Abstract
Plant growth-promoting rhizobacteria (PGPR) generally exert their effects through enhancement of plant nutrient status and/or phytohormone production. The effects of PGPR on aromatic plant species are poorly known. We measured plant growth parameters, chlorophyll content, trichome density, stomatal density, and levels of secondary metabolites in peppermint (Mentha piperita) seedlings inoculated with PGPR strains Bacillus subtilis GB03, Pseudomonas fluorescens WCS417r, P. putida SJ04, or a combination of WCS417r + SJ04. The treated plants, in comparison with controls, showed increases in shoot biomass, root biomass, leaf area, node number, trichome density, and stomatal density, and marked qualitative and quantitative changes in monoterpene content. Improved knowledge of the factors that control or affect biosynthesis of secondary metabolites and monoterpene accumulation will lead to strategies for improved cultivation and productivity of aromatic plants and other agricultural crops without the use of chemical fertilizers or pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peppermint (Mentha × piperita) is cultivated worldwide for production of essential oils (EOs) and fresh or dried herbs, and is one of the most important EO crops (Lawrence 2007).
Fresh and dried peppermint herbs are used for teas and for flavoring of foods and beverages. Peppermint EOs are used extensively as aromatic agents (e.g., in chewing gum, candy, toothpaste, mouthwash, and aromatherapy), pharmaceuticals, antimicrobial agents, and ecofriendly pesticides (MIRC 2010). Herbs, extracts, and EOs of peppermint and spearmint have a long history of medicinal usage for therapy or symptomatic treatment of numerous human diseases and disorders (Zheljazkov et al. 2010).
Aromatic plants belonging to the family Lamiaceae are economically important because of their EO production. The EOs are produced exclusively by glandular trichomes (McCaskill and Croteau 1995), which are epidermal structures covering the aerial portion of the plant.
There are two types of glandular trichomes, termed peltate and capitate, located on both the upper and lower leaf surfaces (Werker 2000). Peppermint (M. piperita) has both types. Only the peltate glandular trichomes accumulate monoterpenes. These trichomes contain secretory cells that are responsible for oil synthesis. Nascent EO is secreted into an emerging cavity formed by the separation of a preformed layer of cuticular material (Rios-Estepa et al. 2010). The density of glandular trichomes on leaves in the genus Origanum (oregano, marjoram) has been positively correlated with plant EO content (Bosabalidis 2002). Intensive farming practices for the purpose of high crop yield and quality traditionally involve the extensive use of chemical fertilizers, which are expensive and have negative environmental impacts. During the past few decades, there has been steadily increasing study and utilization of environmentally safe, sustainable, and organic agricultural food and feed production strategies that reduce negative environmental effects (Lind et al. 2004). “Organic agriculture” is a production system that avoids or minimizes the use of synthetic fertilizers, pesticides, and growth regulators, relying instead on biofertilization, crop rotation, crop residues, mechanical cultivation, and biological pest control to maintain soil productivity. Reduced yield is a major problem and concern in organic production systems (Lind et al. 2004). For the numerous medicinal and aromatic plant species that are consumed without further processing, it is important that no synthetic compounds be present in the harvested crop.
Many species of bacteria, most of which are found in the rhizosphere (the narrow region of soil associated with the roots of plants), have beneficial effects on plant growth and on crop yield and quality. Such bacteria, collectively termed “plant growth-promoting rhizobacteria” (PGPR), promote plant growth through both direct and indirect mechanisms (Kloepper 1993; Niranjan et al. 2006; Van Loon 2007). Direct mechanisms of PGPR include production of stimulatory bacterial volatile organic compounds (VOCs) (Wenke et al. 2010) and phytohormones, reduction of plant ethylene level, and enhancement of plant nutrient status (release of phosphates and micronutrients from insoluble sources; nonsymbiotic nitrogen fixation) (Van Loon 2007). Indirect effects of PGPR include functioning as biocontrol agents to reduce diseases, based on enhancement of disease-resistance mechanisms (induced systemic resistance) (Bakker et al. 2007), promotion of other beneficial symbioses, and protection of plants by degrading xenobiotics in contaminated soils (Figueiredo et al. 2010). Depending on the PGPR species, two or more of the above growth-promoting mechanisms may be present (Vessey 2003).
Enhancement of plant growth parameters and secondary metabolic responses in aromatic plants often result from inoculation of beneficial microbes. The effects of PGPR inoculation on sweet marjoram, Italian oregano, sweet basil, and wild marigold vary depending on the inoculated strain. PGPR-inoculated plant species display host response specificity (Banchio et al. 2009, 2010; Cappellari et al. 2013). In most studies, the growth parameters evaluated were increased significantly by P. fluorescens inoculation. Essential oil yield was increased to varying degrees. Studies of plant interactions involving arbuscular mycorrhizal (AM) fungi in aromatic plants haveshown increases of plant growth and EO production (Copetta et al. 2006; Khaosaad et al. 2006; Zeng et al. 2013). Mycorrhization allows the root system to exploit a greater volume of soil by extending the root zone, reaching small soil pores not accessible by root hairs, and acquiring organic phosphates through production of extracellular acid phosphatases (Bouwmeester et al. 2007).
Another strategy for exploiting microbial potential is to combine the attributes of different microbe strains to achieve an outcome that encompasses numerous or complementary beneficial effects (Lucy et al. 2004). Microorganisms display varying degrees of cooperation/ mutualism whereby they benefit themselves or other organisms, and positive outcomes are enhanced (Vessey 2003). Recent studies have addressed the factors that promote or regulate EO production (Chalchat et al. 1997; Hudaib et al. 2002), and the inhibitory effects of various abiotic stresses on EO production (Karousou et al. 1998; Panou-Filotheou et al. 2001). Many experiments have addressed the role of PGPR inoculation in aromatic plants (Banchio et al. 2009; Cappellari et al. 2013; Santoro et al. 2011). However, there have been no comparative studies on the role of inoculation and co-inoculation with native strains on plant growth at the morphological or anatomical level, or on production of secondary metabolites (particularly monoterpenes) in commercially important aromatic plants. It has been suggested that indigenous isolates are preferred in selection of bacteria for inoculation of crop plants, because they are already adapted to the environment and are intrinsically more competitive than are non-indigenous bacteria (Bhattarai and Hess 1993). In general, our knowledge of morphological and physiological features of aromatic plants associated with rhizobacteria remains limited and fragmentary.
We performed a comparative analysis of the effects of M. piperita root inoculation in three PGPR strains (indigenous and non-indigenous; singly and in combination) on plant morphological, anatomical (glandular trichome and stomatal density), and phytochemical features.
Methods and Materials
Bacterial Strains, Culture Conditions, Media, and Treatments
Three bacterial strains previously reported as PGPRs were studied: (i) Pseudomonas fluorescens WCS417r; (ii) Pseudomonas putida SJ04 (a native fluorescent strain isolated from rhizospheric soil collected with a commercial crop of Mentha × piperita (San José) in Villa Dolores, Córdoba,Argentina and tested for plant growth-promoting activity (GenBank KF312464.1); (iii) Bacillus subtilis GB03 (Banchio et al. 2009). Each strain was grown on LB medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl) for routine use and maintained in nutrient broth with 15 % glycerol at −80 °C for long-term storage.
Each bacterial culture was grown overnight at 30 °C with rotation at 120 rpm until reaching exponential phase, washed twice in 0.9 % NaCl by Eppendorf centrifugation (4300×g, 10 min, 4 °C), resuspended in sterile water, and adjusted to a final concentration of ~106 colony forming unit (CFU)/ ml for use as inoculum. Plants were grown in plastic pots (diam 12 cm, depth 22 cm) containing 250 g sterilized vermiculite. Mentha piperita seedlings were planted (one per pot) in vermiculite and inoculated with 1000 μl bacterial suspension. The treatments were: (1) sterile water (control); (2) GB03; (3) WCS417r; (4) SJ04; (5) WCS417r plus SJ04.
For experiments, single colonies of bacteria grown on nutrient agar were transferred to 100-ml flasks containing the appropriate culture medium and grown aerobically on a rotating shaker (150 rpm) for 48 hr at 28 °C. The bacterial suspensions were diluted in sterile water to a final concentration of 106 CFU/ml as determined by optical density measurement. Plants were treated with 1 ml of the resulting suspension for treatments (2), (3), and (4), or with 1 mL each of WCS417r and SJ04 for treatment (5). Ten plants were used for each treatment.
Greenhouse Experiments
Young shoots from M. piperita plants grown in Traslasierra Valley (Córdoba province, Argentina) were surface disinfected by soaking for 1 min in 17 % sodium hypochlorite solution, and rinsed 3X in sterile distilled water. Disinfected shoots were cultured in 100 mL MS culture medium containing 0.7 % (w/v) agar and 1.5 % (w/v) sucrose (Murashige and Skoog 1962). All culture media contained 30 g/L sucrose and 7.5 g/L agar. Stage I (initial shoot-tip culture): After shoot culture for 30 day as above, apical meristems with foliar primordia, not showing contamination, were aseptically removed from terminal buds. Explants were cultured in test tubes in 10 ml MS medium with 0.53 μM naphthaleneacetic acid (NAA) and 0.26 μM benzyladenine (BA) (Santoro et al. 2011).
Stage II (growth and multiplication): Plantlets obtained from tips were multiplied by single node culture in MS medium with 0.53 μM NAA and 0.28 μM BA. pH was adjusted to 5.8, and growth regulators were added prior to autoclaving (20 min, 121 °C). Temperature was maintained at 22 °C. Photoperiod was 16 hr/day with ~2000 Lux light radiation from cool white fluorescent tubes.
Stage III (rooting and acclimatization): At d 7 of culture, rooting plantlets were obtained at the in vitro multiplication stage, transplanted directly into vermiculite in a greenhouse, and watered by a micro-irrigation system.
Plants were grown in a growth chamber with controlled conditions of light (16/8 hr L/D cycle), temperature (22 ± 2 °C), and relative humidity (~70 %). Bacterial suspensions as described above were applied to experimental seedlings, and sterile water was applied to control seedlings. All plants received Hoagland’s nutrient medium (20 ml/pot) once per week (Banchio et al. 2008). The experiments were performed under non-sterile conditions.
Experiments were replicated three times (10 pots per treatment; 1 plant per pot). Pots were arranged randomly in the growth chamber. Thirty days after inoculation, plants were removed from pots, roots were washed to remove vermiculite, and standard growth parameters (shoot length, leaf number, node number, shoot fresh weight, root dry weight) were measured. Total leaf areas were calculated based on digitized images of leaves using ImageJ (open source software program developed by the National Institutes of Health, Bethesda, MD, USA) as described previously (Turner et al. 2000).
Total Chlorophyll Determination
The amount of total chlorophyll in plants was determined as described by Arnon (1949). In brief, leaves (~0.1 g fresh weight) were placed in a mortar, 80 % acetone added, and ground to a fine pulp. The resulting extract was transferred to a Buchner funnel containing a pad of Whatman filter paper. Grinding of the leaf pulp was repeated during filtration of the extract to adjust the final volume of the filtrate to 10 ml. The optical density of the chlorophyll extract was measured by spectrophotometry at wavelengths 650 and 665 nm. The amount of total chlorophyll present in the extract was calculated on the basis of mg chlorophyll per g fresh leaf tissue, according to the following equation (Mc Kinney 1938):
Trichome Density and Stomatal Density
A layer of acrylic (synthetic nail coating) was brushed onto the adaxial and abaxial sides of the leaf, dried for a few seconds, then carefully extracted and mounted for microscopy with glycerol/ distilled water 1:10 (D’Ambrogio de Argüeso A 1986). Six leaf blades were processed for each treatment. Trichome density and stomatal density (number per mm2) were calculated from three microscope fields chosen at random for each leaf epidermis. Histological preparations of trichomes and stomata were assessed using a standard Zeiss model 16 microscope. Photomicrographs were taken with a Zeiss Axiophot microscope equipped with image capture and digitization (AxioVision 4.3, with camera AxioCam HRc 200× magnification). Trichomes and stomata were counted using the ImageJ program for image analysis, and their frequency (n/mm2) was calculated as described previously (Barbieri et al. 2012; Mucciarelli et al. 2003). Density was expressed as the ratio between mean number of trichomes or stomata per leaf pair and the corresponding leaf area.
Extraction of EOs
Shoot samples were weighed individually and subjected to hydrodistillation in a Clevenger-like apparatus for 40 min. The volatile fraction was collected in dichloromethane, and β-pinene (1 μl in 50 μl ethanol) was added as an internal standard.
Mentha piperita plants contain ~3 % volatile oils, consisting of >50 different compounds. The major EO components, which comprise ~60 % of total oil volume, are 1,8-cineole, linalool, (−) menthone, (−) menthol, and (+) pulegone. These compounds were quantified in relation to the standard β-pinene added during the distillation procedure as above. Flame ionization detector (FID) response factors for each compound generated essentially equivalent areas (differences <5 %).
Chemical analyses were performed using a Perkin-Elmer Q-700 gas chromatograph (GC) equipped with a CBP-1 capillary column (30 m × 0.25 mm, film thickness 0.25 μm) and a mass selective detector. Analytical conditions: injector temperature 250 °C, detector temperature 270 °C; oven temperature programmed from 60 °C (3 min) to 240 °C at 4 °/min; carrier gas = helium at a constant flow rate of 0.9 mL/min; source 70 eV. Oil components were identified based on mass spectral and retention time data and confirmed by direct comparison with commercial standard compounds (Banchio et al. 2005).
GC analysis was performed using a Shimadzu GC-RIA gas chromatograph fitted with a 30 m × 0.25 mm fused silica capillary column coated with Supelcowax 10 (film thickness 0.25 μm). GC operating conditions: injector and detector temperatures 250 °C; oven temperature programmed from 60 °C (3 min) to 240 °C at 4 °/min; detector = FID; carrier gas = nitrogen at a constant flow rate of 0.9 ml/min.
Statistical Analyses
Data were pooled and subjected to analysis of variance (ANOVA) followed by comparison of multiple treatment levels with controls using Fisher’s post hoc LSD (least significant difference) test. Differences between means were considered significant for P values < 0.05. The Infostat software program, version 2008 (Group Infostat, Universidad Nacional de Córdoba, Argentina) was used for all statistical analyses.
Results
Plant Morphology
The effects of PGPR inoculation on M. piperita growth and development varied depending on the experimental treatment (inoculation with GB03, WCS417r, SJ04, or WCS417r + SJ04) (Fig. 1; Table 1). Each of the growth parameters evaluated was significantly (P < 0.05) altered by each of the four treatments (Table 1). Root dry weights were ~40 % higher for plants in each of the treatment groups than in the control (noninoculated) group (P < 0.05). Root length was altered only by WCS417r treatment. Shoot fresh weight for each of the treatment groups was ~380 mg higher than in the control group (Fig. 1a). This increase was due to a combination of increased leaf number, shoot length (Table 1), and leaf area (25 cm2 higher) (Fig. 1b).
Growth and development parameters of 30-day old Mentha piperita plants in a control group and four treatment groups (inoculated or co-inoculated with PGPR) as described in M&M. a fresh weight. b leaf area. c stomatal density. Different letters above bars within a panel indicate significant differences according to Fisher’s LSD test (P < 0.05)
Leaf chlorophyll a, chlorophyll b, and total chlorophyll concentrations were significantly higher for each of the treatment groups than the control group. All three chlorophyll values were higher for the GB03 group than the other treatment groups (Table 2). Chlorophyll a and b values were higher for the SJ04 group than the WCS417r group. For all three chlorophyll values, the WCS417r + SJ04 co-inoculated group was intermediate between the SJ04 and WCS417r singly-inoculated groups.
Plant Anatomy
Single non-glandular capitate trichomes and peltate glandular trichomes were present on the adaxial and abaxial leaf surfaces. A small capitate glandular trichome consisted of a globose unicellular head attached to the leaf by a two- or three-celled uniseriate stalk. Peltate glandular trichomes consisted of a large eight-celled head, with an enlarged secretory cavity, attached to a one-celled short stalk. Average numbers of trichomes per cm2 for the control group and the four treatment groups are shown in Table 3. Trichomes were significantly more abundant on the abaxial than the adaxial surface. Differences in numbers of capitate trichomes were not significant. Peltate trichomes were present on both surfaces for the treatment groups, but their density was lower on the adaxial surface. The density of peltate trichomes on both surfaces was higher for the treatment groups than the control group (Table 3). Differences in peltate trichome density among the four treatment groups were not significant. Stomatal density was higher for the abaxial than the adaxial surface for all treatments (data not shown). Overall stomatal density increased from 93 stoma/mm2 in the control group to ~140 stoma/mm2 in the treatment groups. The value was higher for the WCS417r + SJ04 group (154 stoma/mm2) than for the other three treatment groups (Fig. 1c).
Secondary Metabolites
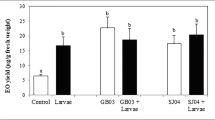
GC analysis of EOs revealed striking differences between the treatment and control groups (Fig. 2). These differences were due mainly to variations in major EO components (Fig. 3) and total EO yield.
Total EO concentrations were in the range of 0.5 % (for control plants) and 2 % (for treated plants) fresh weight, similar to findings in other aromatic species (Werker 2000).
Monoterpene accumulation values (μg per g fresh weight) were 4.69 for the control group, 15–20 for the singly-inoculated groups, and 29.2 for the WCS417r + SJ04 group (~6-fold higher than the control value; P = 0.001) (Fig. 2). Yields of the major EO components ((+) pulegone, (−) menthone, (−) menthol, 1,8-cineole, and linalool) were higher in all four treatment groups than in the control group (Fig. 3). Menthol yield increased to 2.09 μg/g fresh weight (P < 0.05) in the WCS417r + SJ04 group in comparison with 0.22 μg/g fresh weight in the control group and 1.1–1.2 μg/g fresh weight in the other three treatment groups. Similar trends were observed for menthone, 1,8–cineole, linalool, and pulegone; yield was always higher for the WCS417r + SJ04 group than the other three treatment groups. Yields for each of the major EO components were higher for the GB03 group than the WCS417r or SJ04 groups, but the differences between these three singly-inoculated groups were generally not significant.
Discussion
Plant Morphology
Enhanced growth and development following inoculation with PGPR has been reported for many plant species (Gray and Smith 2005; Van Loon 2007; Vessey 2003). The causes of the enhancement vary depending on the species, and may include both direct and indirect mechanisms (Glick 1995; Gupta et al. 2002). In the present study, the magnitude of the effects of PGPR on M. piperita growth and development varied depending on the inoculated strain (GB03, WCS417r, SJ04, or WCS417r + SJ04). Single inoculation with the three PGPR strains evaluated resulted in significant increases in almost all plant growth and morphogenesis parameters measured. The only exception was the lack of effect of SJ04 on root development. Shoot fresh weight was enhanced significantly (~40 %) in each of the treatment groups compared with the control group. This effect appeared to be due primarily to increased leaf area in the WCS417r + SJ04 group, and in part to increased leaf and node number and shoot length in the GB03, WCS417r, and SJ04 groups. These findings rule out the possibility that the enhanced growth resulted simply from increased plant hydration. Root dry weight was higher in the GB03, WCS417r, and WCS417r + SJ04 groups than in the control group. This increase was due primarily to increased number of lateral roots (data not shown). Root length was affected only by WCS417r treatment. Vessey (2003) described various effects of PGPR on root morphology. Zhang et al. (2007) reported that enhanced lateral root formation led to increased root surface area and nutrient uptake potential.
We previously obtained similar results for Origanum majoricum, O. majorana, and Tagete minuta inoculated with WCS417r (Banchio et al. 2010; Cappellari et al. 2013). GB03 enhanced growth parameters of O. majoricum and O. basilicum (basil) (similar to its effects on M. piperita in the present study), but had no effect on development parameters of O. majorana. In vitro studies showed that VOCs emitted by WCS417r and GB03 produced effects similar to those observed here in M. piperita, and were more striking in plants affected by GB03 (Santoro et al. 2011). In the present study, developmental parameters in the WCS417r + SJ04 group were not significantly different from those in the singly-inoculated or control groups.
In this study, plants received Hoagland’s nutrient solution, in which nitrogen and other nutrients are readily available. The growth stimulatory effects observed were therefore not due to phosphate solubilization, sulfate oxidation, or increased nitrate availability (Kloepper 1993). Rather, the enhancement of growth following PGPR inoculation was presumably due to increased production and emission of growth hormones and/or VOCs by the bacteria (Ryu et al. 2005; Santoro et al. 2011; Van Loon 2007). Such components, collectively termed “plant growth regulators”, are organic substances that affect plant physiological processes at extremely low concentrations and play regulatory roles in plant growth and development (Dobbelaere et al. 2003).
Cultures of various PGPR have been shown to produce phytohormones in substantial amounts (Zahir et al. 2004) that promote plant development. Numerous bacterial strains produce auxins and/or ethylene, and some strains have been reported to produce cytokinins and gibberellins (Van Loon 2007). Studies during the past few decades have shown that rhizobacteria are capable of releasing functional VOCs (Vespermann et al. 2007). Zhang et al. (2008) showed that exposure to B. subtilis VOCs enhanced photosynthetic efficiency, chlorophyll content, and cell expansion. We observed increased fresh weight and root dry weight in M. piperita exposed to GB03 and WCS417r VOCs (Santoro et al. 2011).
Plant Anatomy
Trichome distribution is not uniform over the leaf surface; there are distinct differences between the abaxial and adaxial epidermis. Consistent with the findings of Turner et al. (2000), we observed approximately twice as many trichomes on the abaxial as on the adaxial surface. After 30 days, plants in the four treatment groups had greater numbers of peltate trichomes on both the adaxial and abaxial surfaces. Glandular trichome density is controlled by numerous environmental and hormonal factors. Several studies indicate that jasmonates in particular play a role (Ament et al. 2004; Boughton et al. 2005; Lange and Ahkami 2013; Li et al. 2004). Stomata are involved in photosynthesis and transpiration, two essential processes in plants. Increased stomatal density, as observed in our treatment groups, theoretically could enable plants under well-hydrated conditions to increase conductance for gas exchange at the leaf surface and thereby avoid photosynthetic limitation due to suboptimal CO2 supply (Schlüter et al. 2003). The more stomata per unit area, the more CO2 can be taken up, and the more water can be released (Berry et al. 2010).
Chlorophyll concentration is an important parameter in evaluation of plant photosynthetic efficiency and response to environmental stress (Zhu et al. 2012). We observed higher concentrations of chlorophyll a, chlorophyll b, and total chlorophyll in the PGPR-treated groups than in the control group, in agreement with previous findings (Colla et al. 2008). A likely explanation is that PGPR-inoculated plants can absorb more water and nutrients than can non-inoculated plants (Subramanian and Charest 1997) and are, therefore, less susceptible to oxidative stresses that damage the photosynthetic apparatus (Evelin et al. 2009).
Secondary Metabolites
Our previous studies showed that PGPR inoculation increased monoterpene production in various aromatic plant species (Banchio et al. 2009, 2010; Cappellari et al. 2013). In the present study, total EO yield in the four treatment groups was 4- to 6-fold higher (P < 0.001) than in the control group. In our previous studies, P. fluorescens inoculation increased total EO yield (relative to controls) 2.5-fold in O. majoricum, 24-fold in O. majorana, and by 50 % in T. minuta (Banchio et al. 2010; Cappellari et al. 2013), and bacterial VOCs increased 2-fold in M. piperita (Santoro et al. 2011). The present study showed differential effects of GB03 on monoterpene accumulation. We found previously that exposure to B. subtilis VOCs significantly increased EO yield in O. basilicum (Banchio et al. 2009), but had no effect on total monoterpene level or EO yield in O. majoricum or O. majorana seedlings, or in M. piperita (Banchio et al. 2009; Santoro et al. 2011). The effects of rhizobacteria and their VOCs on these secondary metabolites varied depending on the strain, suggesting that the rhizobacteria are recognized by the host plant in a strain-specific manner.
Levels of the major EO components analyzed (1,8-cineole, linalool, (−) menthone, (−) menthol, (+) pulegone) were markedly different in the four treatment groups from the control group. (+) Pulegone was by far the predominant component, accounting for ~50 % of total EOs. Our findings suggest an increase in terpene biosynthesis in the inoculated plants, although we did not directly measure this process. Similar results have been obtained for various aromatic plant species treated with arbuscular mycorrhizal fungi. Gupta et al. (2002) inoculated Mentha arvensis cultivars with the fungus Glomus fasciculatum and observed increases in plant height, shoot growth, and oil content. Khaosaad et al. (2006) reported altered EO concentration in Origanum sp. Copetta et al. (2006) observed altered glandular trichome abundance and EO yield in O. basilicum exposed to mycorrhizal fungi.
Expression of the gene controlling terpenoid biosynthesis in Mentha aquatica increases in response to herbivore feeding; the majority of terpene production is diverted to synthesis of (+)-menthofuran, which has been shown to repel herbivorous mint leaf beetles in bioassay tests (Lamiri et al. 2001). Monoterpene synthesis is promoted by herbivore feeding in Minthostachys mollis (Banchio et al. 2005) and in other plant species, most likely thus protecting damaged leaves from further attack (Harrewijn et al. 2001; Hummelbrunner and Isman 2001).
Essential oil concentrations and composition in plants play several key roles in plant-environment interactions and plant-plant communication. Terpenoids are crucial components in plant defensive responses to abiotic and biotic stresses (Unsicker et al. 2009; Vickers et al. 2009), signaling among plant organs (Heil and Silva Bueno 2007), and plant-plant communication (Baldwin et al. 2006; Lange and Ahkami 2013).
The increases in EO synthesis observed in the present study presumably represent defensive responses to colonization by microorganisms. Several EO compounds in M. piperita exert insecticidal, antifungal, and/or antibacterial effects (Sangwan et al. 2001). Induction of systemic resistance, which can be elicited not only by pathogens and herbivores but also by beneficial microorganisms and certain synthetic compounds, provides plants with an enhanced capacity for rapid and effective activation of cellular defensive responses against pathogen or insect attack (Conrath 2011). Induction of systemic resistance against herbivores also involves “priming” of jasmonate-dependent responses and other ye tunknown mechanisms (Pineda et al. 2010; van Oosten et al. 2008). Priming of plant defenses by beneficial microorganisms has been proposed to be a consequence of the modulation of plant immune systems associated with establishment of the symbiosis, and related changes in defense-related signaling (Pozo and Azcón-Aguilar 2007; Zamioudis and Pieterse 2012). The increased monoterpene concentrations we observed in inoculated plants may result from growth-promoting substances, secreted by PGPR, that affect plant metabolic processes. Increases in stomatal density and chlorophyll content in inoculated plants may be reflected in a higher rate of carbon assimilation and result in increased terpenoid biosynthesis, which requires carbon fixation through photosynthesis (Ghirardo et al. 2011). Terpenoid biosynthesis depends on primary metabolism (photosynthesis) and oxidative pathways for carbon and energy supplies (Singh et al. 1991). Giri et al. (2003) found that net photosynthesis of PGPR-hosting plants is correlated with nutritional status. Factors that increase dry matter production affect the interrelationship between primary and secondary metabolism, leading to increased biosynthesis of secondary products (Shukla et al. 1992). In the present study, trichome density was higher in the treatment groups than in the control group. Oil yield is strongly correlated with the total number and developmental distribution patterns of glandular trichomes, the biosynthetic machinery that quickly and efficiently converts imported carbohydrates into EOs (Lange et al. 2011; Lange and Turner 2012). Rios-Estepa et al. (2010) proposed a mechanism whereby increased expression of a biosynthetic gene induces production of glandular trichomes, facilitating the production and storage of EOs.
In this study, single inoculation with Pseudomonas (WCS417r or SJO4) or B. subtilis GB03 affected plant growth and development, trichome density, and chlorophyll content to similar degrees. Co-inoculation with WCS417r and SJO4 produced the greatest increases in stomatal density and levels of secondary metabolites, indicating a synergistic effect on monoterpene biosynthesis. This finding may reflect a higher number of CFU per seedling; the plants were inoculated with 106 CFU/mL from each strain. On the other hand, previous studies on co-inoculation with various PGPR strains indicated that most of the strains did not have negative effects on each other (Marimuthu et al. 2002; Vestberg and Cassells 2009). Compositional studies of various microbial communities have shown that Pseudomonas species generally are able to adapt to a wide range of environments and to increase population size (Van Loon 2007; Vessey 2003) because of their ability to metabolize a wide range of carbonaceous substances excreted by plant roots, the versatility of their metabolism, and their rapid growth rates (Figueiredo et al. 2010; Lucy et al. 2004; Vestberg and Cassells 2009). Other reports indicate that co-inoculation with certain combinations of PGPR strains has greater or comparable effects on plant protection and plant growth promotion in comparison with separate inoculation (Schisler et al. 1997; Schmidt et al. 2004). Native strains may be preferred in such cases in selection of bacteria for inoculation of crop plants, because they are already adapted to the environment and are more competitive than are non-indigenous strains. In summary, we describe a novel approach to evaluate the effects of specific PGPR strains on M. piperita. Bacterial inoculants are an effective biotechnological tool for stimulating secondary metabolism in plants. Studies of the activities of these inoculants will help clarify certain adaptive processes that are poorly understood at present. There is steadily increasing demand for environmentally safe, sustainable, organic agricultural practices that reduce negative environmental effects associated with food and feed production (Lind et al. 2004). A worldwide shift is underway from traditional inorganic farming methods toward ecofriendly, organic farming methods. For the numerous medicinal and aromatic plant species that are consumed without further processing, it is important that no synthetic compounds be present in the harvested crop. Such crop plants are ideal candidates for development of growth-promoting strategies involving PGPR and biofertilizers.
References
Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135:2025–2037
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Bakker PAHM, Pieterse CMJ, Van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathol 97:239–243
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006) Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 311:812–815
Banchio E, Zygadlo J, Valladares G (2005) Quantitative variations in the essential oil of Minthostachys mollis (Kunth.) Griseb. in response to insects with different feeding habits. J Agric Food Chem 53:6903–6906
Banchio E, Bogino P, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 36:766771
Banchio E, Xie X, Zhang H, Paré PW (2009) Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J Agric Food Chem 5:653–657
Banchio E, Bogino P, Santoro MV, Torres L, Zygadlo J, Giordano W (2010) Systemic induction of monoterpene biosynthesis in Origanum x majoricum by soil bacteria. J Agric Food Chem 58:650–654
Barbieri G, Vallone S, Orsini F, Paradiso R, De Pascale S, Negre-Zakharov F, Maggio A (2012) Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J Plant Physiol 169:1737–1746
Berry JA, Beerling DJ, Franks PJ (2010) Stomata: key players in the Earth system, past and present. Curr Opin Plant Biol 13:233–240
Bhattarai T, Hess D (1993) Yield responses of Nepalese spring wheat (Triticum aestivum L.) cultivars to inoculation with Azospirillum spp. of Nepalese origin. Plant Soil 151:67–76
Bosabalidis AM (2002) Structural features of Origanum sp. In: Kintzios SE (ed) Medicinal and aromatic plants-Industrial profiles, Oregano. The genera Origanum and Lippia. Taylor and Francis, London, pp 11–64
Boughton AJ, Hoover K, Felton GW (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol 31:2211–2216
Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12:224–230
Cappellari L, Santoro MV, Nievas F, Giordano W, Banchio E (2013) Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl Soil Ecol 70:16–22
Chalchat JC, Garry RP, Michet A (1997) Variation of the chemical composition of essential oil of Mentha piperita L. during the growing time. J Essent Oil Res 9:463–465
Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E (2008) Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fert Soils 44:501–509
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum brasilicum L. var. Genovese. Mycorrhiza 16:485–494
D’Ambrogio de Argüeso A (1986) Manual de técnicas en histología vegetal. Buenos Aires, Hemisferio Sur
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Figueiredo MVB, Seldin L, de Araujo FF, Mariano RLR (2010) Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin Heidelberg, pp 21–43
Ghirardo A, Gutknecht J, Zimmer I, Brüggemann N, Schnitzler JP (2011) Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants. PLoS One 6:e17393
Giri B, Kapoor R, Mukerji KG (2003) Influence of Arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biol Fert Soils 38:170175
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 4:109–117
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signalling processes. Soil Biol Biochem 37:395–412
Gupta ML, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular-arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour Technol 81:77–79
Harrewijn P, Van Oosten AM, Piron PGM (2001) Natural terpenoids as messengers: a multidisciplinary study of their production, biological functions, and practical applications. Springer, Kluwer Academic Publisher, Netherlands
Heil M, Silva Bueno JC (2007) Within-plant signalling by volatiles leads to induction and priming of an indirect plant defence in nature. Proc Natl Acad Sci U S A 104:5467–5472
Hudaib M, Speroni E, Di Pietra AM, Cavrini V (2002) GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J Pharm Biomed Anal 29:691–700
Hummelbrunner LA, Isman MB (2001) Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J Agric Food Chem 49:715–720
Karousou R, Grammatikopoulos G, Lanaras T, Manetas Y, Kokkini S (1998) Effects of UV-B radiation on Mentha spicata essential oils. Phytochemistry 49:2273–2277
Khaosaad T, Vierheiling H, Nell M, Zitterl-Eglsser K, Novak J (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16:443–446
Kloepper JW (1993) Plant-growth-promoting rhizobacteria as biological control agents. In: Metting FB (ed) Soil microbial ecology: applications in agricultural and environmental management. Marcel Dekker, New York, pp 255–273
Lamiri A, Lhaloui S, Benjilali B, Berrada M (2001) Insecticidal effects of essential oils against Hessian fly, Mayetiola destructor (Say). Field Crops Res 71:9–15
Lange BM, Ahkami A (2013) Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes—current status and future opportunities. Plant Biotech J 11:169–196
Lange BM, Turner GW (2012) Terpenoid biosynthesis in trichomes—current status and future opportunities. Plant Biotech J 11:2–22
Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM (2011) Improving peppermint essential oil yield and composition by metabolic engineering. Proc Natl Acad Sci U S A 108:16944–16949
Lawrence BM (2007) Mint: the genus mentha. Medicinal and aromatic plants—industrial profiles. CRC Press/Taylor & Francis, Boca Raton
Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA (2004) The tomato homolog of Coronatine-Insensitive 1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16:126–143
Lind K, Lafer G, Schloffer K, Innerhoffer G, Meister H (2004) Organic fruit growing. UK. CABI Publishing, Wallingford
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth promoting rhizobacteria. Anton van Lee Int JG 86:1–25
Marimuthu S, Subbian P, Ramamoorthy V, Samiyappan R (2002) Synergistic effect of combined application of Azospirillum and Pseudomonas fluorescens with inorganic fertilizer on root rot incidence and yield of cotton. J Plant Dis Protect 109:569–577
Mc Kinney G (1938) Some absorption spectra of leaf extract. Plant Physiol 13:128–140
McCaskill D, Croteau R (1995) Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha × piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 197:49–56
MIRC (2010) Mint Industry Res. Council, Great Falls, MT. Available at http://usmintindustry.org/ (accessed March 2014)
Mucciarelli M, Scannerini S, Bertea C, Maffei M (2003) In vitro and in vivo peppermint (Mentha piperita) growth promotion by nonmycorrhizal fungal colonization. New Phytol 158:579–591
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assay with tobacco tissue culture. Physiol Plant 15:473–497
Niranjan RS, Shetty HS, Reddy MS (2006) Plant growth promoting rhizobacteria: potential green alternative for plant productivity. In: Siddiqui ZA (ed) PGPR: Biocontrol and biofertilization. Springer, Netherlands, pp 197–216
Panou-Filotheou H, Bosabalidis AM, Karataglis S (2001) Effects of copper toxicity on leaves of oregano (Origanum vulgare subsp. hirtum). Ann Bot 88:207–214
Pineda A, Zheng SJ, Van Loon JJA, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514
Pozo MJ, Azcón-Aguilar C (2007) Unravelling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Rios-Estepa R, Lange I, Lee JM, Lange BM (2010) Mathematical modeling-guided evaluation of biochemical, developmental, environmental, and genotypic determinants of essential oil composition and yield in peppermint leaves. Plant Physiol 152:2105–2119
Ryu CM, Hu CH, Locy RD, Kloepper JW (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268:285–292
Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 24:3–21
Santoro MV, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Biochem 49:1177–1182
Schisler DA, Slininger PJ, Bothast RJ (1997) Effects of antagonist cell concentration and two strain mixtures on biological control of Fusarium dry rot of potatoes. Phytopathol 87:177–183
Schlüter U, Muschak M, Berger D, Altmann T (2003) Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J Exp Bot 54:867–874
Schmidt CS, Agostini F, Simon AM, Whyte J, Townend J, Lifert C, Killham K, Mullins C (2004) Influence of soil type and pH on the colonization of sugar beet seedlings by antagonistic Pseudomonas and Bacillus strains, and on their control of Pythium dampingoff. Eur J Plant Pathol 110:1025–1046
Shukla A, Abad Farooqi AH, Shukla YN, Sharma S (1992) Effect of triacontanol and chlormequat on growth, plant hormones andartemisinin yield in Artemisia annua L. Plant Growth Regul 11:165–171
Singh N, Luthra R, Sangwan RS (1991) Mobilization of starch and essential oil biogenesis during leaf ontogeny of lemongrass (Cymbopogon flexuosus Stapf.). Plant Cell Physiol 32:803–811
Subramanian KS, Charest C (1997) Nutritional, growth, and reproductive responses of maize (Zea mays L.) to arbuscular mycorrhizal inoculation during and after drought stress at tasselling. Mycorrhiza 7:25–32
Turner GW, Gershenzon J, Croteau RB (2000) Development of peltate glandular trichomes of peppermint. Plant Physiol 124:665–680
Unsicker S, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12:479–485
Van Loon LC (2007) Plant response to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Van Oosten VR, Bodenhausen N, Reymond P, Van Pelt JA, Van Loon LC, Dicke M (2008) Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol Plant Microbe Interact 21:919–930
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volátiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73:5639–5641
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vestberg M, Cassells AC (2009) The use of AMF and PGPR inoculants singly and combined, to promote microplant establishment, growth and health. In: Varma A, Kharkwal AC (eds) Symbiotic fungi: principles and practice. Springer, New York, pp 337–360
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
Werker E (2000) Trichome diversity and development. In: Hallahan DL, Gray JC (eds) Advances in botanical research. Academic, San Diego, pp 37–75
Zahir AZ, Arshad M, Frankenberger ET Jr (2004) Plant growth promoting rhizobacteria: application and respectives in agriculture. Adv Agron 81:97–168
Zamioudis C, Pieterse CM (2012) Modulation of host immunity by beneficial microbes. Mol Plant Microbe 25:139–150
Zeng Y, Gu LP, Che DB, Hao ZP, Wang JY, Huang LQ, Yang G, Cui XM, Yang L, Wu ZX, Chen ML, Zhang Y (2013) Arbuscular mycorrhizal symbiosys and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza 23:253–265
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56:264–273
Zheljazkov VD, Cantrell CL, Astatkie T, Ebelhar MW (2010) Productivity, oil content and composition of two spearmint species in Mississippi. Agron J102:129–133
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ 58:186–191
Acknowledgments
This study was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), MinCyT Córdoba, and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. CT, EB, and WG are Career Members of CONICET. LC and MVS received fellowships from CONICET- MinCyT. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
del Rosario Cappellari, L., Santoro, M.V., Reinoso, H. et al. Anatomical, Morphological, and Phytochemical Effects of Inoculation with Plant Growth- Promoting Rhizobacteria on Peppermint (Mentha piperita). J Chem Ecol 41, 149–158 (2015). https://doi.org/10.1007/s10886-015-0549-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-015-0549-y