Abstract
Pale swallow-wort (Vincetoxicum rossicum) and black swallow-wort (V. nigrum) are two invasive plant species in the northeastern United States and eastern Canada that have undergone rapidly expanding ranges over the past 30 years. Both species possess a highly bioactive phytotoxin -(−) antofine in root tissues that causes pronounced inhibition in laboratory bioassays of native plant species co-located in habitats where swallow-wort is found. To further evaluate the allelopathic potential of -(−) antofine, we: determined its concentration in young plant tissues; used in situ approaches to assess antofine stability, potential activity of degradation products, activity in sterile and nonsterile soil; and determined accumulation and concentration in hydroponic cultivation and field collected soil samples. Extracts of seeds and young seedlings were found to have approximately 2–3 times the level of -(−) antofine in comparison to root extracts of adult plants. Breakdown products of antofine accumulated rapidly with exposure to light, but more slowly in the dark, at ambient temperatures, and these products did not retain biological activity. Extraction efficiencies of control soil spiked with -(−) antofine were low but easily detectable by HPLC. Soil samples collected over two growing seasons at four different sites where either pale swallow-wort or black swallow-wort populations are present were negative for the presence of -(−) antofine. Dose response curves using sterile and nonsterile soil spiked with -(−) antofine demonstrated a requirement for at least 20–55 × greater -(−) antofine concentrations in soil to produce similar phytotoxic effects to those previously seen in agar bioassays with lettuce seedlings. Sterile soil had a calculated EC50 of 686 μM (250 μg/g) as compared to nonsterile soil treatments with a calculated EC50 of 1.88 mM (640 μg/g). When pale swallow-wort and black swallow-wort adult plants were grown in hydroponic cultivation, −(−) antofine was found in root exudates and in the growing medium in the nM range. The concentrations in exudate were much lower than that needed for biological activity (μM) although they might be an underestimate of what may accumulate over time in an undisturbed rhizosphere. Based on these various results, it remains uncertain as to whether -(−) antofine could play a significant allelopathic role for invasive swallow-worts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pale swallow-wort (Vincetoxicum rossicum (Kleopow) Barbarich = Cynanchum rossicum (Kleopow) Borhidi) and black swallow-wort (V. nigrum (L.) Moench = C. louiseae Kartesz & Gandhi) have emerged as invasive plant species in the northeastern United States and southeastern Canada over the past 30 years (DiTommaso et al. 2005; Lawlor 2000). They are both herbaceous, perennial, twining vines related to milkweeds (Apocynaceae, subfamily Asclepiadoideae). The major distinguishing characteristic is the coloration of the flowers, which are pink to maroon for pale swallow-wort, native to the Ukraine and European Russia, while those of black swallow-wort, native to southwestern Europe, are dark purple to black (Markgraf 1972). Possibly introduced as ornamentals, both are now present in 21 states from New England to Maryland and west to Nebraska and Kansas, with additional reports from California, and the Canadian provinces of Ontario and Quebec (DiTommaso et al. 2005; EDDMapS 2014; Kartesz 1999; USDA 2009).
Invasive plants typically exhibit a collective suite of biological properties that can contribute effectively to their invasiveness in a new environment, and this appears to be the case for swallow-worts. Both are competitive in multiple habitats because swallow-worts are highly adaptive to varying light and soil pH environments within both native and disturbed areas (Averill et al. 2010, 2011; DiTommaso et al. 2005; Magidow et al. 2013; Sheeley and Raynal 1996). Both species possess high fecundity, particularly in high light environments (Averill et al. 2011; Smith et al. 2006). Part of the competitiveness of pale swallow-wort could be due to the sequestering of nutrients from the soil due to the large production of root biomass in comparison to aerial stems and leaves (Cappuccino 2004; Milbrath 2008; Smith et al. 2006).
Allelopathy also might play a role in the invasiveness of swallow-worts. The phytochemical -(−) antofine was identified as a potent phytotoxin capable of inhibiting seed germination and root growth of several co-located native species at μM concentrations in in vitro bioassays (Gibson et al. 2011). Although several hypotheses have been proposed to explain the invading success of some plant species in their new environment based on competitive advantages (Lorenzo et al. 2013), only the novel weapons hypothesis suggests that allelochemicals released from the invading species can significantly inhibit the growth of native species in their new area of expansion (Hierro and Callaway 2003; Inderjit et al. 2008b). There is evidence of allelopathy in some species, but the allelochemicals responsible have not always been identified, and there have been conflicting reports, perhaps due to inaccurate or inappropriate methodologies (Blair et al. 2006; Callaway and Aschehoug 2000; Callaway and Ridenour 2004; Duke et al. 2009; Inderjit et al. 2008a; Perry et al. 2007; Weir et al. 2003, 2009). Some recent studies have shown a relatively low contribution of allelopathy to an invasive plant’s competitiveness in four different plant systems (Del Fabbro et al. 2014; Uddin et al. 2014). Others have indicated a demonstrable field consequence of allelopathy in some plants, such as artemisinin from Artemisia annua (Jessing et al. 2014), Japanese knotweed (Fallopia japonica) effects on restoration (Dommanget et al. 2014), Bothriochloa spp. litter and leachates on native grasses (Greer et al. 2014), and gallic acid from Phragmites australis (Rudrappa et al. 2009; Uddin et al. 2014), although the levels of gallic acid produced are in dispute (Weidenhamer et al. 2013). Thus, the abundance of novel chemistries in invasive exotic plants (Cappuccino and Arnason 2006) and numerous in vitro studies illustrating significant phytotoxic effects of root exudates as well as changes in microflora (Ambika 2013; Bais et al. 2006; Inderjit et al. 2008b; Inderjit and Callaway 2003; Koocheki et al. 2013) still provide the impetus to evaluate allelopathic contributions to invasiveness.
Whether -(−) antofine plays an active role for swallow-worts within the soil and rhizosphere that significantly aids in the displacement of native plant populations is as yet unproven. This study was undertaken to further evaluate the allelopathic potential for -(−) antofine using a variety of approaches: analysis of field-collected soil samples from four different soil types throughout two growing seasons; antofine stability and potential activity of degradation products; concentration in young plant tissues; activity in sterile and nonsterile soil; and accumulation and concentration due to release from root tissues.
Methods and Materials
Stability Studies
-(−) Antofine used in this test, and in other tests below as an internal standard, was purified from pale swallow-wort roots as previously described in Gibson et al. (2011) and stored at −20 °C until needed. To assess breakdown due to light or ambient temperature, 500 μg samples of purified -(−) antofine were dissolved in 0.5 ml of MeOH in tared vials and stored at 25 °C in a growth chamber with 12:12 h (L:D) exposure or in vials wrapped in foil for up to 8 d. Samples were collected in duplicate after 2 and 8 d, and stored at −20 °C until analyzed by HPLC. In addition, soil (collected from Great Gully Nature Preserve in an area without swallow-wort present, see Table 1) or sand (Silica Sand, natural grain, U.S.Silica Co.) samples (4 g into 125 ml Erlenmeyer flasks) were spiked with 500 μg -(−) antofine. Duplicate flasks were harvested immediately, and the remaining flasks then placed into a growth chamber at 25 °C with a 12:12 h (L:D) cycle and harvested at monthly intervals for 3 mo (N = 2 per treatment). Each flask was extracted with 20 ml CH2Cl2, dried in vacuo, transferred to a tared vial and dried under nitrogen, then stored at −20 °C prior to analysis.
Soil Sampling
Soil samples were collected monthly from June through October in 2010 and in 2011 from two locations in New York with a pale swallow-wort infestation (Great Gully Nature Preserve and Robert G. Wehle State Park) and from two locations with a black swallow-wort infestation (Round Island in Bear Mountain State Park and the Dutchess County Cooperative Extension office in Millbrook, NY) (Table 1). Five soil cores (3 cm diam and approximately 6 cm deep) were collected in a circular pattern around each of 6 swallow-wort plants from each field location each month. Soil cores were approximately 3 cm distant from the plant stems. Soil cores from each plant were combined within a plastic bag and then placed on ice for transport back to the laboratory within 24 h. Soil was sieved through 3 mm mesh to remove stones, large plant debris, and visible roots. Soil was thoroughly mixed, and then approximately 200 g from each individual field sample for a total of 6 replicates per field site per month were placed in a plastic bag and frozen at −20 °C until extraction. A total of 240 separate samples were collected, and each individual sample was analyzed in triplicate.
Soil Extraction
Three experiments were conducted to assess extraction efficiencies prior to processing field soil samples. -(−) Antofine (1 mg dissolved in 1 ml methanol) was used to spike 5 g of soil samples (not infested with swallow-wort) from the Wehle and Great Gully areas and then allowed to dry completely. Three replicates of each sample then were extracted in 10 ml of CH2Cl2 alone, CH2Cl2:MeOH (1:1), or CH2Cl2:(CH3)2CO (1:1) to determine the best solvent to use. Subsequently 5 g each of Great Gully control soil samples were spiked in triplicate over a concentration range of 25 μg to 400 μg -(−) antofine dissolved in 1 ml methanol, dried, and then extracted with 10 ml of CH2Cl2 to assess extraction efficiency. Finally, amounts of 5, 10, and 20 g of uninfested Great Gully soil also were spiked in triplicate with 500 μg -(−) antofine to evaluate extraction efficiency from larger soil volumes using 20 ml CH2Cl2.
Three subsamples of 20 g soil per each field sample from each month of collection (June-October in 2010 and 2011, giving a total of 720 subsamples) were measured and placed into individual 125 ml flasks to which 20 ml of CH2Cl2 were added. An additional 20 g sample was used to calculate soil moisture. The flasks were covered with aluminum foil and placed on a shaker at 60 rpm for 1 h inside a fume hood. At 30 min, the flasks were sonicated for 1 min and then placed back on the shaker for the remaining 30 min. The extract was filtered through Whatman #1 filter paper; the soil in the flask was rinsed with an additional 5 ml of CH2Cl2 that was then filtered and combined. This extract was dried under nitrogen in tared vials and stored at −20 °C until analysis by HPLC.
Seedling Root Growth Bioassays
Bioassays with lettuce seedlings were conducted as described previously (Gibson et al. 2011) to characterize the phytotoxic activity of the major initial breakdown product of -(−) antofine. -(−) Antofine standards of 1.5 mg in 1.5 ml MeOH were prepared for immediate use or had been exposed to light or dark conditions at 25 °C for 7 d as previously described. A serial dilution of the fresh standard and the exposed material was prepared ranging from 12.5 μg/ml to 0.78 μg/ml in MeOH. Negative controls employed MeOH alone. All samples were tested using 20 seeds per plate, and the bioassay was replicated in time. All applied MeOH solutions were air dried onto the surface of assay plates for 1 h, and then 20 ml of 1.6 % agarose in water were added. After 1 h, lettuce seeds (green butterhead, Johnny’s Selected Seeds, Winslow, ME, USA) were placed 20 mm from the “top” edge, and plates were sealed with parafilm, then incubated upright for 7 d at 25 °C and a 16:8 h (L:D) cycle, prior to taking root measurements.
To evaluate the effect of antofine spiking in soil, 1 g quantities of non-infested soil from Round Island were either sterilized in glass tubes and then transferred into single wells of a sterile 24 well titer plate, or added directly into the wells. Antofine concentrations at 4, 10, 20, 50, and 100 μg in 500 μl of deionized water were added into 4 replicate wells, with 500 μl of deionized water as the negative control, and then approximately 5 lettuce seeds (black seeded simpson, Burpee & Co., Warminster, PA, USA) were added to each well. Because no effect on root growth was observed for any antofine concentration within that range (data not shown), an additional antofine concentration series of 0, 62.5, 125, 250, 500, and 1000 μg in 500 μl of deionized H2O was added into 4 replicate wells of either sterile or nonsterile soil, with 500 μl of deionized H2O as the negative control, and then lettuce seeds were added as above. Plates were sealed with parafilm, and each concentration series was replicated in time. Plates were incubated at 25 °C and a 16:8 h (L:D) cycle for 1 wk prior to taking root length measurements.
-(−) Antofine Quantitation from Seedlings and Plantlets
Stratified pale swallow-wort seeds were surface sterilized in 5 % Clorox in deionized H2O for 30 min, followed by several sterile H2O rinses. Seeds were placed into individual wells of 12 well plates using 4 ml of 1/2 strength Hoaglands #2 solution with 1.5 % agar, and placed inside a growth chamber at 16:8 h (L:D) and 25 °C. Seedlings, in replicates of 4 plants each, were harvested at 1 and 2 wk, frozen, and extracted directly by grinding to a powder with mortar and pestle in 10 ml MeOH:CH2Cl2 (1:1). The extract was filtered and dried in vacuo. Residue then was partitioned with 5 ml CH2Cl2:10 ml H2O and repeated 3 times. The CH2Cl2 extracts were combined and dried under nitrogen in tared vials. Stratified black swallow-wort seeds did not germinate sufficiently to provide replicate seedlings and plantlets for analysis in these studies. For comparison, however, our previous studies reported antofine levels in black swallow-wort seeds at approximately one-half of that detected in pale swallow-wort seeds and in black swallow-wort roots at approximately one-third of that detected in pale swallow-wort roots (Gibson et al. 2011).
For plantlets, stratified pale swallow-wort seeds were germinated on sterile germination paper, and then placed on the agar surface of 50 ml of 1/4 strength Hoaglands #2 plant solution containing 1.5 % agar placed within 90 × 70 mm pyrex jars topped with a glass petri dish. Plants were grown in a growth chamber at 16:8 h (L:D) and 25 °C, and 4 replicate samples of 4–5 plants were harvested at 10 wk with few showing root growth. Plantlets were frozen separately and then lyophilized for dry wt. Agar from each jar was frozen and extracted separately. Plants and agar were extracted as described above for seedling extracts. Two-mo-old plantlets also were obtained by planting vernalized seeds in MetroMix professional growing mix (SunGrow) and watered as needed with 1/2 strength Hoagland’s solution until harvested. Plantlets were lyophilized and stored at −20 °C and then split into three replicates. Each sample was ground to a powder, then extracted as described above for seedlings. Analysis of all samples was performed by HPLC as described below.
Hydroponic Culture and Exudate Collection
Pale and black swallow-wort crowns from field grown plants were collected in the fall of 2013. The roots were trimmed to 10–13 cm, and then the plant mass was stored in moist peat moss at 4C. After 4 mo in storage, five root crowns each of pale and black swallow-wort were planted into Perlite using a wooden rack holding 10 inverted 4 L bottles with the bottom removed and the cap end fitted with plastic tubing and clamp. Plants were grown in a greenhouse under artificial lighting at 16:8 h (L:D) and 25±4 °C, and watered as needed with ¼ strength modified Hoaglands #2 nutrient solution. Shoots formed within 2 wk of planting, and collection of root exudates were initiated at 6 wk following planting, when shoots were well established. Plants eventually produced flowers followed by seed pods by week 8 of root exudate collections (13 wk following planting). Leachate was collected weekly or biweekly starting at 6 wk after planting with final collection at 20 wk after planting. Individual exudates were obtained by flooding each inverted bottle containing roots and perlite up to the top layer with approximately 1800 ml of distilled H2O. After 1 h, the leachate was drained from the bottom through the cap end tubing for extraction. Plant biomass was collected for dry weights after final collection. At the termination of collections, perlite from each container was collected and frozen until extraction. The thawed perlite sample from each container was transferred to a 2 L beaker with 1800 ml of methanol to cover, and it soaked for 1 h at room temperature. Each eluant was recovered by filtration through Whatman #1 filter paper, dried in vacuo, and then transferred to a tared vial with MeOH for drying under nitrogen. Samples were stored at −20 °C until analysis.
For extraction of the exudate from each plant, the leachate was chromatographed on Amberlite XAD-16. Preliminary experiments to assess -(−) antofine binding to either XAD-16 or XAD-4 indicated that -(−) antofine was not extracted well with XAD-4, while methanol elution of XAD-16 had a recovery efficiency of 23 %. The resin was first batch extracted in MeOH to remove impurites, equilibrated by several exchanges of H2O, and then slurry-packed into 2.5 cm ID × 9.0 cm polypropylene columns holding 30 ml of resin. After loading of the leachate, columns were rinsed with 100 ml water, and then eluted with 100 ml MeOH. The eluant was dried in vacuo, then transferred to tared vials and dried under nitrogen. Samples were stored at −20 °C until analysis.
HPLC Analysis
All extracts were dissolved in 200 μl MeOH with sonication, and then 100 μl were filtered through a 0.45 μ PVSF filter prior to analysis. Analytical HPLC was carried out similarly to that described in Gibson et al. (2011) using a (ODS)3 column (end capped C18, Phenomenex, 5 μm, 250 mm × 4.6 mm), premixed isocratic mobile phase of methanol: 20 mM ammonium acetate (1:1 v/v), flow rate of 1 ml/min. Primary detection by UV at 260 nm and a wavelength scan of 200 to 350 nm allowed detection of -(−) antofine, −(−) antofine oxide, and dehydroantofine, the major identified breakdown product. PDA scans from 200 to 350 nm were used to verify signal at the expected retention times. Quantitation was calculated based on calibration curves with -(−) antofine standard using integration of peak areas after verification of PDA scans with limit of detection (s/n = 10) established at 0.2 μg on-column (linear range of 0.2–50 μg, r 2= 0.948), and then corrected for extraction efficiency depending on the extraction protocol employed.
Spectrometric Measurements
LRESIMS spectra were acquired by infusion of methanolic solutions at 5 μl/min by a syringe pump (Harvard apparatus) into a Micromass ZMD-4000 spectrometer. Positive-ion-mode spectra were acquired with capillary cone voltages of 3.2 kV and 40 V, respectively; negative-ion-mode spectra were acquired with capillary and cone voltages of 4.0 kV and 20 V, respectively.
Statistical Analysis of Data
Most data were analyzed with one way or two way ANOVAs (P < 0.05) using GraphPad Prism® statistical and graphing software, version 5.0d. The seedling bioassay with antofine-spiked soil was analyzed as a two way ANOVA using SAS 9.3 software; means were separated using Fisher’s protected least significant difference test.
Results
Stability of -(−) Antofine in Light and Ambient Temperatures
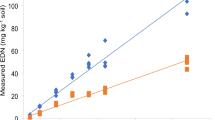
-(−) Antofine, spiked at 500 μg per sample, was more easily recovered from sand than soil when kept at 25 °C for up to 3 mo, although the recovery rate dropped over time in both treatments (Fig. 1). By 2 mo, recovery from nonsterile soil was less than 1 % compared to 15 % recovery at the initial time point. This was significantly lower than from sand, which showed a 22 % recovery compared to initial recovery rates of 34 % (Fig. 1). When -(−) antofine (dissolved in MeOH) was exposed to light, within 2 d we observed a rapid breakdown to dehydroantofine (C23H22NO3, mw 360.2, RT = 5 min; Fig. 2b). Complete disappearance of -(−) antofine occurred by 8 d under light, with further degradation of dehydroantofine into other undefined breakdown products (Fig. 2d). Under dark conditions at 25 °C, degradation of -(−) antofine, corresponding to an approximate 20 % loss, had also occurred by 8 d (Fig. 2c). Thus, both sets of samples degraded over time at ambient temperature.
Recovery of -(−) antofine under ambient conditions in nonsterile sand and soil. A 4 g soil or sand sample in a 125 ml Erlenmeyer flask was spiked with 500 μg of -(−) antofine and placed in a growth chamber at 12:12 h (L:D) and 25 °C, and then extracted with 20 ml CH2Cl2 at monthly intervals. Mean ± SD are shown for each time point (n = 2)
HPLC traces (absorbance profiles at A260nm) showing breakdown of -(−) antofine in presence of light and dark conditions at 25 °C. Starting material was an -(−) antofine standard containing approximately 95 % -(−) antofine (RT = 14.8 min) and a small amount of -(−) antofine oxide (RT = 11.5 min). Each sample consisted of 500 μg of -(−) antofine in 0.5 ml MeOH. A. 2 d dark, B. 2 d light, C. 8 d dark, D. 8 d light
In vitro Testing for Activity of Dehydroantofine
The major initial breakdown product of -(−) antofine was identified as dehydroantofine, but it was not known whether this compound and other breakdown products would retain the phytotoxic activity of the parent compound. Both light and dark-exposed samples stored at ambient temperature up to 7 days showed losses in activity relative to the -(−) antofine standard that was consistent with the amount of loss of -(−) antofine observed in the previous experiment (Fig. 3). Dehydroantofine and other unidentified breakdown products, retained only 10 % of activity of the parent compound at the highest dose tested of the light stored sample, while the dark stored sample still retained about 80 % of activity (Fig. 3).
Dose response profiles, plotted as mean ± SD of the root lengths (mm) of lettuce seeds germinated in the presence of varying concentrations of an -(−) antofine standard and -(−) antofine samples that had been exposed to light or dark conditions for 7 d prior to the experiment. The 0 μg/ml concentration was MeOH only (20 seeds per treatment with two independent replicates)
Extraction Efficiency of -(−) Antofine Spiking from Soils
Initial spiking experiments with purified -(−) antofine gave slightly higher extraction efficiencies in two different non-infested soil samples from Wehle (calcareous silt loam) and Great Gully (Honeoye silt loam) with CH2Cl2 alone in comparison to CH2Cl2:MeOH (1:1) and CH2Cl2:(CH3)2CO (1:1), but solvent type was not significantly different. Extraction efficiency varied with soil type with CH2Cl2 only slightly better than other tested solvents in the high organic soils with an average extraction efficiency of ~15 % of total. CH2Cl2 was chosen for ease of use in solvent extraction for the 4 different soils. At lower spiked concentrations of -(−) antofine (25–100 μg), recovery rates were reduced by half of those at higher concentrations (250–400 μg). The volume of soil extracted (up to 20 g) did not appear to substantially affect sample recovery even with increased solvent.
Analyses of Soil Samples from Two Growing Seasons at 4 Different Infested Sites
We attempted to analyze whether -(−) antofine or its major breakdown product could accumulate to detectable levels in soils during the active growing seasons for these perennial weed species. Analyses of soil samples from all 4 sites over 5 mo (June-October) of two growing seasons (representing a total of 720 samples) were negative for the presence of -(−) antofine and -(−) antofine oxide. Further concentration of samples and higher injection volumes did not aid in detection, while spiking of the samples with -(−) antofine prior to HPLC analysis clearly showed a visible peak. Due to the complication of interfering soil components, it was not possible to assess whether other -(−) antofine degradation products had accumulated to any degree.
Effects of -(−) Antofine Spiking in Soil on Lettuce Seedling Root Growth
-(−) Antofine concentrations up to 125 μg/g soil had no effect on root growth when spiked in either sterile or nonsterile soil, although roots grown in nonsterile soil grew significantly longer (data not shown). Higher concentrations of -(−) antofine (from 125 μg to 1000 μg) were inhibitory in both sterile and non-sterile soil compared to the control (Fig. 4), with greater inhibition of root growth to applied dose in sterile than in non-sterile soil except at 1000 μg of -(−) antofine (interaction of soil treatment and concentration; F 5,80 = 3.08, P = 0.014). Sterile soil had a calculated EC50 of 686 μM (250 μg/g) as compared to nonsterile soil treatments with a calculated EC50 of 1.88 mM (640 μg/g).
Dose response profiles, plotted as mean ± SD of the root lengths (mm) of lettuce seeds germinated in the presence of varying concentrations of an -(−) antofine standard spiked into 1 g of sterile or non-sterile soil. The 0 μg/g concentration was deionized water only (each treatment consisted of 4 wells containing 5 seeds each with two independent replicates). Means denoted by the same letter are not significantly different (Fisher’s protected least significant difference test, P > 0.05)
Concentrations of -(−) Antofine in Seedlings and Plantlets
Analysis of 1 wk and 2-week-old pale swallow-wort seedlings revealed concentrations of total -(−) antofine at 0.07 % and 0.18 % dry weight, respectively (Table 2). Greenhouse-grown seedlings (2 mo) had similar concentrations of -(−) antofine to that from 1 wk old seedlings. At 10 wk of agar cultivation, the average plantlet concentration of -(−) antofine was 1.1 % dry weight. Root growth under the growth chamber conditions in artificial medium was spare, and no -(−) antofine was detectable in the agar medium itself (Table 2). When calculated on a total activity of the biomass using EC50 from laboratory bioassays (Hiradate et al. 2010), the total allelopathic potential activity of these young tissues is high (Table 2). When total activity is calculated using the EC50s obtained using those from -(−) antofine effects in sterile or nonsterile soil (686 μM and 1.88 mM, respectively), however, only the cultured plantlets or seeds contain -(−) antofine at concentrations that are likely capable of allelopathic potential in nonsterile conditions, using a value of 3 as a typical range of established allelochemicals (Hiradate et al. 2010) (Table 2).
-(−) Antofine in Root Exudates from Perlite Cultivation
Exudates of both pale and black swallow-wort revealed small levels of -(−) antofine recovered from XAD extraction over a 15 wk growing period (Fig. 5). In general, pale swallow-wort exudates contained -(−) antofine for all weeks tested, with the highest concentration detected (average of ~40 μg/total exudate) at the 14 wk time point following a 3 wk delay in collection. Black swallow-wort exudates were sometimes lower but also showed an increase at the 14 wk time point similar to that from pale swallow-wort exudates. At week 15, when roots were removed, the perlite growth medium also contained detectable levels of -(−) antofine at ~20 μg/total exudate. Measured -(−) antofine concentration from exudates ranged from 1 to 400 nM over the time course, and if corrected for XAD extraction efficiency of -(−) antofine from dilute solution, it would range from 30–700 nM.
Recovery of -(−) antofine from exudates collected from pale (PSW) or black (BSW) swallow-wort plants grown in hydroponic culture over a 4 mo period. Exudates were concentrated using Amberlite XAD16, then eluted with MeOH, and analyzed by HPLC. The sample collection at 15 wk represents amount of -(−) antofine recovered from the extracted perlite growth medium. Mean ± SD are shown for each time point (N = 5)
Discussion
In this study, we investigated -(−) antofine’s concentration in tissues of different swallow-wort life stages, root exudates, and soils, as well as its activity under different combinations of light and soil conditions, in order to advance our understanding of its potential role as an allelopathic agent for swallow-worts. Douglass et al. (2011) had suggested potential allelopathy from swallow-wort residues and dried tissues when tested in laboratory bioassays, although the causal agent was not identified. Our earlier study demonstrated that -(−) antofine was a highly bioactive phytochemical, present at higher levels in root tissues as compared to shoot tissues, and that it was capable of inhibiting germination and root growth at μM concentrations of native plant species typically found in habitats where swallow-worts have invaded (Gibson et al. 2011). Furthermore, we found that seeds of pale swallow-wort contained -(−) antofine at concentrations of 1.1 % (fresh weight) in comparison to root concentrations of 0.05 % (dry weight), which were higher than those in black swallow-wort (Gibson et al. 2011). Here, we demonstrated that seedlings and young plantlets of pale swallow-wort also contain higher levels of the phytotoxin than roots of adult plants. These young tissues, as well as seeds, would possess an even higher total activity relative to that contained in roots (Hiradate et al. 2010), implying allelopathic potential (Table 2). Total activity was a range similar to that reported for Juglans sp.(juglone), Mucuna pruriens (L-DOPA), and Spirea thunbergii (cinnamyl-glucopyranose derivatives) (Hiradate et al. 2010), and higher than Carduus nutans and C. acanthoides (aplotaxane) (Silva et al. 2014), where total activity values of 3 or higher are considered to have significant allelopathic potential. Douglass et al. (2011) used agar containing actively-growing 5 day old germinated swallow-worts as the source material and demonstrated phytotoxicity and reduced germination to some indicator plant species. Their findings likely resulted from seed- and plantlet-released -(−) antofine.
Our laboratory experiments to evaluate potential breakdown of -(−) antofine demonstrated that degradation occurs even at ambient temperatures in the dark, and at a higher rate with light exposure, over time. Breakdown products, including the identified initial product dehydroantofine, poorly retained phytotoxicity compared to -(−) antofine. Although our laboratory studies indicated that extraction of -(−) antofine from soils, particularly those with high organic matter, would be low, we attempted to analyze soil from 4 different populations at monthly intervals over two seasons. All were negative for -(−) antofine. It seems likely that, if -(−) antofine or dehydroantofine were present in the samples, it would not persist for long periods of time under ambient temperatures, even without exposure to light, based on our assessments of -(−) antofine stability under laboratory conditions. Potential microbial degradation (discussed below) also could contribute to the absence of antofine in soil samples.
We found that -(−) antofine was much less inhibitory when applied to non-sterile soils as compared to sterile ones. Dose response curves using sterile and nonsterile soil spiked with -(−) antofine demonstrated a requirement for at least 20–55 × greater -(−) antofine concentrations in soil, respectively, for obtaining EC50s than that previously seen in agar bioassays with lettuce (34 μM, Gibson et al. 2011). The fact that about three times as much antofine was required in non-sterile soil to produce equivalent inhibition to that in sterile soil suggests inactivation or breakdown of -(−) antofine by microorganisms. Thus, when calculated based on EC50s from soil, only seeds and young plantlets would have threshold values of total activity capable of allelopathic potential in nonsterile soil, which we used to mimic the natural environment.
In this study, root exudates obtained from hydroponic culture of adult plants contained measurable levels of -(−) antofine, but the concentrations were much lower than those found to cause inhibition to native competing plant species and test plants in previous laboratory bioassays (Gibson et al. 2011) and in those conducted as part of this study. These concentrations from exudates, however, may be underestimates of accumulation in undisturbed rhizospheres based on the extraction efficiencies and the potential breakdown of released -(−) antofine under these conditions. Additionally, this study was not designed to assess concentration gradients that might occur with pulsed release from the root surface. We did observe an increase in levels as exudate collections were extended by two and 3 wk intervals, suggesting that greater accumulation in soil can occur with time.
Definining the role of allelopathy in invasiveness is fraught with multiple challenges. Blum (2014) has detailed the major problems in comparing single factor dose responses in laboratory assays with those allelopathic interactions that are likely to occur under field conditions. Part of the difficulty in assessing whether allelopathy plays an ecologically relevant role is due to the inherent analytical techniques employed (Blair et al. 2006, 2009) and the potentially complex interactions with microbiota and neighboring plants in the soil environment that complicate the ability to monitor allelochemical concentrations. In general, concentrations of allelochemicals in soil and the surrounding environment typically are much lower than those needed to observe phytotoxic effects under laboratory conditions. This point has been raised to discount any role of allelopathy in plant-plant interactions, but the measured concentrations are limited by the analytical methods themselves. Concentrations may be underestimated due to extraction efficiencies or breakdown of active chemicals in the soil due to environmental conditions or microbial action. In this study, we observed a marked difference with -(−) antofine spiked in sterile as compared to nonsterile soil, with an ~3× increase in EC50, indicating that spiked -(−) antofine was either unavailable or had been degraded more readily. We did demonstrate that high concentrations of -(−) antofine (at 125–1000 μg) resulted in significant inhibition of root growth in both sterile and nonsterile soil treatments. However, these concentrations are well in excess of that detected in root exudates, thus appearing to weaken the case for a significant role for antofine in allelopathy. Concentration gradients also may play a role, as allelochemicals are dispersed from the root surface into a three dimensional environment. Some have suggested that pulsed releases of allelochemicals, such as that from decaying plant matter, wash off from surfaces, or slow release over longer periods of time, might result in higher overall accumulation (Jessing et al. 2014; Silva et al. 2014; Smith and Reynolds 2014). Indeed, Weidenhamer et al. (2014) have shown large spatial and temporal variations in thiophene production through the use of microextraction sampling, supporting the idea that allelochemical concentrations can vary substantially. Recent studies have shown that allelopathic compounds appear to recruit beneficial microbes such as endophytes (Aschehoug et al. 2014) and mycorrhizal fungi (Bongard et al. 2013), and that allelochemicals can act to suppress pathogens (Kaur et al. 2009; Zhang et al. 2009). Antofine’s reported antimicrobial properties may assist in swallow-wort establishment and persistence (Gibson et al. 2011; Mogg et al. 2008). Other reports have noted alterations in overall soil microbe populations, as reviewed in Rout and Callaway (2012); these microbial shifts may aid in invasiveness relative to native species (Bongard et al. 2013), or act to control an invasive plant (Barto et al. 2010; Lankau 2009), especially over time, as reported in long-term field populations of Heracleum mantegazzianum (giant hogweed) (Dostál et al. 2013).
The results of this study leave open the question of -(−) antofine’s role in the allelopathy of swallow-worts. Its breakdown under ambient conditions and greatly reduced activity in nonsterile soil are balanced in part by the fact that measurable albeit low amounts of -(−) antofine accumulate in exudates and that seeds and young plant tissues have high specific allelopathic potential. At present, this study does suggest that -(−) antofine might contribute to early seedling establishment, but its subsequent function in the life history of swallow-worts remains unclear.
References
Ambika SR (2013) Multifaceted attributes of allelochemicals and mechanism of allelopathy. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy. Springer Verlag, Berlin HeidelberG, pp 389–405
Aschehoug ET, Callaway RM, Newcombe G, Tharayil N, Chen S (2014) Fungal endophyte increases the allelopathic effects of an invasive forb. Oecologia 175:285–291
Averill KM, DiTommaso A, Mohler CL, Milbrath LR (2010) Establishment of the invasive perennial Vincetoxicum rossicum across a disturbance gradient in New York State USA. Plant Ecol 211:65–77
Averill KM, DiTommaso A, Mohler CL, Milbrath LR (2011) Survival, growth, and fecundity of the invasive swallowworts (Vincetoxicum rossicum and V. nigrum) in New York State. Invasive Plant Sci Manag 4:198–206
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Barto K, Friese C, Cipollini D (2010) Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. J Chem Ecol 36:351–360
Blair AC, Nissen SJ, Brunk GR, Hufbauer RA (2006) A lack of evidence for an ecological role of the putative allelochemical (±)-Catechin in spotted knapweed invasion success. J Chem Ecol 32:2327–2331
Blair AC, Weston LA, Nissen SJ, Brunk GR, Hufbauer RA (2009) The importance of analytical techniques in allelopathy studies with the reported allelochemical catechin as an example. Biol Invasions 11:325–332
Blum U (2014) Some issues and challenges when designing laboratory bioassays. In: Blum U (ed) Plant-plant alelopathic interaction II. Switzerland, Springer International, pp 77–129
Bongard CL, Navaranjan G, Yan W, Fulthorpe RR (2013) Fungal colonization of the invasive vine Vincetoxicum rossicum and native plants. Plant Ecol Evol 146:45–52
Callaway RM, Aschehoug ET (2000) Invasive plant versus their new and old neighbores: a mechanism for exotic invasion. Science 290:521–523
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Cappuccino N (2004) Allee effect in an invasive alien plant, pale swallow-wort Vincetoxium rossicum (Asclepiadaceae). Oikos 106:3–8
Cappuccino N, Arnason JT (2006) Novel chemistry of invasive exotic plants. Biol Lett 2:189–193
Del Fabbro C, Güsewell S, Prati D (2014) Allelopathic effects of three plant invaders on germination of native species: a field study. Biol Invasions 16:1035–1042
DiTommaso A, Lawlor FM, Darbyshire SJ (2005) The biology of invasive alien plants in Canada.2. Cynanchum rossicum (Kleopow) Borhidi [= Vincetoxicum rossicum (Kleopow Barbar.] and Cynanchum louiseae (L.) Kartesz & Gandhi [= Vincetoxicum nigrum (L.) Moench]. Can J Plant Sci 85:243–263
Dommanget F, Evette A, Spiegelberger T, Gallet C, Pacé M, Imbert M, Navas M-L (2014) Differential allelopathic effects of Japanese knotweed on willow and cottonwood cuttings used in riverbank restoration techniques. J Environ Manag 132:71–78
Dostál P, Müllerová J, Pyšel P, Pergl J, Klinerová T (2013) The impact of an invasive plant changes over time. Ecol Lett 16:1277–1284
Douglass CH, Weston LA, Wolfe D (2011) Phytotoxicity and potential allelopathy in pale (Cynanchum rossicum) and black swallowwort (C. nigrum). Invasive Plant Sci Manag 4:133–141
Duke SO, Blair AC, Dayan FE, Johnson RD, Meepagala KM, Cook D, Bajsa J (2009) Is (−)-Catechin a novel weapon of spotted knapweed (Centaurea stoebe)? J Chem Ecol 35:141–153
EDDMapPS (2014) Early detection & distribution mapping system (http://www.eddmaps.org/). The University of Georgia—Center for Invasive Species and Ecosystem Health
Gibson DM, Krasnoff SB, Biazzo J, Milbrath LR (2011) Phytotoxicity of antofine from invasive swallow-worts. J Chem Ecol 37:871–879
Greer MJ, Wilson GWT, Hickman KR, Wilson SM (2014) Experimental evidence that invasive grasses use allelopathic biochemicals as a potential mechanism for invasion: chemical warfare in nature. Plant Soil. doi:10.1007/s1104-014-2209-3
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasions. Plant Soil 256:25–39
Hiradate S, Ohse K, Furubayashi A, Fujii Y (2010) Quantitative evaluation of allelopathic potentials in soils: total activity approach. Weed Sci 58:258–264
Inderjit, Callaway RM (2003) Experimental designs for the study of allelopathy. Plant Soil 256:1–11
Inderjit, Pollock JL, Callaway RM, Holben W (2008a) Phytotoxic effects of (±) -catechin in vitro, in soil, and in the field. PLoS ONE 3:e2536
Inderjit, Seastedt TR, Callaway RM, Pollock JL, Kaur J (2008b) Allelopathy and plant invasions: traditional, congeneric, and bio-geographical approaches. Biol Invasions 10:875–890
Jessing KK, Duke SO, Cedergreen N (2014) Potential ecological roles of artemisinin produced by Artemisia annua L. J Chem Ecol 40:100–117
Kartesz JT (1999) A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. In: Kartesz JT, Meacham CA (eds) Synthesis of the North American Flora, Version 1.0
Kaur H, Kaur R, Kaur S, Baldwin IT, Inderjit (2009) Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PLoS ONE 4:e4700
Koocheki A, Lalegani B, Hosseini S (2013) Ecological consequences of allelopathy. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy. Springer Verlag, Berlin Heidelberg, pp 23–38
Lankau R (2009) Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biol Invasions 12:2059–2068
Lawlor FM (2000) Herbicidal treatment of the invasive plant Cynanchum rossicum and experimental post control restoration of infested sites. State University of New York, Syracuse, p 78
Lorenzo P, Hussain MI, González L (2013) Role of allelopathy during invasion process by alien invasive plants in terrestrial ecosystems. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy: current trends and future applications. Springer, Berlin Heidelberg, pp 3–21. doi:10.1007/978-3-642-30595-5_1
Magidow LC, DiTommaso A, Ketterings QM, Mohler CL, Milbrath LR (2013) Emergence and performance of two invasive swallowworts (Vincetoxicum spp.) in contrasting soil types and soil pH. Invasive Plant Sci Manag 6:281–291
Markgraf F (1972) Vincetoxicum N.M. Wolf. In: Tutin TG, Heywood VJ, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europeae, vol 3. Diaapensiaceae to Myoporaceae. Cambridge University Press, Cambridge, pp 71–73
Milbrath LR (2008) Growth and reproduction of invasive Vincetoxicum rossicum and V. nigrum under artificial defoliation and different light environments. Botany 86:1279–1290
Mogg C, Petit P, Cappuccino N, Durst T, McKague C, Foster M, Yack JE, Arnason JT, Smith ML (2008) Tests of the antibiotic properties of the invasive vine Vincetoxicum rossicum against bacteria, fungi and insects. Biochem Syst Ecol 36:383–391
Perry LG, Thelen GC, Ridenour WM, Callaway RM, Paschke MW, Vivanco JM (2007) Concentrations of the allelochemical (±)-catechin in Centaurea maculosa soils. J Chem Ecol 33:2337–2344
Rout ME, Callaway RM (2012) Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Ann Bot 110:213–222
Rudrappa T, Choic YS, Levia DF, Legates DR, Lee KH, Bais HP (2009) Phragmites australis root secreted phytotoxin undergoes photo-degradation to execute severe phytotoxicity. Plant Signal Behav 4506–513
Sheeley SE, Raynal DJ (1996) The distribution and status of species of Vincetoxicum in eastern North America. Bull Torrey Bot Club 123:148–156
Silva FML, Donega MA, Cerdeira AL, Corniani N, Velini ED, Cantrel CL, Dayan FE, Coelho MN, Shea K, Duke SO (2014) Roots of the invasive species Carduus nutans L. and C. acanthoides L. produce large amounts of aplotaxene, a possible allelochemical. J Chem Ecol 40:276–284
Smith LM, Reynolds HL (2014) Light, allelopathy, and post-mortem invasive impact on native forest understory species. Biol Invasions 16:1131–1144
Smith LL, DiTommaso A, Lehmann J, Greipsson S (2006) Growth and reproductive potential of the invasive exotic vine Vincetoxicum rossicum in Northern New York State. Can J Bot 84:1771–1780
Uddin MN, Robinson RW, Caridi D, Al Harun MAY (2014) Suppression of native Melaleuca ericifolia by the invasive Phragmites australis through allelopathic root exudates. Am J Bot 101:479–487
USDA NRCS (2009) The PLANTS Database (http://plants.usda.gov). National Plant Data Center, Baton Rouge, LA 70874–4490 USA. 2009
Weidenhamer J, Li M, Allman J, Bergosh RG, Posner M (2013) Evidence does not support a role for gallic acid in Phragmites australis invasion success. J Chem Ecol 39:323–332
Weidenhamer JD, Mohney BK, Shihada N, Rupasinghe M (2014) Spatial and temporal dynamics of root exudation: how important is hetrogeneity in allelopathic interactions? J Chem Ecol 40:940–952
Weir TL, Bais HP, Vivanco JM (2003) Intraspecific and interspecific interactions mediated by a phytotoxin, (−) catechin, secreted by the roots of Centaurea maculosa (spotted knapweed). J Chem Ecol 29:2397–2412
Weir TL, Bais HP, Vivanco JM (2009) Retraction note- Intraspecific and interspecific interactions mediated by a phytotoxin, (−) catechin, secreted by the roots of Centaurea maculosa (spotted knapweed). J Chem Ecol 35:860
Zhang S, Jin Y, Tang J, Chen X (2009) The invasive plant Solidago canadensis L. suppresses local soil pathogens through allelopathy. Appl Soil Ecol 41:215–222
Acknowledgments
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendor that may also be suitable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibson, D.M., Vaughan, R.H. & Milbrath, L.R. Invasive Swallow-worts: An Allelopathic Role for -(−) Antofine Remains Unclear. J Chem Ecol 41, 202–211 (2015). https://doi.org/10.1007/s10886-015-0552-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0552-3