the stimulus between consecutive contacts. Each of 15 replicates per experiment employed a new set of 10 males and test stimuli.
Ten instead of 1 FE of candidate BP components were bioassayed taking into account that body scales may be better pheromone dispensers than Teflon® decoys, or that live female A. lineatella may replenish their pheromone components over time, whereas we administered only a single application of test stimulus at the beginning of each 15-min bioassay. The experimental protocol did not allow more than three replicates per day, so group bioassays instead of single-insect bioassays were conducted. This ensured that some males responded to test stimuli in each bioassay despite the lack of sonic signals females emit in response to sonic signals from males (Hart et al., Gries laboratory, unpublished data).
Two synthetic GP components [E5-10:OAc (100 ng) and E5-10:OH (10 ng)] were tested alone or in combination with the following: (1) body extract of females at 10 FE (experiment 1); (2) a complete synthetic blend of candidate BP components, consisting of two acetates [octadecyl acetate (18:OAc), (Z)-11-eicosenyl acetate (Z11-20:OAc)], four methylated hydrocarbons [11-methyltricosane (11me-23Hy), 2-methyltetracosane (2me-24Hy), 11-methylpentadecane (11me-25Hy), 13-methylheptacosane (13me-27Hy)], and eight straight-chain hydrocarbons [docosane (22Hy), tricosane (23Hy), tetracosane (24Hy), pentacosane (25Hy), hexacosane (26Hy), octacosane (28Hy), nonacosane (29Hy), and tricontane (30Hy)] (experiment 2); (3–6) BP minus the two acetates 18:OAc and Z11-20:OAc (experiment 3), BP minus all hydrocarbons (experiment 4), BP minus methylated hydrocarbons (experiment 5), or BP minus straight-chain hydrocarbons (experiment 6).
BP blends lacking acetates (experiment 3) or methylated hydrocarbons (experiment 5) were not effective in increasing the number of body contacts, so follow-up experiments explored which acetate (experiments 7 and 8) or methylated hydrocarbon(s) (experiments 9–12) contributed to behavioral activity of the BP blend. 18:OAc appeared more effective than Z11-20:OAc (experiments 7 and 8), and 11me-23Hy was the single-most effective methylated hydrocarbon (experiment 11), so additional experiments were run to investigate which enantiomer of 11me-23Hy was behaviorally active by testing GP alone or in combination with 18:OAc plus (S)-11-methyltricosane [(S)-11me-23Hy] (experiment 13), (R)-11-methyltricosane [(R)-11me-23Hy] (experiment 14), or both (1:1; experiment 15). With the presence of both the R- and S-enantiomers of 11-methyltricosane needed for males to respond (experiment 15), experiment 16 tested GP plus female body extract vs. GP plus synthetic 18:OAc and (R)- and (S)-11me-23Hy at equivalent ratios and quantities. Paired mean contacts of paired stimuli by male moths were analyzed statistically using paired t tests (Zar, 1996). All statistical analyses were performed with JMP® Version 4 (SAS Institute, Cary, NC, USA).
Results
Gas chromatographic–electroantennographic detection analyses of body extracts from female A. lineatella revealed small amounts (<0.2 ng) of the two sex pheromone components E5-10:OAc and E5-10:OH and numerous compounds that elicited responses from male antennae and several that did not (Figure 2). In GC-MS analyses, two of these EAD-active compounds with fragmentation ion m/z 61 (indicative of an acetate functionality) and with molecular ions m/z 312 and m/z 338 were identified as octadecyl acetate (18:OAc) and an eicosenyl acetate, respectively. Dimethyl disulfide treatment (Dunkelblum et al., 1985) of the latter without prior isolation yielded an adduct with GC-MS fragmentation ions m/z 173 and m/z 259, indicative of a double bond at C11. This compound was thus postulated and, through comparative GC-MS of an authentic standard, confirmed to be (Z)-11-eicosenyl acetate (Z11-20:OAc).
Representative recording (N = 5) of flame ionization detector (FID) and electroantennographic detector (EAD: male Anarsia lineatella antenna) responses to 10 equivalents of body extract of female A. lineatella. Chromatography: splitless injection; injector and FID: 240°C, DB-5 column (30 m × 0.32 mm ID); temperature program: 50°C (2 min), then 15°C/min to 280°C (10 min). Compound abbreviation [with amounts per 1 female equivalents (FE) in parenthesis] as follows: 22Hy = docosane (7.4 ng); 18:OAc = octadecyl acetate (7.7 ng); 23Hy = tricosane (6.5 ng); 11me-23Hy = 11-methyltricosane (3.8 ng); Z11-20:OAc = (Z)-11-eicosenyl acetate (3.8 ng); 24Hy = tetracosane (1.8 ng); 2me-24Hy = 2-methyltetracosane (15.0 ng); 25Hy = pentacosane (9.1 ng); 11me-25Hy = 11-methylpentacosane (1.0 ng); 26Hy = hexacosane (3.1 ng); 27Hy = heptacosane (20 ng); 13me-27Hy = 13-methylheptacosane (19.0); 28Hy = octacosane (3.6 ng); 29Hy = nonacosane (19.9 ng); 30Hy = triacontane (2.9 ng).
Mass spectra of other EAD-active compounds in female body extracts suggested that they were saturated hydrocarbons. Four of these had retention indices (Van den Dool and Kratz, 1963) indicative of methyl branches. Their mass spectra revealed fragmentation ions diagnostic of methyl branch positions (Pomonis et al., 1980; Francke et al., 1987, 1988; Gries et al., 1991, 1993, 1994) and suggested that they were 11me-23Hy, 2me-24Hy, 11me-25Hy, and 13me-27Hy, respectively. Comparative GC-MS of insect-produced and authentic standards confirmed the structural assignments.
Laboratory Experiments with Candidate Contact Pheromone Components
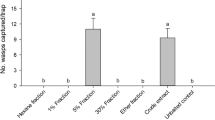
Teflon® decoys impregnated with body extracts from female A. lineatella at 10 FE plus GP components provoked more decoy contacts by male A. lineatella than GP components alone (Figure 3; experiment 1). These results could not be attributed to small (<0.2 ng) amounts of the sex pheromone components E5-10:OAc and E5-10:OH in body extracts of female moths because both treatment and control stimuli contained 100 and 10 ng, respectively, of synthetic E5-10:OAc and E5-10:OH.
Mean (±SE) number of contacts made by male A. lineatella in experiments 1–6 (15 replicates each) with a Teflon® decoy impregnated with various test stimuli. In each experiment, an asterisk (*) indicates a significant preference for a particular stimulus; paired t test, P < 0.05. Abbreviations as follows: GP = synthetic gland pheromone components [(E)-5-decen-1-yl acetate (100 ng) and (E)-5-decen-1-ol (10 ng)]; female body extract = body extract of female A. lineatella tested at 10 female equivalents; BP = synthetic body pheromone components consisting of two acetates [18:OAc, Z11-20:OAc], four methylated hydrocarbons (Hy) [11me-23Hy, 2me-24Hy, 11me-25Hy, 13me-27Hy], and eight straight-chain aliphatic hydrocarbons [22Hy, 23Hy, 24Hy, 25Hy, 26Hy, 28Hy, 29Hy, 30Hy]. For full names of chemicals, see caption of Figure 2.
A synthetic blend of all candidate BP components at ratios and concentrations equivalent to 10 FE plus GP provoked more contacts by males than GP alone (Figure 3; experiment 2). Synthetic BP blends lacking straight-chain hydrocarbons were still bioactive (Figure 3; experiment 6), but BP blends lacking acetates (Figure 3; experiment 3), all hydrocarbons (Figure 3; experiment 4), or all methylated hydrocarbons (Figure 3; experiment 5) were not. Neither Z11-20:OAc nor 18:OAc alone significantly enhanced the attractiveness of GP (Figure 4; experiments 7 and 8), but the opposite was true for 18:OAc combined with four methylated hydrocarbons (Figure 4; experiment 9).
Mean (±SE) number of contacts made by male A. lineatella in experiments (exp.) 7–12 (15 replicates each) with a Teflon® decoy impregnated with various test stimuli. In each experiment, an asterisk (*) indicates a significant preference for a particular treatment; paired t test, P < 0.05. Abbreviations as in captions of Figures 2 and 3.
Deleting a group of two or single methylated hydrocarbons from the BP blend determined that only 11me-23Hy, in addition to 18:OAc, is needed to retain the blend's behavioral activity (Figure 4; experiments 10–12).
The R- and S-enantiomers of 11me-23Hy in combination, but not singly, are BP pheromone components (Figure 5; experiment 15). A BP blend with the S-enantiomer alone was benign (Figure 5; experiment 13) and was inhibitory with the R-enantiomer alone (Figure 5; experiment 14). The three-component BP blend consisting of 18:OAc, (R)-11me-23Hy, and (S)-11me-23Hy was as effective as a body extract in provoking contacts by male A. lineatella (Figure 5; experiment 16).
Mean (±SE) number of contacts made by male A. lineatella in experiments 13–16 (15 replicates each) with a Teflon® decoy impregnated with various test stimuli. In each experiment, an asterisk (*) indicates a significant preference for a particular treatment; paired t test, P < 0.05. (R)- and (S)-11me-23 = (R)- and (S)-11-methyltricosane, respectively. Other abbreviations as in captions of Figures 2 and 3.
Discussion
Significantly more captures of male A. lineatella in traps baited with conspecific virgin females than in those baited with a two-component synthetic sex pheromone (Schlamp, 2005) suggested that females use additional communication signals to attract mate-seeking males. Our data indicate that close-range communication signals include pheromone components from the females' body surface that may provoke contact by males.
Although numerous compounds were extractable from the females' body surface and elicited responses from male antennae (Figure 2), the body pheromone (BP) seems to comprise only three components: 18:OAc, (R)-11me-23Hy, and (S)-11me-23Hy (Figures 4 and 5). Positive responses by males only to BP blends containing both the R- and S-enantiomers of 11me-23Hy (Figure 5; experiments 13–15), and even inhibition of response to blends containing only the R-enantiomer (Figure 5; experiment 14), indicate that (R)- and (S)-11me-23Hy are BP components of female A. lineatella.
Varying levels of responding insects to control stimuli from one experiment to another were likely because of the fact that we proceeded with experiments even at low atmospheric pressure, which is not conducive to high levels of response. Treatment stimuli also differed in their attractiveness, further modifying the overall level of response between experiments. However, with the same control stimulus retained in each experiment, we could assess the relative strength of a treatment stimulus within and between experiments.
Methyl (R)- and (S)-3-hydroxy-2-methylpropanoates (99% ee) were chosen as starting materials for the syntheses of the enantiomers of 11-methyltricosane by a route that did not affect the chiral center. When the Grignard coupling of mesylate 7 with n-undecylmagnesium bromide was performed, an inseparable mixture of desired hydrocarbon 8 and the Grignard reagent dimer by-product n-docosane formed. To obtain pure 8, we coupled mesylate 7 with 10-undecen-1-ylmagnesium bromide. The unsaturated reaction products were converted into their respective epoxides, and mono-epoxide 10 was separated from the di-epoxide derived from the Grignard by-product. Regenerated (by de-epoxidation) olefin 9 was then hydrogenated to give the final compound 8. Polarimetric studies of final products 8 and 12 did not yield measurable values of optical rotation.
Body pheromone components may serve as ultimate cues to confirm the proper species, and sex, of a prospective mate. The two acetates, 18:OAc and Z11-20:OAc, are indeed present only in body extracts of female but not male A. lineatella (data not shown), suggesting that they may help males recognize females. In contrast, 11me-23Hy is present in body extracts of both males and females (data not shown), suggesting that it is not suitable for mate recognition. However, considering that both enantiomers of 11me-23Hy were required to induce positive responses by males, the presence of only one (R) or both enantiomers may help reveal the signaler's sex. In field experiments (Schlamp, 2005), those contact pheromone components had no effect on long-range attraction of male A. lineatella, indicating that they play a role only at close range before or during courtship.
This type of close- and long-range communication system with components from sex pheromone glands and body scales has been reported in other species of moths. Live female gypsy moths Lymantria dispar or physical models of female L. dispar baited with sex pheromone and covered with female abdominal scales, elicited copulatory responses by males, whereas exposure of males to sex pheromone alone did not (Charlton and Cardé, 1990). Similarly, males of the smaller tea tortrix moth Adoxophyses orana will attempt copulation only in the presence of female-produced sex pheromone and scales (Shimizu and Tamaki, 1980).
In summary, this study has revealed contact pheromone components derived from scales of female A. lineatella, which, together with gland-derived sex pheromone components, induce contacts by males. Contact pheromone components do not enhance the efficacy of sex pheromone in attracting males in the field (Schlamp, 2005). Thus, the superior attractiveness of virgin female A. lineatella as a trap bait compared with synthetic sex pheromone (Schlamp, 2005) must be due to other signals that need to be investigated.
References
H. Arn E. Städler S. Rauscher (1975) ArticleTitleThe electroantennographic detector—a selective and sensitive tool in the gas chromatographic analysis of insect pheromones Z. Naturforsch. 30c 722–725 Occurrence Handle1:CAS:528:DyaE28XislGqug%3D%3D
R. E. Charlton R. T. Cardé (1990) ArticleTitleFactors mediating copulatory behavior and close-range mate recognition in the male gypsy moth Lymantria dispar Can. J. Zool. 68 1995–2004 Occurrence Handle10.1139/z90-281
J. L. Clement (1982) ArticleTitleSexual pheromones in European termites of the genus Reticulitermes: behaviour and specific isolation Biol. Behav. 7 55–68
D. L. J. Clive (1978) ArticleTitleModern organoselenium chemistry Tetrahedron 34 1049–1132 Occurrence Handle10.1016/0040-4020(78)80135-5 Occurrence Handle1:CAS:528:DyaE1cXlsl2jsr0%3D
E. Dunkelblum S. H. Tan P. J. Silk (1985) ArticleTitleDouble-bond location in monounsaturated fatty-acids by dimethyl disulfide derivatization and mass spectrometry application to analysis of fatty-acids in pheromone glands of 4 Lepidoptera J. Chem. Ecol. 11 265–278 Occurrence Handle10.1007/BF01411414 Occurrence Handle1:CAS:528:DyaL2MXksFSktbc%3D
V. Finidori-Logli A.-G. Bagneres D. Erdmann W. Francke J.-L. Clement (1996) ArticleTitleSex recognition in Digliphus isaea Walker (Hymenoptera: Eulophidae): Role of an uncommon family of behaviorally active compounds J. Chem. Ecol. 22 2063–2080 Occurrence Handle1:CAS:528:DyaK28XnsVWrtb0%3D
W. Francke M. Tóth G. Szöcs P. Guerin H. Arn (1987) ArticleTitleIdentification of 5,9-dimethylheptadecane as a sex pheromone of the moth Leucoptera scitella Naturwissenschaften 74 143–144 Occurrence Handle1:CAS:528:DyaL2sXhvFeisb8%3D
W. Francke M. Tóth G. Szöcs W. Krieg H. Ernst E. Buschmann (1988) ArticleTitleIdentifizierung and Synthese von Dimethylalkanen als Sexuallockstoff weiblicher Miniermotten (Lyonetiidae) Z. Naturforsch. 43c 787–789
M. D. Ginzel J. G. Millar L. M. Hanks (2003) ArticleTitle(Z)-9-Pentacosene: Contact sex pheromone of the locust borer, Megacyllene robiniae Chemoecology 13 135–141 Occurrence Handle10.1007/s00049-003-0239-z Occurrence Handle1:CAS:528:DC%2BD2cXhvVGqsrc%3D
G. G. Grant D. Frech L. Macdonald K. N. Slessor G. G. S. King (1987) ArticleTitleCopulation releaser pheromone in body scales of female whitemarked tussock moth, Orgyia leucostigma (Lepidoptera: Lymantriidae): Identification and behavioural role J. Chem. Ecol. 13 345–356 Occurrence Handle10.1007/BF01025894 Occurrence Handle1:CAS:528:DyaL2sXhsFegurY%3D
T. G. Gray R. F. Shepherd D. L. Struble J. B. Byers T. F. Maher (1991) ArticleTitleSelection of pheromone trap and attractant dispenser load to monitor black army cutworm Actebia fennica J. Chem. Ecol. 17 309–316 Occurrence Handle10.1007/BF00994334
G. Gries R. Gries J. H. Borden J. Li K. N. Slessor G. G. S. King W. W. Bower R. J. West E. W. Underhill (1991) ArticleTitle5,11-Dimethylheptadecane and 2,5-dimethylheptadecane: Sex pheromone component of the geometrid moth, Lambdina fiscellaria fiscellaria Naturwissenschaften 78 315–317 Occurrence Handle10.1007/BF01221418 Occurrence Handle1:CAS:528:DyaK3MXlsl2ku7g%3D
G. Gries R. Gries S. H. Krannitz J. Li G. G. S. King K. N. Slessor J. H. Borden W. W. Bowers R. J. West E. W. Underhill (1993) ArticleTitleSex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae) J. Chem. Ecol. 19 1009–1019 Occurrence Handle1:CAS:528:DyaK3sXks1yntL0%3D
R. Gries G. Gries J. Li C. T. Maier C. R. Lemmon K. N. Slessor (1994) ArticleTitleSex pheromone components of the spring Hemlock looper, Lambdina athasaria (Walker) (Lepidoptera: Geometridae) J. Chem. Ecol. 20 2501–2511 Occurrence Handle1:CAS:528:DyaK2MXitFyrsbs%3D
R. Gries G. Khaskin G. Gries R. G. Bennett G. G. S. King P. Morewood K. N. Slessor W. D. Morewood (2002) ArticleTitle(Z,Z)-4,7-Tridecadien-(S)-2-yl acetate: Sex pheromone of Douglas-fir cone gall midge, Contarinia oregonensis J. Chem. Ecol. 28 2283–2297 Occurrence Handle12523568 Occurrence Handle1:CAS:528:DC%2BD38Xos1Knur0%3D
D. O. Hathaway (1981) ArticleTitlePeach twig borer: Field evaluations of concentrations of pheromone and monitoring of populations J. Econ. Entomol. 74 344–345 Occurrence Handle1:CAS:528:DyaL3MXkvFagsrw%3D
M. I. Haverty R. J. Woodrow L. J. Nelson K. Grace (2000) ArticleTitleCuticular hydrocarbons of termites of Hawaiian Islands J. Chem. Ecol. 26 1167–1192 Occurrence Handle10.1023/A:1005479826651 Occurrence Handle1:CAS:528:DC%2BD3cXjvFWrtrw%3D
R. W. Howard C. A. McDaniel G. J. Blomquist (1978) ArticleTitleCuticular hydrocarbons of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae) J. Chem. Ecol. 4 233–245 Occurrence Handle10.1007/BF00988058 Occurrence Handle1:CAS:528:DyaE1cXltVCisLo%3D
L. L. Jackson (1970) ArticleTitleCuticular lipids of insects: II. Hydrocarbons of the cockroaches Periplaneta australasiae, Periplaneta brunnea, Periplaneta fuliginosa Lipids 5 38–41 Occurrence Handle5418208 Occurrence Handle1:CAS:528:DyaE3cXpslOgsQ%3D%3D
M. Kehat L. Anshelevich E. Dunkelblum S. Greenberg (1994) ArticleTitleSex pheromone traps for monitoring the peach twig borer, Anarsia lineatella Zeller: Effect of pheromone components, pheromone dose, field aging of dispenser, and type of trap on male captures Phytoparasitica 22 291–298 Occurrence Handle1:CAS:528:DyaK2MXitFGhtb4%3D
S. W. Kimani W. A. Overholt (1995) ArticleTitleBiosystematics of the Cotesia flavipes complex (Hymenoptera: Braconidae): interspecific hybridization, sex pheromone and mating behaviour studies Bull. Entomol. Res. 85 379–386 Occurrence Handle10.1017/S0007485300036117
G. G. S. King R. Gries G. Gries K. N. Slessor (1995) ArticleTitleOptical isomers of 3,13-dimethylheptadecane: Sex pheromone components of the western false hemlock looper, Nepytia freemani (Lepidoptera: Geometridae) J. Chem. Ecol. 21 2027–2045 Occurrence Handle1:CAS:528:DyaK28Xis1yltA%3D%3D
C. Lange (1993) ArticleTitleOxirane as a chemical ionization case: Reactivity towards different classes of compounds Org. Mass Spectrom. 28 1285–1296 Occurrence Handle10.1002/oms.1210281102 Occurrence Handle1:CAS:528:DyaK2cXis12nsbY%3D
J. S. McElfresh J. G. Millar (1993) ArticleTitleEstablishment and rearing of Anarsia lineatella (Lepidoptera: Gelechiidae) on a meridic diet J. Econ. Entomol. 85 1399–1404
J. G. Millar R. E. Rice (1992) ArticleTitleReexamination of the female sex pheromone of the peach twig borer: Field screening of minor constituents of pheromone gland extracts and of pheromone analogs J. Econ. Entomol. 85 1709–1716 Occurrence Handle1:CAS:528:DyaK2cXktVGnsb0%3D
R. L. Pederson C. W. Lee T. A. Ung H. Ishihara (2003) ArticleTitleProduction of insect pheromones using olefin cross metathesis Abst. Pap. – Am. Chem. Soc. 224 1–2
J. G. Pomonis D. R. Nelson C. L. Flatland (1980) ArticleTitleInsect Hydrocarbons 2. Mass spectra of dimethyalkanes and the effect of the number of methylene units between groups on fragmentation J. Chem. Ecol. 6 965–972 Occurrence Handle10.1007/BF00994653 Occurrence Handle1:CAS:528:DyaL3MXpvFWhtA%3D%3D
R. E. Rice R. A. Jones (1975) ArticleTitlePeach twig borer: field use of a synthetic sex pheromone J. Econ. Entomol. 68 358–360
W. Roelofs J. Kochansky E. Anthon R. Rice R. Cardé (1975) ArticleTitleSex pheromone of the peach twig borer moth (Anarsia lineatella) Environ. Entomol. 4 580–582 Occurrence Handle1:CAS:528:DyaE2MXlt1yqtLc%3D
Schlamp, K. K., 2005. Contact pheromone components and diel periodicity of sexual communication in peach twig borers, Anarsia lineatella (Lepidoptera: Gelechiidae). Master of Pest Management thesis, Simon Fraser University, Burnaby, Canada.
K. Shimizu Y. Tamaki (1980) ArticleTitleReleasers of male copulatory attempt in the smaller tea tortrix moth (Lepidoptera: Tortricidae) Appl. Entomol. Zool. 15 140–150
Sidney, M. C. 2004. Tree-derived stimuli affecting host-selection response of larva and adult peach twig borers, Anarsia lineatella (Zeller) (Lepidoptera: Gelechiidae). Master of Science thesis, Simon Fraser University, Burnaby, Canada.
J. G. Stoffolano E. Schauber C. Yin J. A. Tillman G. J. Blomquist (1997) ArticleTitleCuticular hydrocarbons and their role in copulatory behavior in Phormia regina (Meigen) J. Insect Physiol. 43 1065–1076 Occurrence Handle12770478 Occurrence Handle10.1016/S0022-1910(97)00050-4 Occurrence Handle1:CAS:528:DyaK1cXitl2j
F. Sugawara A. Kobayashi K. Yamashita K. Matsuda (1978) ArticleTitleIdentification of octadecyl and (Z)-11-eicosenyl acetate, major components of defensive secretion of Gastrophysa atrocyanea Motschulsky Agric. Biol. Chem. 42 687–688 Occurrence Handle1:CAS:528:DyaE1cXktl2ltrk%3D
J. Szafranek E. Malinski E. Dubis E. Hebanowska J. Nawrot P. Oksman K. Pihaja (1994) ArticleTitleIdentification of branched alkanes in lipids of Leptinotarsa decemlineata Say and Tribolium destructor by GC-MS: A comparison of main-beam and link-scanned spectra J. Chem. Ecol. 20 2197–2212 Occurrence Handle10.1007/BF02033197 Occurrence Handle1:CAS:528:DyaK2cXmslKjtLg%3D
K. Tarvita L. L. Jackson (1970) ArticleTitleCuticular lipids of insects: I. Hydrocarbons of Leucophaea maderae and Blatta orientalis Lipids 5 35–37
Y. Tsuda M. Kaneda K. Sato M. Kojaki (1981) ArticleTitleIso-heptacosane in tulip pollen and iso-pentacosane in lily pollen Phytochemistry 20 505–506 Occurrence Handle10.1016/S0031-9422(00)84176-X Occurrence Handle1:CAS:528:DyaL3MXksFyjtr4%3D
H. Dool ParticleVan den P. D. Kratz (1963) ArticleTitleA generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography J. Chromatogr. 2 463–471
D. Wagner M. J. F. Brown P. Broun W. Cuevas L. E. Moses D. L. Chao D. M. Gordon (1998) ArticleTitleTask related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus J. Chem. Ecol. 24 2021–2038 Occurrence Handle10.1023/A:1020781508889 Occurrence Handle1:CAS:528:DyaK1MXis12l
J. H. Zar (1996) Biostatistical Analysis Prentice Hall Upper Saddle River, NJ
Acknowledgments
We thank Kaaaly Levan and James Duperron for allowing us to conduct experiments in their orchards and Eberhard Kiehlmann for review of the manuscript. The research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada, and the B.C. Fruit Growers' Association to G. G. and G. J. R. J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlamp, K.K., Gries, R., Khaskin, G. et al. Pheromone Components from Body Scales of Female Anarsia lineatella Induce Contacts by Conspecific Males. J Chem Ecol 31, 2897–2911 (2005). https://doi.org/10.1007/s10886-005-8402-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-8402-3