Abstract
The allelopathic potential of the Eurasian invasive plant Alliaria petiolata has been well documented, with the bulk of the effects believed to be mediated by arbuscular mycorrhizal fungi (AMF). We exposed the herbaceous annual Impatiens pallida, which is native to North America, to fractionated A. petiolata extracts at four developmental stages (germination, presymbiosis growth, symbiosis formation, and symbiosis growth) by using exposure levels expected to be similar to field levels. Surprisingly, we found strong direct effects on I. pallida germination and growth, but no indirect effects on I. pallida growth mediated by AMF. We also observed strong synergistic effects with a complete A. petiolata extract that inhibited I. pallida germination and presymbiosis root growth more than either a glucosinolate or flavonoid enriched fraction alone. In fact, the flavonoid enriched fraction tended to stimulate germination and presymbiosis root growth. In contrast to these strong direct effects, I. pallida plant growth during both the symbiosis formation and symbiosis growth phases was unaffected by A. petiolata extracts. We also found no inhibition of AMF colonization of roots or soils by A. petiolata extracts. We show that AMF can actually ameliorate allelopathic effects of an invasive plant, and suggest that previously observed allelopathic effects of A. petiolata may be due to direct inhibition of plant and fungal growth before symbiosis formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allelopathy has historically been defined as direct inhibition of plant growth by organic compounds produced by a donor plant (Choesin and Boerner 1991; Barkosky et al. 1999). These compounds act through several mechanisms that include causing DNA mutations (Hashimoto et al. 1984; Hashimoto and Shudo 1996; Wu et al. 2000), blocking photosynthesis (Einhellig et al. 1993; Gonzalez and Estevez-Braun 1997), and/or triggering programmed cell death (Bais et al. 2003). While these mechanisms clearly act directly on the target plants, many potential mechanisms of indirect inhibition exist as well (Inderjit and Weiner 2001). For example, compounds released by a donor plant can influence nutrient availability in a way that is more beneficial for the donor plant (Inderjit and Mallik 1999). Also, microbial populations supported by a donor plant can selectively inhibit growth of surrounding plants (Kaminsky 1981), or microbes that benefit surrounding plants can be inhibited by a donor plant (Stinson et al. 2006). Such beneficial microbes include the arbuscular mycorrhizal fungi (AMF), with which more than 80% of plants surveyed associate (Smith and Read 2008). AMF are obligately symbiotic and provide their host plants with mineral nutrients and water in exchange for photosynthate (Smith and Read 2008). Many plants depend on AMF for normal growth, and any inhibition of AMF, therefore, would also indirectly slow plant growth.
Alliaria petiolata (M. Bieb.) Cavara and Grande (garlic mustard), a Eurasian native that is invasive in North America, can inhibit seed germination and growth of native plant species, and AMF and ectomycorrhizal germination and growth (Vaughn and Berhow 1999; Roberts and Anderson 2001; Prati and Bossdorf 2004; Stinson et al. 2006; Callaway et al. 2008; Wolfe et al. 2008; Barto and Cipollini 2009b), although inhibition does not always occur (McCarthy and Hanson 1998; Cipollini et al. 2008; Barto and Cipollini 2009a). Many of these results are based on seed and spore germination assays, or exposures that began before seed and spore germination. Stinson et al. (2006) observed reductions in colonization of woody species exposed to A. petiolata during the symbiosis formation phase, but a herbaceous plant exposed during the symbiosis formation phase was unaffected (Barto and Cipollini 2009a). Allelopathic effects of A. petiolata have been attributed to glucosinolates and isothiocyanates (Vaughn and Berhow 1999), but extracts enriched in alliarinoside and several flavonoid glycosides, known anti-herbivory compounds from A. petiolata (Haribal and Renwick 1998; Haribal et al. 2001), reduced AMF spore viability more than a glucosinolate enriched fraction (Callaway et al. 2008). Plant responses to alliarinoside and the flavonoid glycosides before symbiosis formation have not yet been determined.
We explored the effects of A. petiolata extracts on the four major developmental stages of a plant-mycorrhizae association (1. germination, 2. presymbiosis growth, 3. symbiosis formation, and 4. symbiosis growth), each in a separate experiment. The germination phase includes any stratification requirement up to and including emergence of the radicle from the seed. Growth of the plant before colonization by AMF is included in the presymbiosis growth phase. Although the non-mycorrhizal condition is usually rare in the field, sites with a long invasion history are likely to have low AMF colonization potential (Roberts and Anderson 2001), and this experiment demonstrates how well a re-introduced native grows when exposed to A. petiolata extracts without the benefit of AMF. During the symbiosis formation phase, contact between AMF and the plant is initiated and fungal structures begin to form inside the plant root. Allelopathic compounds could interfere with signaling between host plants and AMF, thereby limiting the formation of the symbiosis. Finally, the symbiosis continues to operate throughout the remainder of the plant’s lifetime in the symbiosis growth phase. Once a symbiosis is formed, fungal structures inside the root are likely to be somewhat insulated from allelopathic effects. However, fungal hyphae in the soil will still be exposed, and limited growth of fungal hyphae would compromise the ability of the fungus to absorb water and nutrients for its plant host. Plants can reject such parasitic associations (Smith and Read 2008), killing the fungus, but also slowing plant growth to less than that found in a mutualistic association.
The objectives of this experiment were to assess the effects of glucosinolate and flavonoid glycoside enriched fractions of A. petiolata, alone and in concert, on growth of a North American native plant and its associated AMF. These effects were assessed across multiple life stages in order to determine the importance of AMF in mediating any observed allelopathic effects. We expected glucosinolate and flavonoid enriched fractions to inhibit growth of I. pallida, primarily indirectly through inhibition of AMF in later life stages.
Methods and Materials
Extracts were prepared from first year A. petiolata plants randomly collected from the Wright State University Nature Preserve (39o48.0′N, 84o1.0′W) during late Spring, when the establishment of mycorrhizal symbioses with host plants is occurring actively. Plant tissues, leaves and roots, either were extracted immediately after collection, or flash frozen and stored at −20°C to maintain a standard lot of material for extract preparation. It is unclear whether secondary metabolites produced by A. petiolata enter the environment as root exudates, leaf leachates, or both, so we used both leaves and roots to prepare our extracts. Glucosinolates were separated from the flavonoid glycosides by using a butanol/water fractionation as described in Callaway et al. (2008), yielding glucosinolate enriched and flavonoid enriched fractions. The glucosinolates and flavonoids were by far the most abundant compounds in their respective fractions, but it was not our intention to purify these compounds because we wanted the combined treatment of glucosinolate and flavonoid enriched fractions to represent the complete A. petiolata phytochemical profile. All experiments were dosed at a rate equivalent to 3.3 mg A. petiolata tissue equivalents per g assay media, a dose that was estimated by assuming equivalent transfer rates of glucosinolates from plants to soils for A. petiolata as reported for Brassicaceae used as biofumigants (Callaway et al. 2008).
Impatiens pallida Nutt. (pale jewelweed) was chosen as the target plant because it grows in the same woodland habitats invaded by A. petiolata and is dependent on AMF for normal growth (K Barto, personal observation). While AMF generally are not thought to be host specific, certain plant-fungus associations are more effective than others (Stampe and Daehler 2003; Johnson et al. 2004), so we used naturally associated AMF of I. pallida. Mycorrhizal inoculum consisted of finely chopped I. pallida roots collected from a population in Yellow Springs, Ohio (39 o47.0′N, 83 o52.5′W). Impatiens pallida seed were collected from the same population for all experiments. Inoculum was prepared in the fall and stored at 5°C until needed.
The seed germination experiment was conducted in small glass dishes (9 cm diam), which were stored at 3°C throughout the experiment. The presymbiosis growth experiment and the symbiosis formation and symbiosis growth experiments were conducted in root-viewing chambers made of 13 × 30 × 0.15 cm glass plates held ½ cm apart by silicon on 3 sides (Friese and Allen 1991). All experimental chambers, glass dishes, and root-viewing chambers, were filled with a 1:1 mix of field soil and sterile coarse sand or the same mix with activated carbon (AC) added at a rate of 20 ml/l soil, as has been used in other experiments with A. petiolata (Prati and Bossdorf 2004). Activated carbon sorbs organic compounds and serves as an additional experimental control to verify that effects are due to allelochemicals (Inderjit and Callaway 2003). Field soil, collected from an A. petiolata free area of the Wright State University Nature Preserve, has a high proportion of clay, and forms impenetrable bricks in the chambers without the addition of sand (K. Barto, personal observation). Root-viewing chambers were covered with foil and stored at a slight angle to encourage root and hyphal growth along one glass plate (Friese and Allen 1991). With the exception of the seed germination experiment, all chambers were placed under fluorescent grow-lights (130 µmol PAR/m2/sec) at ∼22°C in a growth room. Four separate experiments were conducted, examining one developmental stage per experiment.
Germination
We examined the effects of treatments on germination by concurrently adding test fractions and ungerminated seeds to glass dishes. Seed germination was assessed by placing 5 I. pallida seeds on the soil surface, and applying doses every other week. Dishes were covered but not sealed to allow oxygen to circulate. Germination was scored weekly for 6 mo. We used 4 extracts (glucosinolate enriched fraction, flavonoid glycoside enriched fraction, combined fraction, and water control) crossed with 2 activated carbon treatments (with or without). There were 5 replicate chambers for each treatment combination. Final percent germination was analyzed using ANOVA with activated carbon and extract as fixed factors, followed by Tukey’s HSD test where indicated. Data did not need to be transformed to meet assumptions of normality and homogeneity of variances, and were analyzed using R 2.7.1 (R Development Core Team 2008).
Presymbiosis Plant Growth
We examined the presymbiosis growth stage by simultaneously adding test fractions and germinated seeds to root-viewing chambers. Impatiens pallida seeds were germinated in sterile water at 3°C (Leck 1979). Preliminary experiments showed that seedlings quickly became colonized with AMF in field soils, so chambers were treated with 190 mg/l of the fungicide chlorothalonil (Daconil®, Syngenta Crop Protection, Inc, Greensboro, NC, USA) every other day to maintain the uncolonized status of seedlings in this experiment. Chlorothalonil can inhibit plant growth at high does, so we conducted preliminary experiments and determined that this dosage schedule would block mycorrhizal colonization of seedlings without directly impacting I. pallida growth (K. Barto, unpublished data). Presymbiosis seedling growth was assessed by placing one newly germinated I. pallida seed on the soil surface while adding test compounds. Doses were applied once a week throughout the experiment by injecting 5 ml of extract into the chamber through each of three injection sites, located 7.5, 15, and 23 cm from the top of the chamber, along the 30.5 cm side. This volume was sufficient to wet the entire soil volume without forcing water out of the base of the chamber. The roots were traced onto transparency film and digitized every 3 d until a root reached the bottom of the chamber.
Total root length and area of the root system were quantified using ImageJ (NIH: http://rsb.info.nih.gov/ij/). We also measured the box-counting fractal dimension (FD), which quantifies exploration efficiency of the system independently of rhizosphere size (Walk et al. 2004), using the FracLac plugin for ImageJ (http://rsb.info.nih.gov/ij/plugins/frac-lac.html) (Barto and Cipollini 2009b). Height of the aboveground portion of I. pallida also was recorded once a week. Many plants died before the end of the experiment, so we also recorded life span. Growth rates for plant height, root length, and rhizosphere area, along with the box-counting fractal dimension of the root system and the life span of the plants, were analyzed using PROC GLM and ANOVA with activated carbon and extract as fixed factors followed by Tukey’s HSD test where indicated. Data were transformed as necessary to meet assumptions of ANOVA and analyzed with SAS Version 9.1 (SAS Institute Inc., Cary, NC, USA).
Symbiosis Formation
We examined the symbiosis formation stage by injecting compounds into inoculated root-viewing chambers with one growing I. pallida seedling. Impatiens pallida seeds were stratified in water at 3°C to stimulate germination, then planted in chambers. Mycorrhizal inocula, which consisted of chopped roots of field-collected I. pallida, was mixed with the soil at a rate of 1 g inocula/100 g soil before filling chambers. Injections began as soon as seeds were added, and doses were applied once a week throughout the experiment. Mycorrhizal structures were identified non-destructively in the chambers by their fluorescence after excitation with 460 nm light (Friese and Allen 1991). Observations were made with an Eclipse TE 2000-S microscope with a B-2E/C filter cube.
Chambers were observed along horizontal 6 cm transect lines centered at the middle of the chamber and spaced vertically 5 cm apart. The first transect line was 2.5 cm below the top of the chamber, and additional lines were observed as the roots grew down through the chamber. In order to quantify AMF abundance inside roots, we scanned along each transect line and scored uncolonized and colonized roots. Then, we calculated the percentage of colonized roots observed in each chamber. In order to quantify AMF abundance outside of plant roots, we also scanned along each transect line, but due to time constraints we could not quantify hyphal abundance along the entire transect line. Instead, in every third field of view that did not contain a root, we scored for presence of absence of AMF hyphae. Only non-septate hyphae were counted as AMF. Then we calculated the percentage of fields of view containing AMF hyphae.
Height of the above ground portion of I. pallida also was recorded each week. Plants were harvested at the end of the experiment, dried at 30°C to constant mass, and root and shoot dry mass were measured. There were 4 treatments (glucosinolate enriched fraction, flavonoid glycoside enriched fraction, combined fraction, and water control) for each of two carbon amendments (with or without). There were ten replicate chambers per treatment combination yielding 80 chambers total. Data were analyzed as described for the presymbiosis growth experiment, excluding life span. Also, dry mass of the root, shoot, and the root to shoot ratio were analyzed by ANOVA with activated carbon and extract as fixed factors followed by Tukey’s HSD test where indicated. Data did not need to be transformed and were analyzed with SAS Version 9.1 (SAS Institute Inc., Cary, NC, USA). In addition, percent colonization of the root and soil (untransformed) were analyzed using a repeated measures ANOVA with activated carbon and extract as fixed factors using STATISTICA Version 8.0 (StatSoft, Inc.).
Symbiosis Growth
We examined the symbiosis growth stage by injecting compounds into chambers with already established symbioses between I. pallida and its associated mycorrhizal fungi. Established symbioses were generated by growing I. pallida seedlings in root-viewing chambers in field soil containing mycorrhizal inoculum. When seedlings were 4 wk old, colonization was verified by fluorescence microscopy before beginning injections of compounds. Injections were repeated weekly thereafter until the end of the experiment. Fungal development characteristics were monitored as in the symbiosis formation experiment. Plates were monitored weekly with observations beginning when plants were 3 wk old. Plants were harvested at the end of the experiment, dried at 30°C to constant mass, and root and shoot dry mass were measured. There were 4 treatments (glucosinolate enriched fraction, flavonoid glycoside enriched fraction, combined fraction, and water control) for each of 2 carbon amendments (with or without). There were 10 replicate chambers per treatment combination yielding 80 chambers total. Growth rate for plant height, percent colonization of roots and soil, dry mass of the root, shoot, and the root to shoot ratio were analyzed as for the symbiosis formation experiment.
Results
Germination
Impatiens pallida seeds began to germinate after 20 weeks of stratification, and no additional seed germinated after 24 weeks. Exposure to a flavonoid or glucosinolate enriched fraction alone had no significant effect on germination. However, germination was lower in dishes dosed with a combined extract than either control dishes or those receiving a flavonoid enriched fraction (Fig. 1, F3,32 = 3.71, P = 0.021). Activated carbon did not affect germination rates (F1,32 = 0.08, P = 0.779), and there was no interaction between AC and extract (F3,32 = 1.36, P = 0.273).
Percent germination of Impatiens pallida seeds exposed to Allairia petiolata extracts during stratification, means ± 1 SE. Control—Chambers treated with water only. Flav—Chambers treated with a flavonoid enriched fraction. Gluc—Chambers treated with the glucosinolate enriched fraction. Combined—Chambers treated with both flavonoid and glucosinolate enriched fractions. with AC—Chambers contained activated carbon. no AC—Chambers did not contain AC. (N = 5)
Presymbiosis Growth
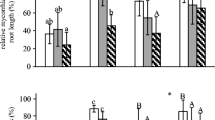
Impatiens pallida plants in chambers with AC grew faster than plants in chambers without AC (Fig. 2a, Table 1). Alliaria petiolata extracts had no effect on I. pallida height (Table 1). Total root length of I. pallida plants increased more quickly in plants grown in soil containing AC than in plants grown without AC (Fig. 2b, Table 1). Root growth varied with extract treatment, and only the combined extract significantly reduced root growth (Fig. 2b, Table 1). Since these plants were not yet colonized by AMF, these impacts represent direct effects of A. petiolata extracts on root growth. Patterns for growth of rhizosphere area followed those of root length (Table 1).
Responses of Impatiens pallida plants exposed to Alliaria petiolata extracts during the presymbiosis growth phase, means ± 1 SE. Control—Chambers treated with water only. Flav—Chambers treated with a flavonoid enriched fraction. Gluc—Chambers treated with the glucosinolate enriched fraction. Combined—Chambers treated with both flavonoid and glucosinolate enriched fractions. with AC—Chambers contained activated carbon. no AC—Chambers did not contain AC. Different letters indicate significant differences by Tukey HSD at α = 0.05. a Height growth rate (N = 9–10). b Root length growth rate (N = 10). c Box counting fractal dimension of the root system (N = 10). d Life span of I. pallida plants (N = 10)
Root structure of I. pallida plants also was affected by A. petiolata extracts. The planar box-counting fractal dimension, which quantifies the exploration efficiency of the root system, was significantly higher in chambers with AC than in chambers without (Fig. 2c, Table 1). As for root length and rhizosphere area, the fractal dimension was highest in chambers that received a flavonoid enriched fraction and lowest in chambers treated with a combined extract (Fig. 2c, Table 1). The interaction between AC and extract also was significant (Table 1), with AC increasing the fractal dimension significantly only in chambers dosed with a glucosinolate enriched fraction (Fig. 2c).
Impatiens pallida plants grown in soil with AC lived longer than plants in soil without AC (Fig. 2d; Table 1). The glucosinolate enriched fraction and the combined A. petiolata extract significantly shortened I. pallida life span, while a flavonoid enriched fraction had no effect (Fig. 2d; Table 1). This inhibition was seen only in chambers without AC (Fig. 2d; Table 1).
Symbiosis Formation
During the symbiosis formation phase, growth rates of I. pallida height, root, and rhizosphere area were unaffected by AC or A. petiolata extract (Table 2). Root structure of I. pallida plants was unaffected by AC (Table 2). However, regardless of AC treatment, root systems of plants exposed to a glucosinolate enriched fraction had significantly higher fractal dimensions (mean ± SE; 1.24 ± 0.015) than those exposed to a combined fraction (1.18 ± 0.020; Table 2). Root systems of control plants (1.21 ± 0.011) or those exposed to a flavonoid enriched fraction (1.19 ± 0.013) had fractal dimensions indistinguishable from either extreme. Shoot and root dry masses of plants at the end of the experiment, and the root to shoot ratio, were unaffected by AC or A. petiolata extracts (Table 2).
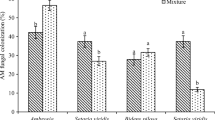
Arbuscular mycorrhizal colonization of I. pallida roots was higher in chambers containing AC than those without throughout the experiment, even though colonization declined with time in all chambers (Fig. 3a, Table 3). Hyphal abundance in the soil was unaffected by A. petiolata extract, but increased with time in chambers containing AC while remaining relatively constant in chambers without AC (Fig. 3b, Table 3).
Responses of Impatiens pallida plants exposed to Alliaria petiolata extracts during the symbiosis formation phase, means ± 1 SE. with AC—Chambers contained activated carbon. no AC—Chambers did not contain AC. a Percentage of root length colonized by AMF over time (N = 9–10). b Percentage of soil area containing AMF hyphae over time (N = 9–10)
Symbiosis Growth
During the symbiosis growth phase, plant height increased more quickly in chambers without AC (mean ± SE; 6.63 ± 0.42 cm/week) than in chambers with AC (5.41 ± 0.25 cm/week; Table 4). Growth rate was unaffected by A. petiolata extracts, and there was no interaction between AC and extract (Table 4). Since I. pallida were given a month in the chambers before dosing began, roots had filled the chambers to such an extent that it would have been extremely difficult to track further development. Root morphology data, therefore, was not collected.
Arbuscular mycorrhizal fungal colonization was higher in roots of plants grown in soils containing AC than in soils without (Fig. 4a, Table 5), but fewer hyphae were found in soils containing AC than in soils without (Fig. 4b, Table 5). Root colonization declined over time (Fig. 4a, Table 5). Root dry mass was higher in chambers without AC (mean ± SE; 0.19 ± 0.026 g) than in chambers with AC (0.15 ± 0.032 g), as was shoot dry mass (without AC: 0.11 ± 0.0076 g, with AC: 0.08 ± 0.0047 g; Table 4). Both were unaffected by A. petiolata extracts (Table 4). The root to shoot ratio was not affected by A. petiolata extracts (Table 4).
Responses of Impatiens pallida plants exposed to Alliaria petiolata extracts during the symbiosis growth phase, means ± 1 SE. with AC—Chambers contained activated carbon. no AC—Chambers did not contain AC. a Percentage of root length colonized by AMF over time (N = 8–10). b Percentage of soil area containing AMF hyphae over time (N = 8–10)
Discussion
As expected, glucosinolate and flavonoid enriched fractions from A. petiolata extracts inhibited the growth of I. pallida. Synergistic effects of the flavonoid and glucosinolate enriched fractions were observed in the germination and presymbiosis growth phases, but not in either symbiosis phase. The flavonoid-enriched fraction had no effect alone, but appeared to enhance the slight toxicity of the glucosinolate enriched fraction, leading to the very low germination or growth rates observed after exposure to a combined fraction. These effects likely are due to the glucosinolates and flavonoids themselves, given their abundance in the extracts, but it is possible that unidentified compounds in the extracts also contributed to effects. Synergistic interactions among allelopathic compounds also occur in Triticum, where phenolic compounds increase cell permeability in root tips, allowing greater uptake of mutagenic benzoxazinoids (Blum et al. 1992; Hashimoto and Shudo 1996). Synergistic mechanisms also operate in Desmodium and Sorghum, which block parasitism by Striga plants by concurrently stimulating germination of Striga seeds while inhibiting further root growth (Chang et al. 1986; Weston et al. 1989; Yoder 2001; Tsanuo et al. 2003). While the specific mechanism of action remains to be elucidated, synergistic effects among compounds produced by A. petiolata clearly limited plant and AMF growth during the early stages of development.
We frequently observed declines in root colonization over time, potentially because as the plants aged and their roots filled the soil volume, assistance from AMF became less necessary. Plants have some control over intraradical fungal growth (Smith and Read 2008) and could have limited fungal development as the plants became root bound. This is supported by the decline in root colonization values in chambers containing AC, as well as control chambers receiving only water. Soil colonization values remained relatively constant throughout the symbiosis growth phase, and even increased in chambers containing AC during the symbiosis formation phase, suggesting that extraradical fungal structures are less dependent on the age or status of the symbiosis than intraradical fungal structures. The plant likely has more control over intraradical fungal structures than extraradical, and the continued growth of extraradical structures may represent growth by the fungus away from its host in the face of decreasing support, via intraradical structures.
Activated carbon sorbs organic compounds and should rescue plants from allelopathic inhibition when incorporated into soils (Inderjit and Callaway 2003). Surprisingly, AC had no effect on germination. Extracts were added to test soils every other week since A. petiolata metabolites generally are not stable in non-sterile soils (Gimsing et al. 2006, 2007; Barto and Cipollini 2009c), but sinigrin has a half-life in excess of 120 d in non-sterile soil water (Tsao et al. 2000). Although low temperatures did not limit degradation of flavonoids (Barto and Cipollini 2009c), it is possible that compounds accumulated over the course of the six month experiment to levels that saturated the AC present in the soils. As expected, AC additions in the presymbiosis experiment rescued plants exposed to A. petiolata extracts. Plants exposed to extracts that were also growing in soils containing AC were not distinguishable from control plants receiving only water. Activated carbon effects during the symbiosis formation and symbiosis growth stages were less consistent, further suggesting that allelopathic inhibition was not as important in these life stages.
We found extensive evidence of inhibition of seed germination and presymbiosis plant growth in the absence of AMF. The lack of these non-AMF mediated inhibitory effects during the symbiosis formation and growth phases suggests that the AMF may actually be protecting the plant from the allelopathic compounds. This is not simply an age effect, where a larger plant is more able to resist inhibition than a small plant, because the presymbiosis and symbiosis formation experiments both began with germinated seed. The only difference was that the soil for the symbiosis formation experiment also contained AMF inocula with no previous exposure to A. petiolata.
In contrast to prior work that demonstrated inhibition of AMF by A. petiolata (Roberts and Anderson 2001; Stinson et al. 2006; Callaway et al. 2008; Barto and Cipollini 2009b), we found no evidence for impacts on AMF. However, earlier research monitored AMF that were not associated with a host plant (Roberts and Anderson 2001; Stinson et al. 2006; Callaway et al. 2008), or began dosing AMF in the presymbiosis phase (Barto and Cipollini 2009b), where we saw inhibition also. When exposures began in the symbiosis formation phase, Barto and Cipollini (2009a) did not see inhibition of I. pallida growth by the same A. petiolata extracts used here. Roberts and Anderson (2001) relied on spore and seed germination assays, and used a dose about eight times higher than that used in our study, so results cannot be compared directly. Stinson et al. (2006) used doses of A. petiolata extracts twice as high as those used here, which could account for the inhibition observed in that study. Ideally, allelopathy studies should use exposure levels equivalent to those found in the field. However, A. petiolata metabolites degrade rapidly, and rarely reach detectable levels in natural soils (Barto and Cipollini 2009c). This likely means that the actual bioactive compounds are degradates of the compounds produced by the plant. We, thus, used doses estimated to be realistic field levels (Callaway et al. 2008). Soil levels under naturally occurring plants likely are even lower than our estimated values, and any impacts not seen at these doses are unlikely to be important in the field.
Stinson et al. (2006) also observed reduced AMF colonization of tree seedlings in conditioned soils, which could be a legacy effect of very low inoculum potential due to direct suppression of spore viability and germination (Roberts and Anderson 2001; Stinson et al. 2006; Callaway et al. 2008), rather than allelopathic inhibition of colonization in the plant. Callaway et al. (2008) used a soil conditioning approach, and found reduced AMF spore viability and infectivity, and reduced emergence of plant seedlings in soil conditioned by A. petiolata. However, all of these endpoints occur before the symbiosis is established. The final biomass of plants grown in conditioned soil was lower than that of plants grown in control soil, suggesting that even after the beneficial mycorrhizal symbiosis becomes established, the direct inhibition incurred during early life stages cannot be overcome.
We suggest that observed allelopathic inhibition by A. petiolata is due to legacy effects of direct inhibition of plant and fungal partners before the symbiosis is established, rather than to inhibition of colonization during an established symbiosis. This may seem like a purely mechanistic distinction because the end result of reduced AMF colonization and reduced plant growth is still the same, but direct inhibition is more easily addressed during restoration, especially when healthy AMF can ameliorate allelopathic effects. The strongest allelopathic effects occur during seed and spore germination and presymbiosis growth, thus suggesting that bypassing these life stages during restorations will be more successful than traditional methods of sowing ungerminated seed. More work is needed to determine whether or not other plant species, especially the woody species used by Stinson et al (2006), also are more sensitive to allelopathic inhibition before associating with mycorrhizal fungi then after the symbiosis has been initiated. Field studies that incorporate germinated seed and fresh AMF inocula also could be used to distinguish between legacy effects and ongoing allelopathic inhibition.
References
Bais, H. P., Vepachedu, R., Gilroy, S., Callaway, R. M., and Vivanco, J. M. 2003. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380.
Barkosky, R. R., Butler, J. L., and Einhellig, F. A. 1999. Mechanisms of hydroquinone-induced growth reduction in leafy spurge. J. Chem. Ecol. 25:1611–1621.
Barto, E. K. and Cipollini, D. 2009a. Density-dependent phytotoxicity of Impatiens pallida plants exposed to extracts of Alliaria petiolata. J. Chem. Ecol. 35:495–504.
Barto, E. K. and Cipollini, D. 2009b. Garlic mustard (Alliaria petiolata) removal method affects native establishment. Invasive Plant Science and Management 2:230–236.
Barto, E. K. and Cipollini, D. 2009c. Half-lives and field soil concentrations of Alliaria petiolata secondary metabolites. Chemosphere 76:71–75.
Blum, U., Gerig, T. M., Worsham. A. D., Holappa, L. D., and King, L. D. 1992. Allelopathic activity in wheat-conventional and wheat-no-till soils: development of soil extract bioassays. J. Chem. Ecol. 18:2191–2221.
Callaway, R. M., Cipollini, D., Barto, K., Thelen, G. C., Hallett, S. G., Prati, D., Stinson, K., and Klironomos, J. 2008. Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055.
Chang, M., Netzly, D. H., Butler, L. G., and Lynn, D. G. 1986. Chemical regulation of distance: characterization of the first natural host germination stimulant for Striga asiatica. J. Am. Chem. Soc. 108:7858.
Choesin, D. N. and Boerner, R. E. J. 1991. Allyl isothiocyanate release and the allelopathic potential of Brassica napus (Brassicaceae). Am. J. Bot. 78:1083–1090.
Cipollini, D. F., Stevenson, R., and Cipollini, K. 2008. Contrasting effects of allelochemicals from two invasive plants on the performance of a nonmycorrhizal plant. Int. J. Plant Sci. 169:371–375.
Einhellig, F. A., Rasmussen, J. A., Hejl, A. M., and Souza, I. F. 1993. Effects of root exudate sorgoleone on photosynthesis. J. Chem. Ecol. 19:369–375.
Friese, C. F. and Allen, M. F. 1991. The spread of VA mycorrhizal fungal hyphae in the soil: inoculum types and external hyphal architecture. Mycologia 83:409–418.
Gimsing, A. L., Poulsen, J. L., Pedersen, H. L., and Hansen, H. C. B. 2007. Formation and degradation kinetics of the biofumigant benzyl isothiocyanate in soil. Environ. Sci. Technol. 41:4271–4276.
Gimsing, A. L., Sørensen, J. C., Tovgaard, L., Jørgensen, A. M. F., and Hansen, H. C. B. 2006. Degradation kinetics of glucosinolates in soil. Environ. Toxicol. Chem. 25:2038–2044.
Gonzalez, J. A. and Estevez-Braun, A. 1997. Phytonematicidal activity of aromatic compounds related to shikimate pathway. Pestic. Biochem. Physiol. 58:193–197.
Haribal, M. and Renwick, J. A. A. 1998. Isovitexin 6″-O-β-D-glucopyranoside: a feeding deterrent to Pieris napi oleracea from Alliaria petiolata. Phytochemistry 47:1237–1240.
Haribal, M., Yang, Z., Attygalle, A. B., Renwick, J. A. A., and Meinwald, J. 2001. A cyanoallyl glucoside from Alliaria petiolata, as a feeding deterrent for larvae of Pieris napi oleracea. J. Nat. Prod. 64:440–443.
Hashimoto, Y. and Shudo, K. 1996. Chemistry of biologically active benzoxazinoids. Phytochemistry 43:551–559.
Hashimoto, Y., Shudo, K., and Okamoto, T. 1984. Mutagenic chemistry of heteroaromatic amines and mitomycin C. Acc. Chem. Res. 17:403–408.
Inderjit and Callaway, R. M. 2003. Experimental designs for the study of allelopathy. Plant Soil 256:1–11.
Inderjit and Mallik, A. U. 1999. Nutrient status of black spruce (Picea mariana [Mill.] BSP) forest soils dominated by Kalmia angustifolia L. Acta Oecol. 20:87–92.
Inderjit and Weiner, J. 2001. Plant allelochemical interference or soil chemical ecology? Perspect. Plant Ecol. Evol. Syst. 4:3–12.
Johnson, D., Vandenkoornhuyse, P. J., Leake, J. R., Gilbert, L., Booth, R. E., Grime, J. P., Young, J. P. W., and Read, D. J. 2004. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol. 161:503–515.
Kaminsky, R. 1981. The microbial origin of the allelopathic potential of Adedostoma fasciculatum H & A. Ecol. Monogr. 51:365–382.
Leck, M. A. 1979. Germination behavior of Impatiens capensis Meerb. (Balsaminaceae). Bartonia:1–14.
Mccarthy, B. C. and Hanson, S. L. 1998. An assessment of the allelopathic potential of the invasive weed Alliaria petiolata (Brassicaceae). Castanea 63:68–73.
Prati, D. and Bossdorf, O. 2004. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am. J. Bot. 91:285–288.
R development core team. 2008. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Roberts, K. J. and Anderson, R. C. 2001. Effect of garlic mustard [Alliaria petiolata (Bieb. Cavara & Grande)] extracts on plants and arbuscular mycorrhizal (AM) fungi. Am. Midl. Nat. 146:146–152.
Smith, S. E. and Read, D. J. 2008. Mycorrhizal Symbiosis. Elsevier Science Ltd London.
Stampe, E. D. and Daehler, C. C. 2003. Mycorrhizal species identity affects plant community structure and invasion: a microcosm study. Oikos 100:362–372.
Stinson, K. A., Campbell, S. A., Powell, J. R., Wolfe, B. E., Callaway, R. M., Thelen, G. C., Hallett, S. G., Prati, D., and Klironomos, J. N. 2006. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 4:0727–0731.
Tsanuo, M. K., Hassanali, A., Hooper, A. M., Khan, Z., Kaberia, F., Pickett, J. A., and Wadhams, L. J. 2003. Isoflavanones from the allelopathic aqueous root exudate of Desmodium uncinatum. Phytochemistry 64:265–273.
Tsao, R., Yu, Q., Friesen, I., Potter, J., and Chiba, M. 2000. Factors affecting the dissolution and degradation of Oriental mustard-derived sinigrin and allyl isothiocyanate in aqueous media. J. Agric. Food. Chem. 48:1898–1902.
Vaughn, S. F. and Berhow, M. A. 1999. Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). J. Chem. Ecol. 25:2495–2504.
Walk, T. C., Van Erp, E., and Lynch, J. P. 2004. Modelling applicability of fractal analysis to efficiency of soil exploration by roots. Ann. Bot. 94:119–128.
Weston, L. A., Harmon, R., and Mueller, S. 1989. Allelopatic potential of sorghum-sudangrass hybrid (Sudex). J. Chem. Ecol. 15:1855–1865.
Wolfe, B. E., Rodgers, V. L., Stinson, K. A., and Pringle, A. 2008. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol. 96:777–783.
Wu, H., Haig, T., Pratley, J., Lemerle, D., and An, M. 2000. Distribution and exudation of allelochemicals in wheat Triticum aestivum. J. Chem. Ecol. 26:2141–2154.
Yoder, J. I. 2001. Host-plant recognition by parasitic Scrophulariaceae. Curr. Opin. Plant Biol. 4:359–365.
Acknowledgements
We thank Eusondia Arnett, Steph Enright, and Cherissa Rainey for help in the lab, and Sarah Tebbens and Chris Barton for helpful discussions about fractal analysis. Helpful comments from two anonymous reviewers improved the manuscript. Funding was provided by an Environmental Protection Agency Greater Research Opportunities Fellowship to K Barto (#91673701).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barto, K., Friese, C. & Cipollini, D. Arbuscular Mycorrhizal Fungi Protect a Native Plant from Allelopathic Effects of an Invader. J Chem Ecol 36, 351–360 (2010). https://doi.org/10.1007/s10886-010-9768-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9768-4