Abstract
Understanding allelopathy has been hindered by the lack of methods available to monitor the dynamics of allelochemicals in the soil. Previous work has demonstrated the feasibility of using polydimethylsiloxane (PDMS) microtubing (silicone tubing microextraction, or STME) to construct sampling devices to monitor the release of lipophilic allelochemicals from plant roots. The objective of this study was to use such sampling devices to intensively monitor thiophene fluxes beneath marigolds over several weeks to gain insight into the magnitude of temporal and spatial heterogeneity in these fluxes. Marigolds were grown in rhizoboxes (20.5 x 20.5 x 3.0 cm) with 16 individual STME samplers per box. Thiophene sampling and HPLC analysis began 45 days after planting. At the end of the study, roots around each sampler were analyzed by HPLC. Results confirmed the tremendous spatial and temporal heterogeneity in thiophene production seen in our previous studies. STME probes show that thiophene concentrations generally increase over time; however, these effects were sampling-port specific. When sampling ports were monitored at 12 h intervals, fluxes at each port ranged from 0 to 2,510 ng day−1. Fluxes measured over daylight hr averaged 29 % higher than those measured overnight. Fluxes were less than 1 % on average of the total thiophene content of surrounding roots. While the importance of such heterogeneity, or “patchiness”, in the root zone has been recognized for soil nutrients, the potential importance in allelopathic interactions has seldom been considered. The reasons for this variability are unclear, but are being investigated. Our results demonstrate that STME can be used as a tool to provide a more finely-resolved picture of allelochemical dynamics in the root zone than has previously been available.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The understanding of allelopathy has been hindered by the lack of methods to monitor the dynamics of allelochemicals in the soil. Previous work has demonstrated the feasibility of using polydimethylsiloxane (PDMS) microtubing to construct sampling devices to monitor the release of lipophilic allelochemicals in soil. Such devices provide measurements of soil allelochemicals which are proportional to soil fluxes, and thus provide a new means to test hypotheses about the roles of these compounds in plant-plant interactions. Mohney et al. (2009) used 1 m lengths of PDMS microtubing (silicone tubing microextraction, STME) to monitor thiophenes exuded by marigold roots. The microtubing was placed in soil with the two ends exposed so that solvent could be passed through the tubing to extract samples for HPLC analysis. PDMS has been used in various other forms (PDMS-coated optical fiber, PDMS solid phase microextraction fibers, PDMS-coated stir bars, wires coated with PDMS, and PDMS sheets) to successfully extract thiophenes from the root zone of marigolds (Mohney et al. 2009; Weidenhamer et al. 2009) and also the highly phytotoxic quinone, sorgoleone, from the root zone and root hairs of sorghum (Dayan et al. 2009; Weidenhamer 2005).
Because PDMS selectively sorbs nonpolar compounds, marigolds have served as a good model system for the development of this analytical method due to the highly lipophilic character of the principal thiophenes. The highly phytotoxic nature of thiophenes is well-known (Campbell et al. 1982), and allelopathic effects have been demonstrated for one Tagetes species (Barto et al. 2011), although the major effects reported in the literature for T. patula and T. erecta are nematocidal (Evenhuis et al. 2004). Barto et al. (2011) used PDMS microtubing samplers to demonstrate movement of hydrophobic thiophenes through the common mycorrhizal network (CMN) in soil. Thiophenes were detected in root exclusion zones beneath Tagetes tenuifolia plants at concentrations up to four times higher when a CMN was present, and bioassays showed that these concentrations were high enough to inhibit the growth of a bioassay species (lettuce). PDMS-based techniques have recently been applied to the analysis of other allelochemicals in soil (Barto and Cipollini 2009; Jessing et al. 2013), and they have been widely used in the analysis of anthropogenic pollutants in the environment (Gouliarmou and Mayer 2012; Gouliarmou et al. 2013; Jahnke et al. 2012).
In our previous work (Mohney et al. 2009; Weidenhamer et al. 2009), we have in most cases observed tremendous variability of thiophene concentrations in both time and space measured in marigold root zones. For example, Weidenhamer et al. (2009) found that the amount of 5-(3-buten-1-ynyl)-2,2′-bithienyl (BBT) measured on individual 5-cm wire probes coated with PDMS varied from 0–15.5 μg for the Tagetes patula cultivar ‘Janie Primrose’ when these probes were inserted into soil beneath these plants in outdoor garden beds for 24 h. Mohney et al. (2009) used similar probes to monitor thiophenes beneath Tagetes erecta plants grown in PVC pipes (7.5 cm ID, 44 cm tall). Four weeks after planting, the total amounts of BBT and α-terthienyl (α-T, the other major thiophene produced by marigolds) detected per pipe (based on the sum of 24 different probes) varied from 1,860 to 11,200 ng. Large variation also was seen from probe to probe and by depth. The one exception to this result was in the first application of STME, where 1 m lengths of tubing were distributed through a fairly large volume of soil, possibly reducing the variability observed by an integrated measurement from this large volume (Mohney et al. 2009).
The importance of temporal and spatial heterogeneity (“patchiness”) in the rhizosphere and root zone is recognized for soil nutrients (Hinsinger et al. 2005, 2011; Lambers et al. 2006). Roots of plants are known to exploit such patchiness by preferentially growing in soil macropores (Passioura 1991) and several non-mycorrhizal plant species have been found to produce short-lived root clusters which can be effective at extracting phosphorus when its available concentrations are very low (Lambers et al. 2006). Variation of properties such as soil pH, moisture, nutrient content, microbial populations, and allelochemical concentrations in time and space and from point to point along a root system is likely to have profound consequences for plant-plant interactions (Blum 2011), and the importance of developing analytical tools that can sensitively measure such variation without disturbance of the soil has received increasing emphasis (Ens et al. 2010; Inselsbacher et al. 2011; Rudolph et al. 2013).
Studies of allelopathy generally have focused on the properties and analysis of bulk soil, due to the limitations of previous methods of soil extraction (Weidenhamer 2007). When variability has been considered, there has generally been a focus on seasonal effects (de Scisciolo et al. 1990; Muscolo and Sidari 2006; Weidenhamer and Romeo 1989), and recently the importance of biogeographic variation of the soil environment has been identified (Hierro et al. 2005; Thorpe et al. 2009; Wardle et al. 2011). In split-root experiments, Blum and co-workers (Lehman et al. 1994; Lyu and Blum 1990;) demonstrated that plant responses to simple phenolic acids such as ferulic acid and p-coumaric acid are proportional to the fraction of the root system exposed to these compounds. Inderjit et al. (2011) noted that issues of temporal and spatial heterogeneity on smaller scales in the root zone are important for studies of the roles of allelochemicals, but there is an almost complete paucity of information about microscale variation of allelochemical concentrations in the soil.
A significant advantage of the STME method that we have previously developed is the ability to repeatedly sample a volume of soil without disturbance. By modifying the design of the STME samplers, it is possible to analyze allelochemical concentrations at particular locations beneath a plant. The objective of this study was to use such sampling devices to intensively monitor thiophene fluxes beneath marigolds over several weeks to gain insight into the magnitude of temporal and spatial heterogeneity of these fluxes.
Methods and Materials
Rhizobox and STME Sampler construction
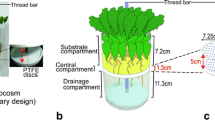
Four Plexiglas® rhizoboxes were constructed (20.5 x 20.5 x 3.0 cm). In each box, four equally spaced sampling ports (4.2 cm apart) were drilled through the boxes at four depths (2, 6.25, 10.5, and 4.75 below the soil surface), giving a total of 16 sampling ports for STME samplers per box (Fig. 1). Four drainage holes were drilled at the bottom of each rhizobox. At each sampling port, an empty 1 ml polyethylene syringe wrapped with 1.1 m of Silastic® tubing (polydimethylsiloxane, 0.30 mm ID x 0.64 mm OD, Fisher Scientific catalog no. 11-189-14) was placed to monitor root exudation (Fig. 2). Each sampler had 1.0 m of silicone tubing exposed to the root zone, while the remaining tubing was threaded through small holes drilled into the syringe barrel and served as inlet and exit ports for the sampling procedure. The samplers spanned the rhizobox so that the inlet and exit ports were on opposite sides of the box (Fig. 2). Prior to use, microtubing was cleaned by soaking in methanol for 24 hr and dried at 70 ° C.
Schematic cross-section of rhizobox showing silicone tubing microextraction (STME) samplers and method of sample collection. Methanol was pumped through the silicone tubing which was wrapped around an empty 1 ml polyethylene syringe for support, and collected in an HPLC autosampler vial. After sampling, air was used to purge the tubing of excess solvent
Growth Media and Plant Cultivation Conditions
Plants were grown in a greenhouse under natural sunlight during March – June 2012. In general, daytime temperatures ranged from 21-32o C and nighttime temperatures from 16-22 ° C. Rhizoboxes were filled with a 1:1 (v:v) mixture of sand and a peat/vermiculite-based growing medium (Redi-earth® plug and seedling mix), and were placed in large tubs with lids cut to fit the boxes so that only the tops of the boxes were exposed to light. Aluminum foil was used further to seal all light gaps to prevent possible degradation of light-sensitive thiophenes. Seeds of Tagetes patula cv. ‘Nemagone’ (W. Atlee Burpee & Co., Warminster, PA,USA) were germinated in Petri dishes on March 8 2012 and transferred to the rhizoboxes. After establishment, plants were thinned to two seedlings per box.
Rhizoboxes normally were watered from the bottom by maintaining them in a shallow pan (<1.0 cm) of water to prevent drying out. Plants received biweekly topical applications of a general purpose fertilizer solution beginning approximately 1 month after planting (Peters Professional® all purpose plant food, 24-8-16 plus B, Cu, Fe, Mn, Mo, and Zn; prepared at recommended rate of 5 ml fertilizer concentrate per 4 L water). The volume applied was sufficient to thoroughly wet the rhizobox. Fertilization was not done immediately before or during sampling.
Sampling Procedure
A syringe pump (KD Scientific, model KDS-230, Holliston, MA) was used to sample the microtubing by pushing 2 ml of 95 % methanol (in water, v/v) through each tube at a rate of 0.25 ml min−1 followed by a 500 μl bolus of air to expel any residual solvent. The resulting extract was collected for HPLC analysis as it exited the tubing. This extraction method has been found sufficient to extract >96 % of the thiophenes on the silicone tubing, and this was confirmed by repeated sampling of a sampling port which yielded > 5 μg total thiophenes. Sampling began 45 days after planting (DAP), and all boxes were sampled at approximately weekly intervals on 45, 50, 57, 64, and 71 DAP. Roots were present throughout the box by the first sampling date. Following completion of these measurements, eight samplers were chosen for daily monitoring for 4 days (78–81 DAP), and all samplers were extracted at 12 h intervals in a morning-evening-morning sequence on 84–85 DAP to see whether a diurnal pattern to exudation could be detected.
Root Sampling
At the termination of the experiment, roots in the area surrounding each sampler (radius approximately 1 cm) were harvested by using a cork borer to cut through the roots, carefully removing the observable roots, and then washing away the remaining growth medium. After blotting dry, roots were weighed to obtain a fresh root mass and then extracted in 3.0 ml of 95 % methanol with sonication for 15 min.
HPLC Methods
A standard sample of α –terthienyl (α-T) was obtained from Sigma Aldrich. The HPLC system was an Agilent 1100 Series equipped with a binary gradient pump, autosampler and diode array detector. Separation was carried out on an Ultra C18 5 μm particle size column (150 x 4.6 mm), with detection at 360 nm. BBT and α-T were identified based on both retention times and UV spectra using the diode array detector (Weidenhamer et al. 2009). Analysis of unused PDMS materials verified that these were free from contaminants when extracted and analyzed by HPLC. As described previously, BBT was quantified based on the ratio of the molar absorptivity of BBT to that of α-T at 360 nm (Weidenhamer et al. 2009). Detector response was linear for α-T up to a concentration of 100 mg L−1. The mobile phase was 95:5 methanol:water (v:v), at a flow rate of 1 mL min−1. All PDMS extracts were analyzed within 24 h of extraction, and were stored in amber autosampler vials to prevent degradation from light exposure. PDMS extracts that could not be analyzed immediately were refrigerated (4 ° C) until analysis. No degradation of thiophenes was observed during this brief storage. All samples were syringe filtered (0.22 μm nylon filter) prior to analysis, and injected in duplicate with the two runs averaged. Typical run to run variation was less than 1 %. The injection volume was 75 μl. The quantitation limit was 0.25 ng per injection, which corresponds to a concentration of 6.7 ng per 2.00 ml extract. For the purposes of data analysis, values below this concentration were treated as zero even if trace amounts were observed.
Microscopy Methods
Fluorescence microscopy of marigold roots was performed using an Olympus IX71 microscope with a DAPI filter (340–390 nm excitation bandwidth and 420 nm long-pass emission filter). Samples were covered with distilled water and imaged using 10X, 40X, and 60X objectives. Images were captured with a SPOT camera and software (SPOT Imaging Solutions, Sterling Heights, MI).
Data Analysis
Data for the 16 sampling ports per box were analyzed both separately and by averaging the two inner and two outer locations at each depth. Data were analyzed using the designs of experiments by methodologies including ANOVA and multiple linear regression. After examining residuals obtained from preliminary models, a log (x + 1) transformation was applied to response variables in order to establish normality. Adding 1 to data made zero values available for analyses with the log transformation. The statistical software SPSS 19.0 for Windows was utilized to run these procedures on the transformed sample data. The homoscedasticity of the data was verified.
Results
STME Sampling Shows High Variability in Both Time and Space
Table 1 reports the amounts of the major marigold thiophene BBT recovered by each STME sampler at a depth of 2 cm on 45–84 DAP. Results of the univariate analysis of variance for these data are shown in Table 2. Variability between rhizoboxes, depths, ports, and DAP were all highly significant, as well as several of the two-way interaction terms. Similar results were seen for α-T (Table 3). When establishing models for BBT and α-T, it was assumed that higher order interaction terms (3-way and above) were not important to explain the variations of BBT and α-T. This is because the experiment was not replicated in terms of DAP.
Within a given rhizobox, adjacent samplers often yielded very different results (Table 1). For example, at 84 DAP, inner port A of rhizobox 2 measured 2189 ng BBT, while the other inner port measured only 14 ng BBT. Both BBT and α-T are highly lipophilic and therefore expected to be not greatly mobile in soil solution. Thus, these results reflect real variations in thiophene release over these small distances. Between rhizoboxes, variation also was quite high. For the inner sampling ports, amounts of BBT detected at 84 DAP were in close agreement only for rhizobox 3 (302 and 325 ng), while for other boxes the difference between inner port samplers was at least three-fold (rhizobox 1) up to more than one hundred fifty-fold (rhizobox 2).
Over the course of the experiment, the amount of thiophenes detected by individual samplers fluctuated greatly. The highest amount of thiophenes detected on an individual sampling port was for rhizobox 3 outer port A at 2 cm depth, which was one of the selected ports sampled 78–81 DAP (data not shown). At 78 DAP, the total amount of BBT measured was 7,173 ng, along with 4,227 ng α-T (11,400 ng total). This was more than a 10-fold increase in the amount of thiophenes detected on this port compared to the 71 DAP measurement (Table 1). By contrast, outer port B at 2 cm depth in this rhizobox showed a 28 % decrease in thiophene content over the same time period, reflecting the highly port-specific nature of these findings.
Overall Trends in Thiophene Release
Despite the high variability at a small scale, the amounts of thiophenes measured overall by STME sampling tended to increase with time, as did the relative proportion of α-T in the root exudate (Figs. 3, 4, 5, 6). For example, the overall trend for the amounts of BBT measured by STME samplers over time shows an increase (Figs. 4, 6b), although statistical analysis showed that the relationship of BBT and DAP was box-specific (box*DAP, P = 0.002, Table 2). The overall trend in the amounts of α-T (Fig. 3, Fig. 6a) also showed an increase with time, although the relationship of α-T and DAP was port-specific (port*DAP, P = 0.014, Table 3). The percentage of α-T in root exudates also increased gradually over time for most depths and sampler locations (Figs. 5, 6c). However, the statistical analysis showed that the relationship of percentage of α-T and DAP was port-specific (port*DAP, P = 0.017, results not shown). The highest amounts of thiophenes detected were measured in the shallowest samplers (2 cm depth, Figs. 3a, 4a); however the effect of depth was box-specific for both BBT (box*depth, P < 0.001; Table 2) and α-T (box*depth, P < 0.001; Table 3).
Measurement of Flux Rates
When STME samplers were monitored frequently (12 h intervals), the magnitude of thiophene flux ranged from 44 to 1,078 ng day−1. Based on our two samplings, there is some evidence of a diurnal pattern to these fluxes, although that requires further study (Table 4). The average thiophene flux in the evening (84.5 DAP) was 197 ng day−1, while the average thiophene flux measured in the morning was 29 % lower, 153 ng day−1. This difference was statistically significant (DAP, P = 0.008; Table 5). As seen in the average overall trends, the highest thiophene fluxes were measured at the shallowest depth (Table 4), and depth was a significant variable in the statistical analysis of these data (P < 0.001, Table 5). The highest measured flux by an individual sampler was 2,510 ng day−1 for rhizobox 2 inner sampling port A at 2 cm depth (all individual measurements are provided in Supplemental Table S1).
Root Thiophene Content And Root Exudates
There was no correlation of thiophene content of marigold roots around each sampler (either on a μg g−1 or total μg basis) to the amount of thiophenes measured by STME samplers. For both BBT and α-T, the relationship of root concentration to measured amounts of each thiophene was not significant (data not shown). The root mass and average thiophene content of marigold roots is reported by depth on both a per gram and total amount basis in Table 6. Concentrations of both BBT and α-T were highest around the shallower (2 cm and 6.25 cm) samplers, while due to much greater root mass at the bottom of the rhizoboxes, the total thiophene content of roots around the deepest samplers (149 μg) was almost as great as that found at 2 cm (163 μg, Table 6). The thiophene fluxes (Table 4) measured at 84.5 (evening measurement, daytime flux) and 85 DAP (morning measurement, nighttime flux) are less than 1 % on average of the total thiophene content of surrounding roots (Table 5), but differences in flux rates were not correlated with root thiophene content.
Within marigold roots, thiophenes are localized in resin ducts. Resin ducts containing the highly fluorescent thiophenes are visualized easily by fluorescence microscopy (Fig. 7). Fluorescence at the tips of root hairs suggests that there may be some amount of active exudation to the soil.
Discussion
Further Development of the STME Method
While the STME method clearly is useful in analysis of lipophilic root exudates, and provides significant advantages in permitting the repeated, undisturbed sampling of the same soil volume, there are a number of questions associated with this new method that remain to be explored. The silicone tubing of the samplers creates a sink for thiophenes that is not normally present in the soil. The regular flushing of the samplers will reinvigorate the samplers as sinks, but the samplers will also serve as a source for thiophenes to surrounding soil when concentrations on the silicone exceed those in the surrounding soil. Previous work (Weidenhamer et al. 2009) has shown that wire solid phase root-zone extraction (SPRE) probes constructed by inserting stainless steel wire into 10-cm lengths of a slightly larger diameter tubing than that used in this experiment lost 25 % of the amount of α-T loaded onto a probe over a 24 hr period when placed in control soils. Thus, these samplers do not function as traps to permanently remove thiophenes from the system. This, combined with the presence of other sinks for thiophenes in the soil (microbial degradation, transport through the soil common mycorrhizal network, absorption to soil organic matter and clays, and uptake of thiophenes by other plants and organisms in field situations) means that the amounts of thiophenes measured represent an underestimate of the actual amounts of thiophenes flowing through this system. Examination of how STME samplers change microbial distributions in soil (e.g., whether microbes colonize the surface of the samplers or are concentrated around the samplers) is another significant question that needs to be addressed.
By contrast with methods that solely measure point-in-time concentrations of compounds, STME samplers have distinct advantages (Mohney et al. 2009; Williamson and Weidenhamer 1990). Direct soil extractions can be biased due to the extraction of root fragments, and the magnitude of this potential bias is suggested by our data on root thiophene content in comparison to the amounts measured by the sampling devices (Table 6). In addition, to the extent that toxicity is a function not only of static concentrations (which reflect the balance of input and output rates at a given point in time) but also flux rates (Williamson and Weidenhamer 1990), the STME samplers provide a means to begin to estimate the dynamic nature of inputs of root exudate compounds in the soil. While using the STME samplers every few days provides a relative measure of the amount of thiophenes that have been released over that time period in a particular volume of soil, the results of repeated samplings on 12 h intervals (Tables 4, S1) suggest that short-interval sampling can provide a much better picture of short-term flux rates.
Potential Mechanism for Heterogeneous Release of Thiophenes from Marigold Roots Because these thiophenes are highly lipophilic, their movement in soil by both diffusion and transpiration effects will be limited. We previously verified the low diffusion potential of α-T by placing PDMS probes loaded with a known amount of α-T in either sand or a 1:1 sand:growth media mixture identical to that used in these experiments. Diffusion away from the loaded PDMS probes was measured and shown to be very slight when using clean PDMS probes to monitor the distance and quantity of thiophene transport (Mohney et al. 2009). Thus the detection of very high quantities of thiophenes around particular samplers implies that the thiophenes have been released in the immediate vicinity of the sampler. Additionally, the moisture levels in the rhizoboxes were maintained at constant levels by the method of watering in part to minimize large gradients in soil moisture. However, such gradients may be important in the field and could have significant impacts on the heterogeneous distribution of root exudates.
Poli et al. (1995) reported the occurrence of thiophenes within marigold roots in two arrays of six reservoirs that abut the endodermis. The presence of high concentrations of sulfur in these reservoirs in comparison to surrounding tissue was confirmed by Sacchetti et al. (2001) using X-ray microanalysis. Our observations (Fig. 7) confirm the occurrence of such reservoirs within the root, and the presence of these reservoirs suggests a possible mechanism for the release of localized, high concentrations of thiophenes by the roots. If such a channel were breached by physical breakage of the root, or by a root herbivore or microbial attack, release of channel contents into the soil could create zones of extremely high thiophene content at the point of attack. While we did not introduce root herbivores into our system, the rhizoboxes were not a sterile environment so the impact of soil microorganisms on the possible release of thiophenes from the root is certainly plausible but unknown. The rhizobox construction and stable position of the sampling devices should have prevented root breakage to a major extent. Our microscopic observations also suggest the possibility of exudation from the tips of root hairs. Further experiments are needed to understand the mechanism of release of thiophenes from marigold roots.
Potential Ecological Importance of Temporal and Spatial Heterogeneity of Allelochemical Distribution in the Root Zone
While differences in soil properties at a broad geographic scale are now recognized as important determinants of plant growth, including the effectiveness of allelopathic root exudates (Thorpe et al. 2009), the impact of smaller-scale differences in chemical properties as seen in this study has not been considered, in part due to the lack of tools to map allelochemical dynamics in soil. Roots modify soil properties in a number of ways that create gradients of concentrations of inorganic and organic soil components, differences in pH, microbial populations, and diurnal movements of water in and out of the root constantly change local conditions in the rhizosphere and root zone (Hinsinger et al. 2005, 2011). Heterogeneity of exudation or release of allelochemicals into the soil may be mitigated somewhat by the impact of the common mycorrhizal network (CMN) of hyphae in soil, which can extend the bioactive range of root-exuded chemicals and facilitate transport from high concentration regions to lower concentration regions of soil (Barto et al. 2011, 2012). Transport of allelochemicals through the CMN may also protect allelochemicals from rapid degradation by moving them away from microbial “hot zones” in the rhizosphere.
Others (Belz and Hurle 2005; Tharayil et al. 2009, 2010) have reported diurnal fluctuations in the exudation of allelochemicals, and Thayaril and Triebwasser (2010) suggest that the pulsing of allelochemical release may be important in overcoming biotic and abiotic degradation mechanisms in soil. While we have measured greater release of thiophenes over daylight hr, the magnitude of diurnal variation seems generally modest in comparison to other variation observed in this study.
Studies of resource competition among plants show that small-scale heterogeneity in soil nutrients may determine competitive outcomes and be a factor in successional changes (Campbell et al. 1991; Gross et al. 1993). Whether heterogeneity in allelochemical concentrations may impact allelopathic interactions has not been explored. There will likely be differences among allelochemicals depending on whether they are taken up and translocated throughout the plant or bind to the root and exert toxic effects at the site of exposure (Dayan et al. 2009). Smaller scale patchiness in allelochemical distributions may allow establishment of otherwise sensitive species in zones of low allelochemical concentration if that patchiness persists over time. The impact of the type of heterogeneity of allelochemical distribution observed in this study on plant growth responses needs to be investigated. Other than the split-root experiments conducted by Blum and co-workers (Lehman et al. 1994; Lyu and Blum 1990) with simple phenolic acids, the potential effects of heterogeneous distribution of toxic allelochemicals in soil have not been explored.
Hinsinger et al. (2011) emphasize that the complexity of rhizosphere processes necessitates the use of modeling informed by data to developing models for how rhizosphere complexity may impact plant growth. For example, Darrah (1991) showed that when root exudates were released at high rates in localized areas for a short amount of time, there was less degradation of the exudates by microorganisms than if the exudates were released uniformly along the root. Thus, release of localized, high concentrations of allelochemicals could increase the impact of allelopathic compounds on competitors. Previous modeling studies have provided useful insights into the effect of plant density on allelopathic interactions (San Emeterio et al. 2007; Sinkkonen 2007) and it is likely that modeling of heterogeneous allelochemical fluxes may yield other useful insights.
In summary, STME provides a useful new window on the temporal and spatial chemical dynamics of the rhizosphere. Our results demonstrate that STME can provide a much more finely-resolved picture of the dynamics of allelochemicals in the root zone than has previously been available. The fluxes and distribution patterns of organic compounds produced by root exudates in soil (substrates) and the potential implications of such fluxes and distribution patterns to plant-plant allelopathic interactions are little understood but may have major implications in the field. Understanding their significance will likely be crucial to translation of research on allelopathy into practical applications for weed-control in agriculture and management of invasive allelopathic plants.
References
Barto K, Cipollini D (2009) Half-lives and field soil concentrations of Alliaria petiolata secondary metabolites. Chemosphere 76:71–75
Barto EK, Hilker M, Müller F, Mohney BK, Weidenhamer JD, Rillig MC (2011) The fungal fast lane: common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS One 6:e27195
Barto EK, Weidenhamer JD, Cipollini D, Rillig MC (2012) Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci 17:633–637
Belz RG, Hurle K (2005) Differential exudation of two benzoxazinoids one of the determining factors for seedling allelopathy of triticaceae species. J Agric Food Chem 53:250–261
Blum U (2011) Plant-plant interactions: phenolic acids, cover crops, and weed emergence. Springer Science and Business Media, Dordrecht
Campbell G, Lambert J, Arnason JT, Towers GHN (1982) Allelopathic properties of α-terthienyl and phenylheptatriyne, naturally occurring compounds from species of Asteraceae. J Chem Ecol 8:961–972
Campbell BD, Grime JP, Mackey JML, Jalili A (1991) A trade-off between scale and precision in resource foraging. Oecologia 87:532–538
Darrah PR (1991) Models of the rhizosphere. Plant Soil 133:187–199
Dayan FE, Howell J, Weidenhamer JD (2009) Dynamic root exudation of sorgoleone and its in planta mechanism of action. J Exp Bot 60:2107–2117
DE Scisciolo B, Leopold DJ, Walton DC (1990) Seasonal patterns of juglone in soil beneath Juglans nigra (black walnut) and influence of J. nigra on understory vegetation. J Chem Ecol 16:1111–1130
Ens EJ, French K, Bremner JB, Korth J (2010) Novel technique shows different hydrophobic chemical signatures of exotic and indigenous plant soils with similar effects of extracts on indigenous species seedling growth. Plant Soil 326:403–414
Evenhuis A, Korthals G, Molendijk L (2004) Tagetes patula as an effective catch crop for long-term control of Pratylenchus penetrans. Nematol 6:877–881
Gouliarmou V, Mayer P (2012) Sorptive bioaccessibility extraction (SBE) of soils: combining a mobilization medium with an absorption sink. Environ Sci Technol 46:10682–10689
Gouliarmou V, Collins CD, Christiansen E, Mayer P (2013) Sorptive physiologically based extraction of contaminated solid matrices: incorporating silicone rod as absorption sink for hydrophobic organic contaminants. Environ Sci Technol 47:941–948
Gross K, Peters A, Pregitzer KS (1993) Fine root growth and demographic responses to nutrient patches in four old-field plant species. Oecologia 95:61–64
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol 168:293–303
Hinsinger P, Brauman A, Devau N, Gérard F, Jourdan C, J-P L, LE Cadre E, Jaillard B, Plassard C (2011) Acquisition of phosphorus and other poorly mobile nutrients by roots. where do plant nutrition models fail? Plant Soil 348:29–61
Inderjit, Wardle DA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662
Inselsbacher E, Öhlund J, Jämtgård S, Huss-danell K, Näsholm T (2011) to monitor organic and inorganic nitrogen compounds in soil. Soil Biol Biochem 43:1321–1332
Hnke A, Mayer P, Mclachlan MS (2012) Sensitive equilibrium sampling to study polychlorinated biphenyl disposition in Baltic Sea sediment. Environ Sci Technol 46:10114–10122
Jessing KK, Cedergreen N, Mayer P, Libous-bailey L, STROBE BW, Rimando A, Duke SO (2013) Loss of artemisinin produced by Artemisia annua L. to the soil environment. Ind Crop Prod 43:132–140
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lehman ME, Blum U, Gerig TM (1994) Simultaneous effects of ferulic andp-coumaric acids on cucumber leaf expansion in split-root experiments. J Chem Ecol 20:1773–1782
Lyu SW, Blum U (1990) Effects of ferulic acid, an allelopathic compound, on net P, K, and water uptake by cucumber seedlings in a split-root system. J Chem Ecol 16:2429–2439
Mohney BK, Matz T, Lamoreaux J, Wilcox DS, Gimsing AL, Mayer P, Weidenhamer JD (2009) In situ silicone tube microextraction: a new method for undisturbed sampling of root-exuded thiophenes from marigold (Tagetes erecta L.) in soil. J Chem Ecol 35:1279–1287
Muscolo A, Sidari M (2006) Seasonal fluctuations in soil phenolics of a coniferous forest: effects on seed germination of different coniferous species. Plant Soil 284:305–318
Passioura JB (1991) Soil structure and plant growth. Soil Res 29:717–728
Poli F, Sacchetti G, Bruni A (1995) Distribution of internal secretory structures in Tagetes patula (Asteraceae). Nord J Bot 15:197–205
Rudolph N, Voss S, Moradi AB, Nagl S, Oswald SE (2013) Spatio-temporal mapping of local soil pH changes induced by roots of lupin and soft-rush. Plant Soil 269:669–680
Sacchetti G, Romagnoli C, Bruni A, Poli F (2001) Secretory tissue ultrastructure in Tagetes patula L. (Asteraceae) and thiophene localization through X-ray microanalysis. Phyton 41:35–48
San emeterio L, Damgaard C, Canals RM (2007) Modelling the combined effect of chemical interference and resource competition on the individual growth of two herbaceous populations. Plant Soil 292:95–103
Sinkkonen A (2007) Modelling the effect of autotoxicity on density-dependent phytotoxicity. J Theor Biol 244:218–227
Tharayil N, Triebwasser DJ (2010) Elucidation of a diurnal pattern of catechin exudation by Centaurea stoebe. J Chem Ecol 36:200–204
Tharayil N, Bhowmik P, Alpert P, Walker E, Amarasiriwardena D, Xing B (2009) Dual purpose secondary compounds: phytotoxin of Centaurea diffusa also facilitates nutrient uptake. New Phytol 181:424–434
Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645
Wardle DA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662
Weidenhamer JD (2005) Biomimetic measurement of allelochemical dynamics in the rhizosphere. J Chem Ecol 31:221–236
Weidenhamer JD (2007) New approaches to analyse allelochemicals in soil. Allelopathy J 19:135–142
Weidenhamer JD, Romeo JT (1989) Allelopathic properties of Polygonella myriophylla. J Chem Ecol 15:1957–1970
Weidenhamer JD, Boes PD, Wilcox DS (2009) Solid-phase root zone extraction (SPRE): a new methodology for measurement of allelochemical dynamics in soil. Plant Soil 322:177–186
Williamson GB, Weidenhamer JD (1990) Bacterial degradation of juglone. J Chem Ecol 16:1739–1742
Acknowledgements
Evan Dort assisted with rhizobox construction and establishment of the experiment. Margaret McCully provided advice on microscopy techniques. Udo Blum provided helpful comments on experimental design and on the manuscript, and two anonymous reviewers provided helpful comments which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 71 kb)
Rights and permissions
About this article
Cite this article
Weidenhamer, J.D., Mohney, B.K., Shihada, N. et al. Spatial and Temporal Dynamics of Root Exudation: How Important is Heterogeneity in Allelopathic Interactions?. J Chem Ecol 40, 940–952 (2014). https://doi.org/10.1007/s10886-014-0483-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0483-4