Abstract
The novel weapons hypothesis states that some invasive weed species owe part of their success as invaders to allelopathy mediated by allelochemicals that are new to the native species. Presumably, no resistance has evolved among the native species to this new allelochemical (i.e., the novel weapon). In their native habitat, however, the plants that co-evolved with these invasive species have theoretically evolved defenses that obviate the allelochemical advantage. Previous studies have claimed that catechin is such a novel weapon of spotted knapweed (Centaurea stoebe = C. maculosa), an invasive species in the non-native habitat of North America. These studies indicated that (−)-catechin is more phytotoxic than (+)-catechin. Other studies have not found sufficient catechin in field soils to support this theory. We report that (−)-catechin and (+)-catechin are essentially equal, but poorly phytotoxic to a variety of plant species in bioassays without soil. In a dose/response experiment with Montana soils, we found the lowest dose for a growth reduction of two native Montana grasses (Koeleria macrantha and Festuca idahoensis) by a racemic mixture of (±)-catechin that ranged from about 25 to 50 mM, concentrations, orders of magnitude higher than expected in nature. Autoclaving the soil before adding the catechin did not affect the activity of catechin. We found (−)-catechin to be a potent antioxidant, in contrast to a previous claim that it acts as an allelochemical by causing oxidative stress. Our findings suggest that catechin is not a novel weapon of spotted knapweed and that other allelochemical(s) or alternative mechanisms must be found to explain the success of this species as an invader in North America.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some invasive plant species are apparently allelopathic and may owe part of their success in new habitats to this trait (Weston and Duke 2003). Claims have been made that the success of Centaurea stoebe (= C. maculosa; spotted knapweed) in North America results from allelopathy via the release of (−)-catechin [(2S,3R)-(−)-catechin] (Bais et al. 2002, 2003; Weir et al. 2003; Thelen et al. 2005; Callaway and Ridenour 2004; Inderjit et al. 2008). This compound was reported to be exuded from the roots of spotted knapweed, and it was identified as the putative allelochemical of this invasive species (Bais et al. 2002). The (+)-catechin enantiomer [(2R,3S)-(+)-catechin] is also produced by the plant, but was initially reported to be nonphytotoxic (Bais et al. 2002) and later 1.5 to two times less active than (−)-catechin (Veluri et al. 2004, erratum).
Catechin is a commonly found flavonoid present in fruits, wine, vegetables, and cocoa products, as well as in many nonfood plants. Grape seeds contain a significant amount of (+)-catechin and (−)-epicatechin. Most foods contain the (+)-enantiomer of catechin, but chocolate mainly contains (−)-catechin together with (−)-epicatechin. (+)-Catechin is the most commonly found enantiomer in nature. Both enantiomers possess antioxidant properties. The two enantiomers have identical nuclear magnetic resonance (NMR) spectra and high-performance liquid chromatography (HPLC) retention times and identical R f values in thin layer chromatography (TLC). The only difference is in optical rotation and the CD spectra, where these two compounds rotate plane polarized light equally in opposite directions. The CD spectra of these enantiomers are antipodal. The mirror image differences in molecular shape can result in differences in biological activity (e.g., Donovan et al. 2006).
Multiple studies have examined various aspects of the role of (–)-catechin as an allelochemical (Bais et al. 2003; Weir et al. 2003, 2005; Veluri et al. 2004; Callaway et al. 2005; Perry et al. 2005; Thelen et al. 2005). This work, in part, laid the groundwork for the novel weapons hypothesis, which states that the success of some exotic invasive plant species may be due to the production of allelochemicals that native species have never encountered and, thus, to which they have not evolved defenses (Callaway and Ridenour 2004).
Blair et al. (2005, 2006, 2009) questioned the findings of (−)-catechin as the allelochemical responsible for the successful invasion of spotted knapweed. They found that the methods for catechin extraction described in previous papers (Bais et al. 2002, 2003; Weir et al. 2003) could not extract catechin from liquid media and produced low recovery efficiencies from soil. With methods that were much more efficient for catechin extraction, they quantified levels of production up to two orders of magnitude less with plants grown in media than stated in earlier publications. A grass species (Festuca idahoensis) native to some of the spotted knapweed-infested areas of North America was only slightly affected by racemic catechin concentrations 20-fold higher than those previously reported to cause 100% mortality (Blair et al. 2005). Lastly, no measurable concentrations of catechin could be detected in soil samples from two spotted knapweed sites in Montana, USA. The last issue was countered with the argument (Inderjit et al. 2006) that catechin can be found only at certain times in these soils and that Blair et al. (2005) had sampled at the wrong time of the year (October, 2004). Blair et al. (2006) sampled three field sites through the summer and fall of 2005 and detected catechin at only one time point at two of the field sites at levels, three orders of magnitude lower than what they had previously reported to reduce growth of F. idahoensis. Later, Perry et al. (2007) found little or no catechin in 402 soil samples from 11 C. stoebe sites, except on one sampling date, when all of the samples from one site had levels of catechin of 0.65 ± 0.45 (SD) mg g−1. In this paper, the authors were less firm than they were previously in their hypothesis that catechin plays a role in the allelopathy of spotted knapweed.
Although many natural compounds can be shown to be phytotoxic in the laboratory or greenhouse, to prove allelopathy, it is ultimately necessary to demonstrate the presence of the compound under natural field conditions at concentrations high enough to have an impact on neighboring plants (Cheng 1995; Romeo 2000). In solution, catechins can be phytotoxic to plants, including algae (Buta and Lusby 1986; D’Abrosca et al. 2006), but there is inadequate evidence of its activity in soil. In this paper, we present evidence that is consistent with the findings of Blair et al. (2005, 2006, 2009) and others (Tharayil et al. 2008). We report that both (+)- and (−)-catechin are weak phytotoxins on a variety of plant species in liquid or agar cultures and that they are essentially inactive as phytotoxins in soil.
Methods and Materials
Plant Material
Lettuce seeds (Lactuca sativa L. cv. Iceberg A) were obtained from Burpee Seed Company and creeping bentgrass seeds (Agrostis stolonifera var. Penncross) from Turf-Seed (Hubbard, OR, USA). Duckweed (Lemna paucicostata Hegelm. 6746) was from a stock maintained in our laboratory since it was brought to us from Japan (Matsumoto and Duke 1990). These species were selected because we have used them to evaluate thousands of synthetic and natural compounds for phytotoxicity (e.g., Dayan et al. 2000, Michel et al. 2004).
Arabidopsis thaliana (accession Columbia-0/Redei-L206440) seeds were obtained from Lehle Seeds (Round Rock, TX, USA). Arabidopsis was used because the original papers that claimed (−)-catechin to be a novel weapon used it as one of their test species (Bais et al. 2002, 2003).
Seeds of junegrass (Koeleria macrantha) and Idaho fescue (F. idahoensis) were obtained from Western Native Seed (P. O. Box 188, Coaldale, CO, USA). These species were selected because they are native to the areas infested by spotted knapweed in North America, and they had been reported previously to be sensitive to catechin (Bais et al. 2003).
Chemicals
The three catechins used [(+)-catechin hydrate, (−)-catechin, and (±)-catechin hydrate] were obtained from Sigma-Aldrich, for experiments in which small amounts were needed. The enantiomeric structures of (−)- and (+)-catechin in different lots of these chemicals were verified by circular dichroism spectroscopy (JASCO, Model J-715). All concentrations of catechins used in nonsoil bioassays were prepared by serial dilution from 10 mM stock solutions prepared in 5% (v/v) acetone. Epicatechin, catechinic acid, mannitol, and quercitin also were purchased from Sigma-Aldrich.
For soil studies, (−)–catechin was synthesized by adding 5 g of (−)-epicatechin to N2 gas-saturated aqueous 5% NaOH (w/v) (75 ml) and stirring under N2 for 10 min at 65°C. The mixture was cooled on ice, acidified to about pH 3 with about 60 ml of ice cold 2 N HCl, and extracted with ethyl acetate (3 × 200 ml). The organic layer was dried over anhydrous Na2SO4, and the viscous solution (ca 30 ml) was applied to a Sephadex LH-20 column (35 mm id × 40 cm) equilibrated with ethanol/H2O (85:15, v/v). The fractions were monitored by TLC and HPLC using (+)-catechin as the reference. The two enantiomers have the same retention time. (−)-Catechin-enriched fractions were pooled and concentrated to obtain 1.5 g of material. This was further purified with a C-18 gravity column (12 cm × 3.4 cm id) equilibrated with 10% MeOH and water. (−)-Catechin-enriched fractions were pooled, evaporated at 40°C to remove MeOH, freeze dried, and crystallized with methanol and CH2Cl2 to yield 420 mg of white needle-like crystals. The identity of the compound was confirmed as catechin by HPLC and NMR, and the absolute configuration was determined by comparison of CD spectra with those of (+)- and (−)-catechin.
Bioassays without Soil
Lettuce seeds were surface disinfected with a 10% Chlorox® solution (6.15% w/v sodium hypochlorite) for 20 min, followed by 1 h of continual rinsing with sterile distilled water. Seeds were air-dried overnight in a Nuaire Biological Safety cabinet. Sterile techniques were used when handling.
Two milliliters of the appropriately diluted solutions of (−), (+), or (±)-catechin (initially dissolved in acetone to give a 5% acetone concentration in the final dilution) were added under sterile conditions to a 9-mm circle of sterile filter paper (Whatman no. 1) in a sterile, disposable Petri dish (Falcon no. 351005 Optilux Petri dish 100 × 20 mm). A solvent control set of dishes was prepared in the same manner using 5% acetone. In one experiment, the pH values of the test solutions were adjusted with either 0.1 M NaOH or 0.1 M HCl, depending on the pH desired. Fifteen lettuce seeds were added to the moistened paper. The dish was covered, sealed with a strip of parafilm, wrapped in aluminum foil to prevent light from entering, and then placed in a dark incubator (Percival Scientific, Model E-30LED3) at 25°C for a 7-day incubation period. Growth was in darkness in order to obtain maximal hypocotyl growth. Four replicates were prepared for each catechin concentration and the control. After 7 days, the dishes were removed, unwrapped from the foil, and placed under light in another incubator (Percival Scientific, Model CU-36L5) at 25°C for 1 day in order for chloroplast development to occur. After the 24-h light cycle, the dishes were removed from the incubator, and the root and hypocotyl lengths were determined. In all treatments, including the control, there were a few ungerminated and/or stunted seedlings, so only the ten most robust plants in each dish were measured. Averages from the measurements from the ten plants per dish were determined. The average of the four means and SE of the means from the four dishes were plotted. Bentgrass bioassays were conducted as previously described (Dayan et al. 2000).
Highly phytotoxic compounds, especially those that cause photooxidative damage, eventually cause loss of chlorophyll. Chlorophyll analysis was done by extracting at least 10 mg of lettuce cotyledon or bentgrass leaf tissue with 2 ml of dimethyl sulfoxide (DMSO) for 1 h at 60°C to 62°C according to the method of Hiscox and Israelstam (1980). There was no further diluting necessary prior to spectrophotometric analysis (Shimadzu UV-3101 PC UV-VIS-NIR). The chlorophyll–DMSO extracts were cooled to room temperature and absorbance at 645 and 663 nm measured with DMSO as the blank. Chlorophyll concentration was determined by the equation of Arnon (1949). The average of the chlorophyll content in lettuce cotyledons or bentgrass leaves was determined.
Duckweed plants were grown and treated with catechins as previously described for herbicides (Michel et al. 2004). Growth was determined nondestructively by image analysis of frond area as described in detail by Michel et al. (2004). Each treatment was conducted in quadruplicate, with SEs of means generated from the data.
A. thaliana plants were treated with catechin in a manner similar to that of Bais et al. (2003). Seeds were sterilized by the method of Baerson et al. (2005) and then placed in filter paper-lined, 6-cm Petri dishes in 3 ml of half strength MS medium (Murashige and Skoog 1962) under a 16:8-h L:D photoperiod at 21°C. The plates were sealed with 3 M surgical tape. Plants were grown for 10 days under these conditions, and then the most robust plants were transferred to 24-well plates (three seedlings per well) containing half strength MS medium with different amounts of (+), (−), or (±)-catechin (initially dissolved in acetone to give a 5% final acetone concentration) or control wells. There were 12 wells of three plants each for each treatment. After 4 days, root lengths were determined, and then all plants were replaced in 24-well plates with half strength MS medium without catechins for 4 days.
In another experiment, square Petri dishes (9 × 9 × 1.5 cm) were used for A. thaliana bioassays as before (Baerson et al. 2005) with the following modifications. Seeds were germinated and seedlings grown on sterile, semisolid Gamborg’s B5 (Gamborg et al. 1968) at a pH of 5.7 with minimal organics (Sigma-Aldrich) supplemented with 10 g/l sucrose. The media of some of the plates was amended with (+)-catechin, (+/−)-catechin, or (−)-catechin. Acetone was used as a solvent where each plate contained 1.0% (v/v) acetone. The controls contained the same amount of acetone. Plants were grown in a controlled environment chamber (Percival) under a photoperiod of 16 h light illuminated at 100 μmol m−2 s−1 photosynthetically active radiation (PAR) and 8 h dark at 22°C for 14 d. Photographs were taken at day 14.
Bioassays in Soil
Metromix 350 potting soil (Hummert International, Earth City, MO, USA) was used in some studies. This mixture contains horticultural grade vermiculite, peat moss, processed bark ash, nutrients, dolomite limestone, and a wetting agent.
In other studies, soil from near the towns of Nelson Gulch and Jens, Montana collected in October, 2005 was used. GPS coordinates for the two sites are: Nelson Gulch 46°34.373′ N 112°8.820′ W 4,273 m and Jens Exit 46°36.359′ N 113°00.832′ W 4,136 m. Further details of the collection sites are provided in Blair et al. (2006). These soils were shipped to Oxford, MS, USA, where they were stored at room temperature until use in bioassays. The storage time varied from days to months, depending on when the experiment was done. The Montana soils were screened to remove rocks and larger organic fragments with a sieve used for sifting flour and then finally screened with a USA standard testing sieve (Tyler 35 mesh equivalent, 425 μm). Ten grams of air-dried soil was placed in 6-cm Petri dishes with different amounts of (±)-catechin or (−)-catechin. Catechins were incorporated by sifting the soils several times with the appropriate amount of the dry chemical. In some studies, the soils were autoclaved (three cycles of 30 min at 121°C, followed by 30 min cooling) before use to eliminate potential effects of soil microflora on catechin. Seeds of junegrass or Idaho fescue pretreated with 0.2% (w/v) KNO3 to induce uniform germination were planted (25 seeds/dish) in soils from Nelson or Jens soil to which 5 ml of distilled water were added. The dishes were then placed in growth chambers at 24°C with 14-h photoperiods and 70 μmol m−2 s−1 PAR. The five most robust plants from each dish were selected, and plant height, fresh weight, and, in some cases, dry weight were determined 10 to 13 days after planting.
Determination of Free Radical Quenching of Catechins
The method of Cespedes et al. (2001) was used to detect ROS quenching of natural compounds. Silica gel TLC plates (GF254 250 µm, Analtech) were spotted with approximately 2.5 μl (2 mg/ml in methanol) of (+)-catechin, (−)-epicatechin, (−)-catechin, the antioxidant quercetin (Hollman et al. 1997; Zhang et al. 2008), and the nonquenching compound mannitol. Plates were developed in toluene/acetone/methanol (6:3:1) with two drops of acetic acid per 10 ml. After eluting, TLC plates were dried and sprayed with a 0.2% 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (w/v) in methanol. Plates also were exposed to iodine vapor. The plates were photographed 30 min after spraying. Antioxidant activity was evidenced by appearance of yellow spots against a purple background.
Analysis of Data
Experiments were repeated in time and space. Data were analyzed with the SAS Software release 9.1 (SAS Institute, Cary, NC, USA). Analysis of variance was performed for each compound concentration, and means were tested with Duncan multiple range test. SEs are also provided to show the variation associated with particular means. Data from dose–response experiments were analyzed with the add-on package for dose–response curves (drc; Ritz and Streibig 2005), for R version 2.2.1 (R Development Core Team 2005) using a four-parameter logistic function. Means and SEs were obtained using the raw data, and I 50 values were one of the parameters in the regression curves. The regression curves were imported into SigmaPlot version 10 (Systat Software, San Jose, CA, USA).
Results
Soil-free bioassays
When A. thaliana was bioassayed in a manner similar to that of Bais et el. (2002, 2003), (+)-, (−)-, and (±)-catechin significantly inhibited root growth at 0.33 and 1 mM, with (−)-catechin being slightly more active than (+)-catechin (Fig. 1). As described by Bais et al. (2003), roots darkened after exposure to catechins (Fig. 2). When plants were transferred to a catechin-free medium after 4 days of catechin exposure, root growth resumed (Fig. 2). The new root growth was not dark, and the darkening in the old roots was less intense. Healthy root hairs were seen in all treatments (Fig. 3) after removal from catechin.
Root lengths of A. thaliana plants grown for 10 days in MS medium and then transferred to 24-well plates for 4 days containing the indicated media. Error bars are ±1 SE of the mean. Means with the same letters above them are not statistically different at the α = 0.05 level, using Duncan’s multiple range test
A. thaliana plants grown for 10 days in MS medium and then transferred to 24-well plates for 4 days to the indicated media, after which all treatments were transferred to media without catechins. Arrows in the first column of photographs indicate darkened root tips. Arrows in the second column of photographs indicate white root tips after 2 days without catechin
In agar, there was little or no growth reduction of A. thaliana roots or shoots when grown continuously on 0.17 or 0.35 mM (−) or (+) catechin, respectively (Fig. 4). There was marked root growth reduction at 0.7 mM, accompanied by browning of the roots. The effects were similar for both enantiomeric forms of catechin.
In solution, catechin can degrade to epicatechin and catechinic acid under some conditions (Kiatgrajai et al. 1982). We found neither of these compounds to be phytotoxic to lettuce, bentgrass, or duckweed at concentrations up to 1 mM (data not shown).
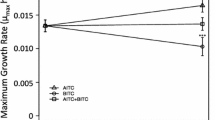
With bentgrass, there was a slight and similar reduction in chlorophyll with both (−)- and (+)-catechin at 3.3 mM (Fig. 5). Studies with duckweed found that both enantiomeric forms of catechin reduced growth at 3.3 mM and that the dose–response curves were similar for both enantiomers (Fig. 6). There was a slight reduction of lettuce hypocotyl growth by 333 μM (+)-catechin (Fig. 7a). Growth of lettuce roots was affected slightly, but significantly, by both enantiomers of catechin at 33 μM and more strongly by 333 μM (−)-catechin (Fig. 7b). Lettuce seedlings, germinated and grown for 8 days in 3.3 mM racemic catechin, were not appreciably stunted (Fig. 8), but browning of the hypocotyl and roots occurred. Chlorophyll levels of the cotyledons of lettuce were not affected at any concentration of any form of catechin tested (data not shown). The stability of catechin in water is affected by pH (Courbat et al. 1977; Hashida et al. 2003; Ho et al. 1995; Blair et al. 2005; Labrouche et al. 2005) with catechin becoming less stable above pH 5. We found no pronounced effect of pH between 5 and 8 on activity of 3.3 mM catechin on lettuce after 7 days (Fig. 9). Racemic catechin at 3.3 mM slightly stimulated growth of hypocotyls at pH 7 and 8 and roots at pH 6.
Effects of different concentrations of (−)- and (+)-catechin on growth of hypocotyls (a) and roots (b) of lettuce seedlings grown in distilled water with or without the catechin after 8 days of exposure. Error bars are ±1 SE of the mean. Those means with letters over them are significantly different from the corresponding control at α = 0.05, using Duncan’s multiple range test. The bar with an asterisk in it is significantly different from the same concentration (−)-catechin treatment at α = 0.05, using Duncan’s multiple range test
Effect of 3.3 mM (±)-catechin at different pH values in distilled water (adjusted with NaOH or HCl) on growth of 8-day-old lettuce roots and cotyledons. Error bars are ±1 SE of the mean. Those bars with letters over them are significantly different from the corresponding control at α = 0.05, using Duncan’s multiple range test
Studies in Soil
There was no marked effect on growth of either Idaho fescue or junegrass by a racemic mixture of catechin in Metromix 350 potting soil at concentrations up to 10 mg/g dry weight after 13 days of growth (Fig. 10). Analysis of the dose–response curves using a four-parameter logistic function calculated nearly identical I 50 values for catechin on Idaho fescue and junegrass, at 15.4 and 15.6 mg/g, respectively. The potential concentration of the combined two enantiomers in the soil at 15 mg/g dry weight is 12.5 mM. At this concentration and above, growth was significantly reduced, but the plants remained green and retained turgor.
Effect of different concentrations of (±)-catechin in Metromix 350 potting soil on growth of Idaho fescue (F. idahoensis) or junegrass (Koeleria macrantha) after 13 days of growth. Error bars are ±1 SE of the mean. Those means with letters over them (a for Idaho fescue and b for junegrass) are significantly different from the corresponding control at α = 0.05, using Duncan’s multiple range test. The inset shows the R plots of data used to generate the I 50 values
Soils contain microflora that can convert phytotoxins to more active or less active compounds. Autoclaving can eliminate this possibility. By using autoclaved and nonautoclaved soils from Jens (Fig. 11a) and Nelson (Fig. 12a), we found little or no effect of a racemic mixture of catechin on growth of Idaho fescue plants after 10 days at concentrations below 10 mg/g dry soil (ca 8.33 mM). There was no difference between results with autoclaved and nonautoclaved soil, except at 30 and 100 mg/g of soil (ca 25 and 83 mM, respectively) in Jens and Nelson soils, respectively, and these differences were small. In nonautoclaved Nelson soil, 15 mg/g dry weight (ca 12.5 mM) of (−)-catechin had no significant effect on growth (Fig. 11A).
Effects of different concentrations of (±)-catechin and 10 mg/g of soil (−)-catechin in autoclaved or nonautoclaved Jens soils planted with Idaho fescue immediately after amendment with the catechin (a) or 2 weeks after amendment (b). Measurements were taken 10 days after planting. Error bars are ±1 SE of the mean. Those means with letters over them are significantly different from the corresponding control at α = 0.05, using Duncan’s multiple range test. Those bars with an asterisk in them are significantly different from the same nonautoclaved soil treatments at α = 0.05, using Duncan’s multiple range test
Effects of different concentrations of (±)-catechin in autoclaved or nonautoclaved Nelson soils planted with Idaho fescue immediately after amendment with the catechin (a) or 2 weeks after amendment (b). Measurements were taken 10 days after planting. Error bars are ±1 SE of the mean. Those means with letters over them are significantly different from the corresponding control at α = 0.05, using Duncan’s multiple range test. Those bars with an asterisk in them are significantly different from the same nonautoclaved soil treatments at α = 0.05, using Duncan’s multiple range test
We considered the possibility that, over a longer time period, catechin could be converted to a more toxic compound by soil microflora, so we incubated the catechin in autoclaved or nonautoclaved moist soil for 2 weeks before planting Idaho fescue and then repeated the experiment (Figs. 11b and 12b). The relative effects of racemic catechin were similar to results from soil that had not been preincubated, but plants grew better in autoclaved than nonautoclaved soil after this 2-week incubation period in nonamended soils and those with less than 3 and 30 mg of catechin/g of soil (ca 2.5 and 25 mM, respectively) in Jens and Nelson, respectively. Idaho fescue grown in (−)-catechin-augmented Nelson soil for 12 days was stunted by 10 and 30 mg/g of soil dry weight (ca 8.33 and 25 mM, respectively; Fig. 13), but the plants were still green and turgid.
Finally, we showed that (−)-catechin is apparently as active as an antioxidant as (+)-catechin, (−)-epicatechin, and quercitin, all known to be antioxidants (Fig. 14).
Antioxidant activity of 1 (−)- epicatechin, 2 (−)-catechin, 3 (+)-catechin, 4 quercetin, 5 mannitol. a Plate was sprayed with anisaldehyde TLC spray reagent to visualize the compounds. b Plate was sprayed with 0.2% DPPH in methanol and allowed to stand for 30 min. Yellow spots on the purple background show the presence of antioxidant activity. c Plate was exposed to iodine vapor
Discussion
Our findings indicate that (+)- and (−)-catechin are not highly phytotoxic compounds in either soil-free or soil studies. Their level of phytotoxicity is much lower than that associated with many other natural phytotoxins that we have assayed in identical soil-free bioassays, such as 2β-angeloyloxy-10β-hydroxyfuranoeremophilane from the Eurasian plant Ligularia macrophylla, the chaparrinone-type quassinoids from Ailanthus spp., or the triketone leptospermone from Leptospermum scoparium with an IC50 values between 1 and 4 μM for duckweed or lettuce (Dayan et al. 1999, 2007; Cantrell et al. 2007). With respect to the novelty of the compounds, catechin is a fairly common compound, being found in plant species native to the Americas (e.g., cacao), as well as the rest of the world (e.g., tea, lentils, grapes) for which no allelopathic properties have been claimed.
The recent literature states widely varying phytotoxicity results for catechins. For example, Weir et al. (2003) report 100% mortality of Idaho fescue at 50 ppm (ca 0.17 mM), while Blair et al. (2005) only report reduced growth of this same species at 1,000 ppm (ca 3.1 mM). We found 3.3 mM (±)-catechin to have no effect on root growth of lettuce, and Tharayil et al. (2008) reported no effect of (±)-catechin on lettuce growth up to the limits of its solubility. However, Buta and Lusby (1986) reported lettuce root growth reduction of about 70% by 1 mM (+)-catechin. Some of these disparities could be due to differences in growing conditions, method of treatment, and/or biotype or variety assayed. The bioassay conditions of Buta and Lusby (1986) were not provided, making explanation of differences in results impossible. We found catechins to stunt roots of A. thaliana grown continuously on agar with concentrations greater than 0.35 mM (100 μg/mL; Fig. 4). Bais et al. (2002) found 0.17 mM (+)-catechin to have little or no effect on A. thaliana root growth, whereas (−)-catechin was a strong inhibitor. Simões et al. (2008) found (+)-catechin to strongly inhibit root growth at concentrations above 0.35 mM when the plants were transferred into a liquid culture containing (+)-catechin that was shaken. In a similar experiment to those of Bais et al. (2002) and Simões et al. (2008), we found 1 mM catechin of either enantiomer to reduce A. thaliana root growth (Fig. 1), but the effects were temporary if the catechin were removed. In this experiment, the two enantiomers had similar activity, although (−)-catechin was slightly more active. In an erratum to the Bais et al. (2002) paper, the authors reported that (−)-catechin was ∼1.5 to twofold more active than the positive (+) enantiomer. Our results do not support such a difference in activity.
More recently, Broeckling and Vivanco (2008) state that catechin’s instability and ability to form complexes in soil confound the understanding of this compound as an allelochemical. The presence of other phenolic compounds in soil increases catechin’s half-life, but it can also form a short-lived procyanidin dimer in soil, which reduces persistence and phytotoxicity of the compound (Tharayil et al. 2008). This latter paper also found (±)-catechin to be essentially nonphytotoxic to both monocotyledonous and dicotyledonous plants, even though they observed similar root browning to that attributed to necrosis by Bais et al. (2003). Tharayil et al. (2008) attributed the root browning to enzyme-mediated (peroxidases or polyphenol oxidase) polymerization of catechin to brown polymers, an effect that should not contribute to toxicity. We found root browning to be somewhat reversible (Fig. 2).
Errata were published to the papers by Veluri et al. (2004), Weir et al. (2003), and Bais et al. (2002), in which the claims of differential phytotoxicity of (−) and (+) catechin and the degree of phytotoxicity of (−)-catechin were reduced, as well as the levels of catechin produced by individual plants. The same laboratory (Prithiviraj et al. 2007) then reported that low doses of catechin can stimulate growth of A. thaliana. They attributed this effect to induction of low levels of reactive oxygen species (ROS). Stimulatory effects of nontoxic doses of phytotoxins (hormesis) are common (Duke et al. 2006). Prithiviraj et al. (2007) also reported that these doses induced pathogen resistance, an effect observed with low doses of other chemical inducers of ROS (Duke et al. 2007).
In one of the original catechin papers (Bais et al. 2003), the mechanism of action of (−)-catechin was linked to induction of ROS. Weir et al. (2006) produced evidence that oxalate, whether produced by a plant or present in the soil, blocks the phytotoxic activity of (−)-catechin by preventing production of ROS. That ROS is involved in the mode of action of catechins as a phytotoxin is debatable because catechins are well-known antioxidants (e.g., Almajano et al. 2007). In fact, more than 1,500 papers mention catechin as an antioxidant (SciFinder® search, as of October, 2008). We are unaware of any papers that show that (−)-catechin is an antioxidant as we have (Fig. 14), but chirality should not influence this type of activity. Antioxidants quench ROS, rather than producing them.
In soil, we found the activity of catechins to be even less than in soil-free systems, with little or no effects at theoretical concentrations in soil water as high as almost 10 mM. This approximation is probably low, as the molarity in soil would be expected to increase with time as the soil dries, provided the catechin does not degrade. At higher levels, the plants were stunted, but not killed. No one has suggested that spotted knapweed generates levels of catechin in soils that would approach 10 mM. Such high concentrations could cause growth reduction by reducing water potential. The results with regard to catechin were the same in autoclaved soils, suggesting that there is no microbial bioactivation or inactivation. Catechin is unstable in soil, especially at high pH (Furubayashi et al. 2007). Inderjit et al. (2008) also noted that (±)-catechin degrades rapidly in soil, so that the level of catechin available to cause a biological effect may be much smaller than what is actually applied. The same could be said of catechin in solution. If catechin is the causal agent of any effect, its instability would contribute to its very weak effects. Inderjit et al. (2008) found catechin to be more phytotoxic in sand or soil with organic matter mixed in it than in soil without organic matter, and they considered junegrass to be highly sensitive to (±)-catechin in soils from Montana, Romania, and India. This conclusion is in marked conflict with our results with this species. In their study, water stress increased the phytotoxicity of (±)-catechin.
In a field situation, catechin is likely to be found in combination with other potential allelochemicals. Tharayil et al. (2006, 2008) found that the individual half-lives of such compounds in soil are extended by the presence of similar compounds. Synergism of simple phenolic compounds in causing phytotoxicity in the absence of soil has not been found in carefully conducted studies (e.g., Duke et al. 1983; Gerig et al. 1989; Jia et al. 2006).
In summary, our results do not support the view that either enantiomer of catechin is involved in allelopathy of spotted knapweed. Our findings, and the fact that catechin is found in many plant species throughout the world, casts doubt on the concept that catechin is a novel weapon that enables spotted knapweed in North America to succeed against native plant species. Nevertheless, the finding by Ridenour and Callaway (2001), in greenhouse carbon addition experiments, that spotted knapweed may cause allelopathic effects on native species through an allelochemical calls for further study of this phenomenon. Rigorous bioassay-directed isolation of the responsible compound(s) might solve this mystery.
References
Almajano, M. P., Delgado, M. E., and Gordon, M. H. 2007. Albumin causes a synergistic increase in the antioxidant activity of green tea catechins in oil-in-water emulsions. Food Chem 102:1375–1382.
Arnon, D. I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15.
Baerson, S. R., Sánchez-Moreiras, A., Pedrol-Bonjoch, N., Schulz, I. A., Kagan, A. K., Reigosa, M. J., and Duke, S. O. 2005. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one (BOA). J. Biol. Chem 280:21867–21881.
Bais, H. P., Walker, T. S., Stermitz, F. R., Hufbauer, R. A., and Vivanco, J. M. 2002. Enantiomeric-dependent phytotoxic and anti-microbial activity of (±)-catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol 128:1173–1179.
Bais, H. P., Vepachedu, R., Gilroy, S., Callaway, R. M., and Vivanco, J. M. 2003. Allelopathy and exotic plant invasion: from molecules and genes to species interaction. Science 301:1377–1380.
Blair, A. C., Hanson, B. D., Brunk, G. R., Marrs, R. A., Westra, P., Nissen, S. J., and Hufbauer, R. A. 2005. New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol. Lett 8:1039–1047.
Blair, A. C., Nissen, S. J., Brunk, G. R., and Hufbauer, R. A. 2006. A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in Centaurea maculosa invasion process. J. Chem. Ecol 32:2327–2331.
Blair, A. C., Weston, L. A., Nissen, S. J., Brunk, G. R., and Hufbauer, R. 2009. The importance of analytical techniques in allelopathy studies with the reported allelochemical catechin as an example. Biol. Invasions (in press).
Broeckling, C. D., and Vivanco, J. M. 2008. A selective, sensitive, and rapid in-field assay for soil catechin, an allelochemical of Centaurea maculosa. Soil Biol. Biochem 40:1189–1196.
Buta, J. G., and Lusby, W. R. 1986. Catechins as germination and growth inhibitors in Lespediza seeds. Phytochemistry 25:93–95.
Callaway, R. M., and Ridenour, W. M. 2004. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ 2:436–443.
Callaway, R. M., Ridenour, W. M., Laboski, T., Weir, T., and Vivanco, J. M. 2005. Natural selection for resistance to the allelopathic effects of invasive plants. J. Ecol 93:576–583.
Cantrell, C. L., Duke, S. O., Fronczek, F. R., Osbrink, W. L. A., Mamonov, L. K., Vassilyev, J. I., Wedge, D. E., and Dayan, F. E. 2007. Phytotoxic eremophilanes from Ligularia macrophylla. J. Agric. Food Chem 55:10656–10663.
Cespedes, C. L., Hoeneisen, M., Bittner, M., Beccerra, J., and Silva, M. 2001. Comparative study of ovatifolin antioxidant and growth inhibition activities. J. Agric. Food Chem 49:4243–4251.
Cheng, H. H. 1995. Characterization of the mechanisms of allelopathy: modeling and experimental approaches. Amer. Chem. Soc. Symp. Ser 582:132–141.
Courbat, P., Weith, A., Albert, A., and Pelter, A. 1977. Contribution to the study of the behavior of catechin in alkaline medium. Helv. Chim. Acta 60:1665–1675.
D’Abrosca, B., Della Greca, M., Fiorentino, A., Isidori, M., Manaco, P., and Pacifico, S. 2006. Chemical constituents of the aquatic plant Schoenoplectus lacustris: evaluation of phytotoxic effects on the green alga Selanastrum capricornutum. J. Chem. Ecol 32:81–96.
Dayan, F. E., Duke, S. O., Sauldubois, A., Singh, N., McCurdy, C., and Cantrell, C. L. 2007. P-Hydroxyphenylpyruvate dioxygenase is a herbicidal target site for β-triketones from Leptospermum scoparium. Phytochemistry 68:2004–2014.
Dayan, F. E., Romagni, J. G., and Duke, S. O. 2000. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol 26:2079–2094.
Dayan, F. E., Watson, S. B., Galindo, J. C. G., Hernández, A., Dou, J., McChesney, J. D., and Duke, S. O. 1999. Phytotoxicity of quassinoids: physiological responses and structural requirements. Pestic. Biochem. Physiol 65:15–24.
Donovan, J. L., Crespy, V., Oliveira, M., Cooper, K. A., Gibson, B. B., and Williamson, G. 2006. (+)-Catechin is more bioavailable than (−)-catechin: relevance to the bioavailability of catechin from cocoa. Free Radical Res 40:1029–1034.
Duke, S. O., Williams, R. D., and Markhart, A. H. 1983. Interaction of moisture stress and three phenolic compounds on lettuce seed germination. Ann. Bot 52:923–926.
Duke, S. O., Cedergreen, N., Velini, E. D., and Belz, R. G. 2006. Hormesis: Is it an important factor in herbicide use and allelopathy? Outlooks on Pest Manag 17:29–33.
Duke, S. O., Wedge, D. E., Cerdeira, A. L., and Matallo, M. B. 2007. Interactions of synthetic herbicides with plant disease and microbial herbicides. pp 277–296 in Vurro M, Gressel J (eds) Novel Biotechnologies for Biocontrol Agent Enhancement and Management. Springer, Dordrecht, The Netherlands.
Furubayashi, A., Hiradate, S., and Fujii, Y. 2007. Role of catechol structure in the adsorption and transformation reactions of l-DOPA in soils. J. Chem. Ecol 33:239–250.
Gamborg, O. L., Miller, R. A., and Ojima, K. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res 50:151–158.
Gerig, T. M., Blum, U., and Meier, K. 1889. Statistical analysis of the joint inhibitory action of similar compounds. J. Chem. Ecol 15:2403–2412.
Hashida, K., Ohara, S., and Makino, R. 2003. Base-catalyzed reactions of (−)-epicatechin: Formation of enantiomers of base-catalyzed reaction products from (+)-catechin. J. Wood Chem. Technol 23:227–232.
Hiscox, J. D., and Israelstam, G. F. 1980. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot 57:1332–1334.
Ho, Y., Lee, Y. L., and Hsu, K. Y. 1995. Determination of (+)-catechin in plasma by high-performance liquid-chromatography using fluorescence detection. J. Chromatog. B- Biomed. Appl 665:383–389.
Hollman, P. C. H., van Trijp, J. M. P., Buysman, M. N. C. P., Gaag, M. S. v. d., Mengelers, M. J. B., de Vries, J. H. M., and Katan, M. B. 1997. Relative bioavailability of the antioxidant flavanoid quercetin from various foods in man. FEBS Lett 418:152–156.
Inderjit, C. R. M., and Vivanco, J. M. 2006. Can plant biochemistry contribute to understanding of invasion ecology? Trends Plant Sci 11:574–580.
Inderjit, P. J. L., Callaway, R. M., and Hoben, W. 2008. Phytotoxic effects of (±)-catechin In vitro, in soil, and in the field. PLoS ONE 3(7): e2536. doi:10.1371/journal.pone.0002536.
Jia, C., Kudsk, P., and Mathiassen, S. K. 2006. Joint action of benzoxazinone derivatives and phenolic acids. J. Agric. Food Chem 54:1049–1057.
Kiatgrajai, P., Wellons, J. D., Gollob, L., and White, J. D. 1982. Kinetics of epimerization of (+)-catechin and its rearrangement to catechinic acid. J. Org. Chem 47:2910–2912.
Labrouche, F., Clark, A. C., Prenzler, P. D., and Scollary, G. R. 2005. Isomeric influence on the oxidative coloration of phenolic compounds in a model white wine: comparison of (+)-catechin and (–)-epicatechin. J.Agric. Food Chem 53:9993–9998.
Matsumoto, H., and Duke, S. O. 1990. Acifluorfen-methyl effects on porphyrin synthesis in intact Lemna pausicostata Hegelm. 6746 plants. J. Agric. Food Chem 38:2066–2071.
Michel, A., Johnson, R. D., Duke, S. O., and Scheffler, B. E. 2004. Dose-response relationships between herbicides with different modes of action and growth of Lemna paucicostata—an improved ecotoxicological method. Environ. Toxicol. Chem 23:1074–1079.
Murashigi, T., and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15:473–497.
Perry, G. L., Thelen, G. C., Ridenour, W. M., Weir, T. L., Callaway, R. M., Pashke, M. W., and Vivanco, J. M. 2005. Dual role for an allelochemical: (±)-catechin from Centauria maculosa root exudates regulates conspecific seedling establishment. J. Ecol 93:1126–1135.
Perry, G. L., Thelen, G. C., Ridenour, W. M., Callaway, R. M., Paschke, M. V., and Vivanco, J. M. 2007. Concentrations of the allelochemical (±)-catechin in Centaurea maculosa soils. J. Chem. Ecol 33:2337–2344.
Prithiviraj, B., Perry, G. L., Badri, D. V., and Vivanco, J. M. 2007. Chemical facilitation and induced pathogen resistance mediated by root-secreted phytotoxin. New Phytol 173:852–860.
Ridenour, W. M., and Callaway, R. M. 2001. The relative importance of allelopathy in interference: the effects of an invasive weed on a native bunchgrass. Oecologia 126:444–450.
Ritz, C., and Streibig, J. C. 2005. Bioassay Analysis using R. Journal of Statistical Software 12, 1-22. URL http://www.bioassay.dk.
R Development Core Team, 2005. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www. R-project.org. ISBN 3-900051-07-0.
Romeo, J. T. 2000. Raising the beam: moving beyond phytotoxicity. J. Chem. Ecol 26:2011–2014.
Simões, K., Du, J., Kretzschmar, F. S., Broekling, C. D., Stermitz, F. S., Vivanco, J. M., and Braga, M. R. 2008. Phytotoxic catechin leached by seeds of the tropical weed Sesbania virgata. J. Chem. Ecol 34:681–687.
Tharayil, N., Bhowmik, P. C., and Xing, B. 2006. Preferential sorption of phenolic phytotoxins to soil: implications for altering the availability of allelochemicals. J. Agric. Food Chem 54:3033–3040.
Tharayil, N., Bhowmik, P. C., and Xing, B. 2008. Bioavailability of allelochemicals as affected by companion compounds in soil matrices. J. Agric. Food Chem 56:3706–3713.
Thelen, C. C., Vivanco, J. M., Newingham, B., Good, W., Bais, H. P., Landres, P., Caesar, A., and Callaway, R. M. 2005. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol. Lett 8:209–217.
Veluri, R., Weir, T. L., Bais, H. P., Stermitz, F. R., and Vivanco, J. M. 2004. Phytotoxic and antimicrobial activities of catechin derivatives. J. Agric. Food Chem 52:1077–1082.
Weir, T. L., Bais, H. P., and Vivanco, J. M. 2003. Intraspecific and interspecific interactions mediated by a phytotoxin (−)-catechin, secreted by the roots of. Centauria maculosa (spotted knapweed). J. Chem. Ecol 29:2397–2412.
Weir, T. L., Bais, H. P., Stull, V. J., Callaway, R. M., Thelen, G. C., Redenhour, W. M., Bhamidi, S., Stermitz, F. R., and Vivanco, J. M. 2006. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta 223:785–795.
Weston, L. A., and Duke, S. O. 2003. Weed and crop allelopathy. Crit. Rev. Plant Sci 22:367–389.
Zhang, J., Stanley, R. A., Adaim, A., Melton, L. D., and Skinner, M. A. 2008. Free radical scavenging and cytoprotective activities of phenolic antioxidants. Molec. Nutrition Food Res 50:996–1005.
Acknowledgment
We thank C. Duncan for soil collections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duke, S.O., Blair, A.C., Dayan, F.E. et al. Is (−)-Catechin a Novel Weapon of Spotted Knapweed (Centaurea stoebe)?. J Chem Ecol 35, 141–153 (2009). https://doi.org/10.1007/s10886-008-9587-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9587-z